1 Department of Cardiology, Harbin Medical University, 150000 Harbin, Heilongjiang, China
2 Cardiovascular Medical Department, The Fourth Affiliated Hospital of Harbin Medical University, 150000 Harbin, Heilongjiang, China
3 Department of Cardiology, Affiliated Zhongshan Hospital of Dalian University, 116000 Dalian, Liaoning, China
†These authors contributed equally.
Abstract
Differences between female and male patients may influence the outcomes of transcatheter aortic valve replacement (TAVR). However, knowledge regarding known sex differences in TAVR procedures among Chinese people remains limited. Therefore, this study aimed to investigate the impact of sex-related differences on reverse left ventricular (LV) remodeling following TAVR in the Chinese population.
Patients with severe symptomatic aortic stenosis (AS) who underwent TAVR at the Heart Center of the Affiliated Zhongshan Hospital of Dalian University were enrolled. A total of 136 patients who underwent implantation of a self-expandable Venus A valve between 2019 and 2024 were evaluated. We retrospectively compared the clinical outcomes and characteristics of all patients by sex.
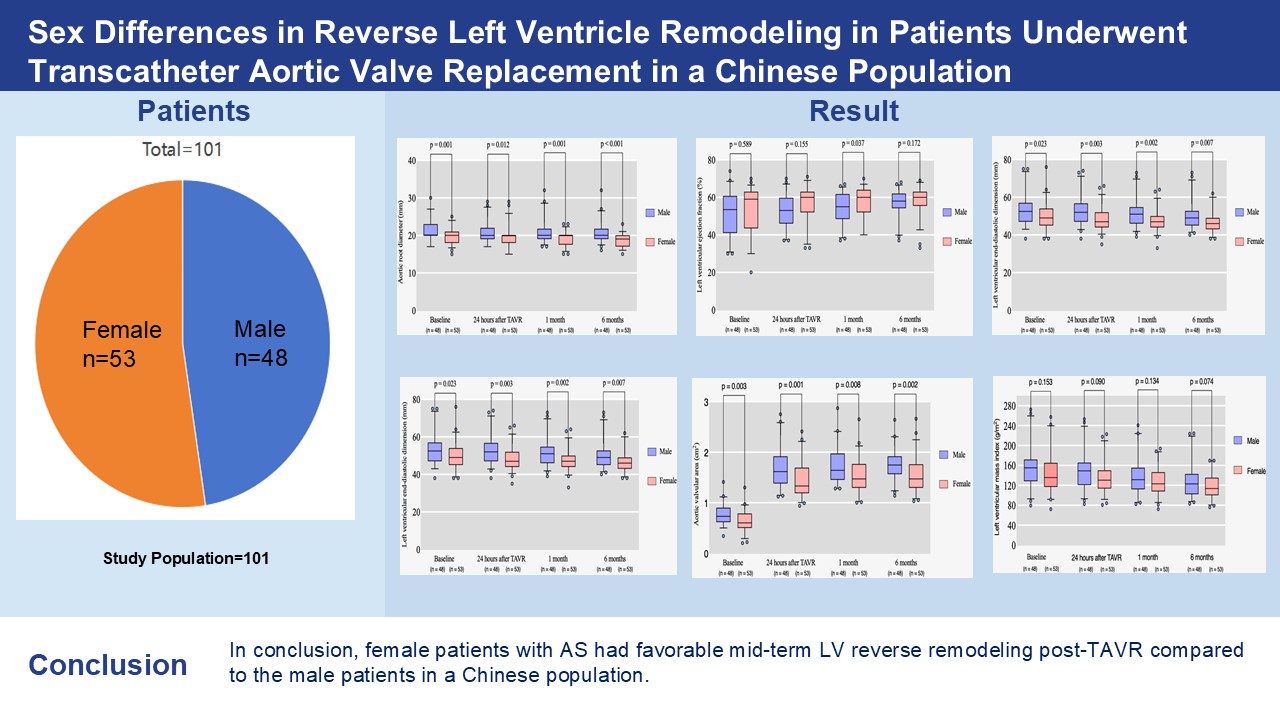
In our study, females presented with a smaller body surface area (BSA) (1.68 ± 0.15 m2 vs. 1.90 ± 0.14 m2, p < 0.001), aortic valve area (AVA) (0.64 ± 0.22 cm2 vs. 0.77 ± 0.20 cm2, p = 0.003), left ventricular end-diastole diameter (LVEDD) (49.72 ± 7.37 mm vs. 53.33 ± 8.36 mm, p = 0.023), as well as interventricular septum in diastole (IVSD) (12.85 ± 2.19 mm vs. 13.88 ± 2.61 mm, p = 0.034) at baseline. Comparatively, males had larger aortic root structures at baseline and a larger size of valve implantation during the procedure (p < 0.05). However, the indexed AVA was not significantly different between the two groups at baseline. Sex-specific outcomes, particularly AVA, LVEDD, aortic root diameter (AO), and IVSD, were significantly different during each follow-up within the first six months (p < 0.05), indicating that females experienced greater improvements in these echocardiographic characteristics after TAVR. Left ventricular ejection fraction (LVEF) only improved significantly at 1-month follow-up in females compared to males (57.77 ± 7.87% vs. 54.40 ± 8.21%, p = 0.037). Multivariable linear-regression analysis showed that being a female patient (Beta: 10.200; 95% CI: 0.075–20.326; p = 0.048), as well as having a higher IVSD (Beta: 2.939; 95% CI: 1.110–4.769; p = 0.002), and higher baseline left ventricular mass index (LVMi) (Beta: 0.409; 95% CI: 0.298–0.521; p < 0.001) were independently associated with greater mid-term LVMi regression post-TAVR.
Female patients with AS exhibited more favorable mid-term LV reverse remodeling post-TAVR compared to male patients in a Chinese population.
Graphical Abstract
Keywords
- aortic stenosis
- transcatheter aortic valve replacement
- sex differences
- left ventricular remodeling
- echocardiography
Transcatheter aortic valve replacement (TAVR) is considered to be an established percutaneous replacement for severe aortic stenosis (AS) in patients at high risk or inoperable patients [1, 2, 3, 4, 5]. More recently, it has been suggested that TAVR is noninferior to surgical aortic valve replacement (SAVR) in patients at low risk and intermediate risk [2, 3, 4]. Recent data suggest that sex-based differences in clinical outcomes do exist. Better midterm and long-term survival after TAVR are associated with female patients despite higher periprocedural complication rates, particularly increased vascular complications. In major TAVR studies, females make up roughly half of the study population. Understanding the outcome characteristics in the female population is crucial, as females often exhibit different tolerance to severe AS compared to males, leading to distinct ventricular remodeling patterns [6, 7, 8]. Left ventricular (LV) reverse remodeling, which is related to mid-term and long-term prognosis, is highly important [9, 10, 11].
Although recent publications have explored sex-specific factors associated with TAVR, the clinical outcomes have not been fully elucidated. A brief study of 305 consecutive TAVR patients revealed no sex-related differences in mortality at 30 days but did detect higher risk of bleeding or periprocedural vascular complication rates in females [12, 13]. AS induces left ventricular overload, resulting in adverse remodeling characterized by cardiac muscle hypertrophy and interstitial collagen deposition, ultimately impairing diastolic and systolic ventricular function. These changes play an important role in LV remodeling [14, 15]. This clinical outcome was associated with sex-related differences, with females exhibiting less myocardial fibrosis, more concentrated LV geometry and better myocardial systolic function [16]. Another observational study of 92 severe AS patients who underwent TAVR, which included 53 females, demonstrated that females experienced faster regression. That study concluded that females could adapt to pressure overload better and could recover faster than males [17]. Further testing of gene and biopsy data from patients with severe LV septal hypertrophy revealed that males had more LV fibrosis than females did [16]. An increase in profibrotic genes may explain why males have reverse less LV remodeling after TAVR.
Several studies have examined sex-related differences in TAVR procedures in Western populations [18, 19, 20, 21]. However, little is known about TAVR outcomes stratified by sex in the Chinese population. Thus, the aim of this study was to evaluate whether there are any sex-related differences in outcomes or reverse LV remodeling in a Chinese population.
A total of 136 patients with severe AS who underwent TAVR at the Heart Center of Affiliated Zhongshan Hospital of Dalian University between 2019 and 2024 were retrospectively evaluated. All patients who underwent TAVR were selected and reviewed by a dedicated team comprised of experienced cardiac surgeons and interventional cardiologists. Assessments of medical history, transesophageal or transthoracic echocardiography, and thoracic computed tomography were used to evaluate AS. These patients were deemed either inoperable or at high risk for SAVR after discussion with the dedicated team. The follow-up data of each patient were collected during a clinical visit or a standardized phone call at one month and six months post-discharge after TAVR. The study was approved by the local ethics committee of the Affiliated Zhongshan Hospital of Dalian University. No annexed industry funding was supplied. This study was driven by the interests of the investigators. Written informed consent was obtained from all patients prior to TAVR, and the study conformed to the Declaration of Helsinki.
TAVR was performed with a self-expanding prosthesis, the Venus A valve (Venus MedTech, Inc., Hangzhou, China). The self-expanding prosthesis possesses a refined supra-annular design devoid of an outer skirt. Retightening, repositioning and even retrieval were not allowed in the delivery catheter system. The Venus A valve has been also widely used in the Chinese population because of the design of a trileaflet valve, which is a made of porcine pericardial tissue and lacks an outer skirt. A 20-F sheath delivery system (Version 20-F Braidin™ Pro guiding catheter; APT Medical, Xiangtan, China) was used to accommodate the prosthesis. A strong radial force was the most dominant feature of the prosthesis and was more suitable for the Chinese population’s calcified anatomy [22]. Three valve sizes were widely used: 23, 26, and 29 mm. The transfemoral approach was the preferred access, besides method, in addition to the transapical approach and transaxillary approach. Several patients were unsuitable for the procedure through the iliofemoral artery. In most cases, percutaneous coronary intervention (PCI) was conducted before the TAVR procedures. PCI was conducted in the same procedural session as TAVR in only 2 male and 1 female patients. The key inclusion criteria were as follows: (1) severe AS patients diagnosed on the recommendation of the European Society of Cardiology/European Association for Cardio-Thoracic Surgery Guidelines (aortic valve area
Transthoracic echocardiographic follow-up was performed at baseline (pre-TAVR), at hospital discharge, and at one and 6 months after TAVR. Two-dimensional Doppler transthoracic echocardiography was expertly performed with a Phillips EPIQ 7 system (Phillips, Eindhoven, Netherlands) by the same echocardiologist. Standard parasternal long-axis views, short-axis views, 4-chamber views, and 2-chamber views were obtained. The Bernoulli simplified equation was used for calculating the mean pressure gradient (MPG) using continuous wave Doppler. The left ventricular end-diastole diameter (LVEDD), interventricular septum in diastole (IVSD), and left ventricular posterior wall thickness (LVPWT) were measured in two dimensions with the parasternal view on the basis of guideline recommendations [23, 24]. The LV mass index (LVMi) was calculated based on Deveraux’s formula in accordance with the joint recommendations of the European Association of Echocardiography companied with and the American Society of Echocardiography as follows: relative wall thickness (RWT) = (LVPWT
All continuous data are expressed as the means
To evaluate the changes of repeated echocardiographic characteristics between males and females, a global and mixed-effects model (main effect of sex and time, and interaction) has now been assessed using repeated-measures two-way ANOVAs, depending on the variable. The primary hypotheses focused on the difference at baseline, 24 hours after TAVR, 1-month follow-up and 6-month follow-up between the male group and female group. In a secondary analysis, the follow-up time point was also included. The follow-up was 24 hours, 1-month, and 6-month after TAVR procedures. Group allocation (male vs. female) was the between-group factor. Time was the within-group factor (24 hours, 1-month, 6-month). Compared with the same group at baseline, ap
Multivariable linear regression models were constructed to identify independent factors associated with left ventricular remodeling parameters. Adjustment variables were selected based on a two-step approach: (1) clinical relevance supported by previous literature; and (2) statistical significance in univariate analysis with a threshold of p
As shown in Fig. 1, of the 136 patients who were referred for enrollment in our study, 35 were excluded. Echocardiograms of 26 patients were not obtained in a timely manner after TAVR during the six-month follow-up because of follow-up at a referral hospital or poor image quality. Four patients died within six months of a cause unrelated to the TAVR procedure. Three patients were excluded because of cardiac-related death during the six-month follow-up. Two patients died during the procedure. The remaining 101 patients (n = 48 men and 53 women) fulfilled the study criteria.
 Fig. 1.
Fig. 1. Study flow chart. TAVR, transcatheter aortic valve replacement.
The baseline characteristics are listed in Table 1. The mean age was 74.5
| All (n = 101) | Male (n = 48) | Female (n = 53) | p value | |
| Age (years, | 74.5 | 74.3 | 74.7 | 0.771 |
| BSA (m2, | 1.78 | 1.90 | 1.68 | |
| NYHA Class III/IV | 81 (80.1) | 40 (83.3) | 41 (77.4) | 0.452 |
| CrCl (mL/minute, | 79.55 | 83.02 | 76.40 | 0.351 |
| Comorbidities: | ||||
| Dyslipidemia | 60 (59.4) | 35 (72.9) | 25 (47.2) | 0.009 |
| Diabetes | 37 (36.6) | 18 (37.5) | 19 (35.8) | 0.863 |
| Hypertention | 63 (62.4) | 30 (62.5) | 33 (62.3) | 0.981 |
| COPD | 5 (5.0) | 5 (10.4) | 0 | 0.051 |
| Stroke | 43 (42.6) | 17 (35.4) | 26 (49.1) | 0.166 |
| PVD | 30 (29.7) | 19 (39.6) | 11 (20.8) | 0.039 |
| CAD | 58 (57.4) | 30 (62.5) | 28 (52.8) | 0.326 |
| Atrial fibrillation | 31 (30.7) | 16 (33.3) | 15 (28.3) | 0.584 |
| Previous valvular replacement surgery | 3 (3.0) | 2 (4.2) | 1 (1.9) | 0.931 |
| Prior CABG | 2 (2.0) | 1 (2.1) | 1 (1.9) | 1.000 |
| Need for urgent aortic valvular intervention | 1 (1.0) | 1 (2.1) | 1 (1.9) | 1.000 |
| Risk evaluation: | ||||
| EuroScore II ( | 6.89 | 6.55 | 7.08 | 0.708 |
| STS mortality ( | 3.97 | 3.73 | 4.18 | 0.511 |
| STS morbimortality ( | 14.54 | 13.21 | 15.74 | 0.094 |
| Electrocardiogram: | ||||
| Sinus | 79 (78.2) | 37 (77.1) | 42 (79.2) | 0.800 |
| Atrial fibrillation | 17 (16.8) | 10 (20.8) | 7 (13.2) | 0.303 |
| Other atrial rhythm | 4 (4.0) | 2 (4.2) | 2 (3.8) | 1.000 |
| Abnormal cardiac electric axis | 54 (53.5) | 25 (52.1) | 29 (54.7) | 0.803 |
| 1° AVB | 16 (15.8) | 10 (20.8) | 6 (11.3) | 0.203 |
| LBBB | 4 (4.0) | 2 (4.2) | 2 (3.8) | 1.000 |
| RBBB | 12 (11.9) | 7 (14.6) | 5 (9.4) | 0.443 |
| LAFB | 5 (5.0) | 5 (10.4) | 0 | 0.053 |
| Echocardiogram: | ||||
| MPG (mmHg, | 50.19 | 47.35 | 52.75 | 0.265 |
| AVA (cm2, | 0.70 | 0.77 | 0.64 | 0.003 |
| AVA/BSA (cm2/m2, | 0.39 | 0.41 | 0.38 | 0.308 |
| LVEF (%, | 52.23 | 51.52 | 52.87 | 0.589 |
| LVEDD (mm, | 51.44 | 53.33 | 49.72 | 0.023 |
| AO (mm, | 20.44 | 21.33 | 19.62 | 0.001 |
| LVPWT (mm, | 12.12 | 12.46 | 11.81 | 0.128 |
| IVSD (mm, | 13.34 | 13.88 | 12.85 | 0.034 |
| LVMi (g/m2, | 151.17 | 157.36 | 145.56 | 0.153 |
| RWT (cm, | 0.51 | 0.51 | 0.51 | 1.000 |
BSA, body surface area; NYHA, New York Heart Association; CrCl, creatinine clearance; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; CAD, coronary artery disease; CABG, coronary artery bypass graft; STS, Society of Thoracic Surgeons; 1° AVB, first-degree atrioventricular block; LBBB, left bundle branch block; RBBB, right bundle branch block; LAFB, left anterior fascicular block; MPG, mean pressure gradient; AVA, aortic valve area; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; AO, aortic root diameter; LVPWT, left ventricular posterior wall thickness; IVSD, interventricular septum in diastole; LVMi, left ventricular mass index; RWT, relative wall thickness; p values in bold are statistically significant.
Table 1 shows the evaluation of the echocardiographic parameters before the TAVR procedure. At baseline, no significant differences were found between the two groups based on the MPG. Additionally, the aortic valve area indexed to the body surface area (AVA/BSA), left ventricular ejection fraction (LVEF), LVPWT, and RWT were comparable between males and females. This study did not find any marked sex-related differences in the LVMi, with the exception of the AVA, LVEDD, IVSD or aortic root diameter (AO). More females had a smaller AVA (0.64
Procedural data of two groups were analyzed in Table 2. As shown in Table 2, no significant differences in paravalvular leak were observed between the two male and female patients. During the procedure, more females were more likely to undergo implantation of size of Venus-A valve and a smaller aortic annulus diameter (22.97
| All (n = 101) | Male (n = 48) | Female (n = 53) | p value | ||
| Venus-A valve size | 0.037 | ||||
| 23 mm | 24 (23.8) | 11 (22.9) | 13 (24.5) | ||
| 26 mm | 53 (52.5) | 20 (41.7) | 33 (62.3) | ||
| 29 mm | 22 (21.8) | 15 (31.3) | 7 (13.2) | ||
| 32 mm | 2 (2.0) | 2 (4.2) | 0 | ||
| Aortic annulus diameter (mm, | 23.91 | 24.97 | 22.97 | 0.001 | |
| Mild paravalvular leak | 7 (6.9) | 2 (4.2) | 5 (9.4) | 0.517 | |
| Moderate/severe paravalvular leak | 7 (6.9) | 4 (8.3) | 3 (5.7) | 0.892 | |
p values in bold are statistically significant.
As shown in Table 3, the patterns of echocardiographic characteristics before and after TAVR were analyzed. The AVA in females was significantly smaller than that in males during every postoperative follow-up [24 hours after TAVR: (1.45
| Male (n = 48) | Female (n = 53) | F value | p value | ||
| MPG (mmHg, | |||||
| Baseline | 47.35 | 52.75 | 1.256 | 0.265 | |
| 24 hours after TAVR | 13.56 | 13.89 | 0.048 | 0.827 | |
| 1 M follow-up | 13.29 | 12.81 | 0.147 | 0.702 | |
| 6 M follow-up | 13.06 | 12.70 | 0.108 | 0.743 | |
| F value | 38.396 | 57.984 | |||
| p value | |||||
| Global test | |||||
| sex (F value, p value) | 0.468, 0.496 | ||||
| time (F value, p value) | 263.221, | ||||
| sex*time (F value, p value) | 1.531, 0.207 | ||||
| AVA (cm2, | |||||
| Baseline | 0.77 | 0.64 | 9.299 | 0.003 | |
| 24 hours after TAVR | 1.70 | 1.45 | 1.585 | 0.001 | |
| 1 M follow-up | 1.72 | 1.53 | 0.882 | 0.008 | |
| 6 M follow-up | 1.76 | 1.55 | 1.148 | 0.002 | |
| F value | 122.245 | 113.104 | |||
| p value | |||||
| Global test | |||||
| sex (F value, p value) | 14.884, | ||||
| time (F value, p value) | 395.596, | ||||
| sex*time (F value, p value) | 1.296, 0.276 | ||||
| AVA/BSA (cm2/m2, | |||||
| Baseline | 0.41 | 0.38 | 1.052 | 0.308 | |
| 24 hours after TAVR | 0.89 | 0.87 | 0.510 | 0.477 | |
| 1 M follow-up | 0.92 | 0.91 | 0.013 | 0.908 | |
| 6 M follow-up | 0.93 | 0.93 | 0.001 | 0.977 | |
| F value | 113.061 | 134.324 | |||
| p value | |||||
| Global test | |||||
| sex (F value, p value) | 0.317, 0.575 | ||||
| time (F value, p value) | 385.871, | ||||
| sex*time (F value, p value) | 0.275, 0.843 | ||||
| LVEF (%, | |||||
| Baseline | 51.52 | 52.87 | 0.294 | 0.589 | |
| 24 hours after TAVR | 53.19 | 55.87 | 2.053 | 0.155 | |
| 1 M follow-up | 54.40 | 57.77 | 4.455 | 0.037 | |
| 6 M follow-up | 56.79 | 58.75 | 1.891 | 0.172 | |
| F value | 6.560 | 6.999 | |||
| p value | |||||
| Global test | |||||
| sex (F value, p value) | 1.912, 0.170 | ||||
| time (F value, p value) | 24.180, | ||||
| sex*time (F value, p value) | 0.831, 0.478 | ||||
| LVEDD (mm, | |||||
| Baseline | 53.33 | 49.72 | 5.340 | 0.023 | |
| 24 hours after TAVR | 52.75 | 48.49 | 9.030 | 0.003 | |
| 1 M follow-up | 51.69 | 47.53 | 10.277 | 0.002 | |
| 6 M follow-up | 50.48 | 46.87 | 7.531 | 0.007 | |
| F value | 4.731 | 5.447 | |||
| p value | 0.004 | 0.002 | |||
| Global test | |||||
| sex (F value, p value) | 8.769, 0.004 | ||||
| time (F value, p value) | 20.490, | ||||
| sex*time (F value, p value) | 0.402, 0.751 | ||||
| AO (mm, | |||||
| Baseline | 21.33 | 19.62 | 12.689 | 0.001 | |
| 24 hours after TAVR | 20.79 | 19.38 | 6.612 | 0.012 | |
| 1 M follow-up | 20.90 | 19.21 | 11.261 | 0.001 | |
| 6 M follow-up | 20.69 | 18.70 | 19.295 | ||
| F value | 1.586 | 57.984 | |||
| p value | 0.198 | 0.003 | |||
| Global test | |||||
| sex (F value, p value) | 16.484, | ||||
| time (F value, p value) | 4.270, 0.006 | ||||
| sex*time (F value, p value) | 0.572, 0.634 | ||||
| LVPWT (mm, | |||||
| Baseline | 12.46 | 11.81 | 2.362 | 0.128 | |
| 24 hours after TAVR | 12.02 | 11.68 | 0.913 | 0.342 | |
| 1 M follow-up | 11.77 | 11.49 | 0.754 | 0.280 | |
| 6 M follow-up | 11.54 | 11.06 | 3.076 | 0.485 | |
| F value | 6.236 | 6.125 | |||
| p value | 0.001 | 0.001 | |||
| Global test | |||||
| sex (F value, p value) | 1.890, 0.172 | ||||
| time (F value, p value) | 18.968, | ||||
| sex*time (F value, p value) | 1.016, 0.386 | ||||
| IVSD (mm, | |||||
| Baseline | 13.88 | 12.85 | 4.621 | 0.034 | |
| 24 hours after TAVR | 13.56 | 12.58 | 5.533 | 0.021 | |
| 1 M follow-up | 12.94 | 12.17 | 4.319 | 0.040 | |
| 6 M follow-up | 12.69 | 11.74 | 7.991 | 0.006 | |
| F value | 8.497 | 8.053 | |||
| p value | |||||
| Global test | |||||
| sex (F value, p value) | 6.322, 0.014 | ||||
| time (F value, p value) | 31.205, | ||||
| sex*time (F value, p value) | 0.374, 0.772 | ||||
| LVMi (g/m2, | |||||
| Baseline | 157.36 | 145.56 | 2.069 | 0.153 | |
| 24 hours after TAVR | 148.20 | 136.40 | 2.933 | 0.090 | |
| 1 M follow-up | 137.10 | 127.51 | 2.280 | 0.134 | |
| 6 M follow-up | 128.46 | 118.09 | 3.260 | 0.074 | |
| F value | 18.796 | 17.800 | |||
| p value | |||||
| Global test | |||||
| sex (F value, p value) | 2.896, 0.092 | ||||
| time (F value, p value) | 73.769, | ||||
| sex*time (F value, p value) | 0.149, 0.930 | ||||
| RWT (cm, | |||||
| Baseline | 0.51 | 0.51 | 0.000 | 1.000 | |
| 24 hours after TAVR | 0.50 | 0.51 | 0.262 | 0.610 | |
| 1 M follow-up | 0.49 | 0.51 | 0.641 | 0.425 | |
| 6 M follow-up | 0.49 | 0.49 | 0.014 | 0.905 | |
| F value | 1.340 | 1.087 | |||
| p value | 0.266 | 0.358 | |||
| Global test | |||||
| sex (F value, p value) | 0.134, 0.715 | ||||
| time (F value, p value) | 2.472, 0.089 | ||||
| sex*time (F value, p value) | 0.707, 0.490 | ||||
MPG, mean pressure gradient; AVA, aortic valve area; BSA, body surface area; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; AO, aortic root diameter; LVPWT, left ventricular posterior wall thickness; IVSD, interventricular septum in diastole; LVMi, left ventricular mass index; RWT, relative wall thickness; TAVR, transcatheter aortic valve replacement. Column 2 and 3: compared with the same group at baseline, ap
 Fig. 2.
Fig. 2. Changes of echocardiographic characteristics at six-month follow-up post-TAVR in male and female patients. Changes in mean pressure gradient (A), aortic valve area (B), aortic valve area/body surface area (C), left ventricular ejection fraction (D), left ventricular end-diastolic dimension (E), aortic root diameter (F), left ventricular posterior wall thickness (G), interventricular septum in diastole (H), left ventricle mass index (I) and relative wall thickness (J) at six-month follow-up post-TAVR in male and female patients. MPG, mean pressure gradient; AVA, aortic valve area; BSA, body surface area; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; AO, aortic root diameter; LVPWT, left ventricular posterior wall thickness; IVSD, interventricular septum in diastole; LVMi, left ventricular mass index; RWT, relative wall thickness; TAVR, transcatheter aortic valve replacement.
For both males and females, as shown in Table 3, the improvements in the MPG, AVA, AVA/BSA and LVMi post-TAVR were significant at every postoperative follow-up compared to those pre-TAVR at baseline (ap
For male patients, the improvements in the LVPWT post-TAVR were significant at every postoperative follow-up compared to those pre-TAVR at baseline (ap
In the male group, the remodeling in the IVSD and LVMi were significant at 1-month post-TAVR compared with baseline (ap
Furthermore, in the Female group, the remodeling in the LVEF, LVEDD were significant at every postoperative follow-up compared to those pre-TAVR at baseline (ap
For female patients, the remodeling in the LVEF, IVSD and LVMi were significant at 1-month post-TAVR compared with baseline (ap
Sex-specific outcomes, especially AVA, LVEDD, AO, IVSD were significantly different during each follow-up within six-month (p
Table 4 presents the results of the multivariable linear-regression analysis, which assessed the independent predictors of greater mid-term LV mass regression post-TAVR. After adjusting for sex, BSA, dyslipidemia, PVD, AVA, AO, IVSD, and baseline LVMi, only sex, IVSD, and baseline LVMi were independently associated with greater mid-term LVMi regression post-TAVR. Multivariable linear-regression analysis showed only female patients (Beta: 10.200; 95% CI: 0.075–20.326; p = 0.048), higher IVSD (Beta: 2.939; 95% CI: 1.110–4.769; p = 0.002), and higher baseline LVMi (Beta: 0.409; 95% CI: 0.298–0.521; p
| Model 1 | Model 2 | |||||
| Beta | 95% CI | p value | Beta | 95% CI | p value | |
| Sex | –1.424 | –12.388 to 9.539 | 0.797 | 10.200 | 0.075 to 20.326 | 0.048 |
| BSA (m2) | –6.089 | –36.496 to 24.317 | 0.692 | 7.982 | –19.458 to 35.422 | 0.565 |
| Dyslipidemia | –2.740 | –13.879 to 8.399 | 0.627 | –1.731 | –9.837 to 6.375 | 0.672 |
| PVD | 5.402 | –6.535 to 17.339 | 0.371 | –3.337 | –11.945 to 5.271 | 0.443 |
| Baseline AVA (cm2) | –9.637 | –35.015 to 15.741 | 0.453 | 6.324 | –12.696 to 25.345 | 0.511 |
| Baseline AO (mm) | 2.431 | 0.325 to 4.537 | 0.024 | 1.354 | –0.257 to 2.965 | 0.099 |
| Baseline IVSD (mm) | 5.670 | 3.716 to 7.624 | 2.939 | 1.110 to 4.769 | 0.002 | |
| Baseline LVMi (g/m2) | 0.474 | 0.381 to 0.568 | 0.409 | 0.298 to 0.521 | ||
Model 1. Crude analysis; Model 2. Adjusted for sex, BSA, dyslipidemia, PVD, baseline AVA, baseline AO, baseline IVSD, baseline LVMi; BSA, body surface area; PVD, peripheral vascular disease; AVA, aortic valve area; AO, aortic root diameter; IVSD, interventricular septum in diastole; LVMi, left ventricular mass index; p values in bold are statistically significant; F-test: F = 15.907, p
 Fig. 3.
Fig. 3. Factors associated with mid-term regression of LVMi (
In conclusion, females with AS had favorable mid-term LV reverse remodeling post-TAVR compared to the males. The thicker IVSD and the more severe LV diastolic function before surgery, the greater the mid-term LV reverse remodeling after surgery, as shown in Fig. 3.
Although the TAVR procedure has been increasingly performed across Asian countries, the use of the TAVR procedure is still common in the Chinese population. The present study provides several valuable insights into the procedure of TAVR and its interaction with sex. This consecutive cohort study evaluated the trends in LV remodeling in patients who underwent isolated TAVR according to sex. The major findings of this study are as follows: (1) At baseline, females had smaller BSA, AVA, LVEDD, IVSD, and lower rates of dyslipidemia and PVD compared to males; (2) Males exhibited larger aortic root structures and received larger prosthetic valves, although indexed AVA did not differ significantly between sexes; (3) Sex-specific differences in echocardiographic parameters (AVA, LVEDD, AO, IVSD) were observed throughout follow-up, with females showing greater improvements post-TAVR; (4) LVEF improved significantly only at 1-month follow-up in females; (5) Females demonstrated more favorable mid-term LV reverse remodeling (
Our study showed that women tend to have a lower incidence of dyslipidemia and PVD at baseline. Sex plays an important role in the development of AS, which leads to sex-specific differences with respect to the modulation of pathological processes [27, 28, 29]. Previous studies have demonstrated that almost half of females with severe AS are asymptomatic, resulting in a lower rate of diagnosis and treatment [30]. A study has shown that older females are associated with a higher incidence of symptomatic heart failure [27]. Consistent with previous findings [17, 31] that males tend to have a higher incidence of comorbidities, our study also found significant sex-specific differences in comorbidities, such as dyslipidemia and peripheral artery disease.
Women with smaller AO at baseline had a smaller size of valve implantation during TAVR were found in our study. A similar conclusion has been reached by a previous study on the very small BSA and aortic annulus in female Asians [32]. As shown in a previous study, the prostheses used for females tended to be smaller in size because of their smaller physical stature [33]. The sex-related differences in AO measurements were in accordance with recent computed tomography (CT) studies, which demonstrated larger diameters in male patients. The diameters were measured across multiplanar planes from the supra-annular to the subannular levels [34, 35]. This should be the focus of interobserver analysis because subjective measurements of AO are needed [36]. Thus, preprocedural measurements of aorta ascendens should be carefully standardized and further refined to understand the influence of sex. A larger type of aortic root structures in male patients might contribute to a higher incidence of valve-in-valve implantation after TAVR. Valve-in-valve implantation has already been known to be a higher technical complication. A previous study has demonstrated that choosing a prosthetic valve with moderate oversizing and more thorough measurement of anatomy for male patients may lead to better surgical outcomes [37].
In our cohort, women had favorable mid-term LV reverse remodeling (
The obstruction resulting from aortic stenosis leads to pressure overload on the LV, prompting the development of concentric myocardial hypertrophy as an adaptive response to reduce wall stress [46, 47]. In the early stages of the disease, these alterations contribute to diastolic dysfunction by diminishing LV compliance, while systolic function remains relatively preserved. However, as the condition progresses, myocardial contractile function and deformation become compromised, ultimately leading to decreased cardiac output. These changes can be effectively monitored through accurate assessment of trans-valvular Doppler velocities and pressure gradients, both of which decrease as systolic function deteriorates significantly. Additionally, the left atrium undergoes morphological changes mirroring those of the LV, enlarging in response to chronic pressure overload [48]. This, in turn, results in elevated pulmonary venous and arterial pressures, culminating in heart failure.
Simard et al. [49] and Treibel et al. [50] reported that extracellular matrix expansion and myocardial fibrosis were detected in males with severe AS [33]. Cardiac magnetic resonance (CMR) and late gadolinium enhancement (LGE) could be used for identifying and quantifying cardiac muscle fibrosis to estimate LV remodeling [51, 52]. The superimposed pressure load on the LV caused by severe AS leads to hypertrophy in musculus cardiacus, resulting in structural changes in the LV. An acute decrease in the MPG caused by the TAVR may lead to LV unloading. The release of pressure overload in the LV may ultimately reverse LV remodeling and improve clinical outcomes [53, 54]. The study of sex-related mechanisms, including cellular, molecular and neurohormonal mechanisms, has been proposed. A previous study indicated not only increased interstitial fibrosis, increased proinflammatory pathway activity and increased profibrotic activation but also showed that there was differential expression of estrogen and androgen receptors [14, 16, 55, 56]. Other studies have shown that the increase in cardiac fibrosis observed in male patients with severe AS is related to increased SMAD family member 2 (SMAD2) phosphorylation and TGF-
The frequency of AS and transthyretin-related amyloid cardiomyopathy (ATTR-CM) increases with age. ATTR-CM can be found in 4 to 16% of the patients with aortic stenosis [58]. This condition profoundly affects the outcome which may lead to sex differences in AS pathology before TAVR and LV reverse remodeling after TAVR. This overlap is not coincidental. Both conditions share common demographic risk factors such as advanced age and male sex, and may present with similar clinical manifestations, including heart failure with preserved ejection fraction (HFpEF), increased LVWT, and low-flow, low-gradient AS [59]. ATTR-CM is an increasingly recognized form of infiltrative cardiomyopathy caused by the deposition of misfolded transthyretin (TTR) protein fibrils in the myocardial extracellular space. There are two main types: wild-type (ATTRwt), which primarily affects elderly individuals, and hereditary or variant (ATTRv), which is linked to TTR gene mutations [58]. ATTR-CM itself does not directly cause aortic valve stenosis, but it is often associated with it, particularly in elderly patients. The connection between the two conditions is believed to arise from shared age-related degenerative processes. In wild-type transthyretin amyloidosis (ATTRwt), misfolded transthyretin proteins are deposited not only in the myocardium but also in valvular tissue, including the aortic valve. This can contribute to valvular thickening, fibrosis, and calcification, which are key pathological features of aortic stenosis. Additionally, chronic pressure overload from AS may accelerate myocardial stress and promote amyloid deposition in the heart, creating a vicious cycle. Thus, in aging individuals—especially men—it is common to find both AS and ATTR-CM coexisting due to overlapping mechanisms involving senile systemic amyloidosis, degenerative valve disease, and age-related cardiac remodeling [60]. The presence of ATTR-CM in patients with AS especially males is clinically significant because it can influence both treatment strategies and prognosis. Patients with dual pathology (AS + ATTR-CM + males) tend to have worse outcomes after valve replacement compared to those with isolated AS, including higher rates of persistent heart failure symptoms, reduced functional recovery, and increased mortality. In the context of the Chinese population, data are limited, but the aging demographics suggest that ATTR-CM may also be underdiagnosed among elderly Chinese patients with AS. As such, further studies are needed to explore its prevalence and clinical impact in this population, and to determine whether systematic screening could improve patient management and outcomes.
In line with our imaging studies, the thicker IVSD and the more severe LV diastolic function before surgery, the greater the mid-term LV reverse remodeling after surgery in our observations. The definition of LV hypertrophy and LV diastolic dysfunction as increasing LVMi. LV pressure overload is caused by AS through a continuous increase in valvular resistance, resulting in structural changes in the LV. The superimposed pressure applied to the LV caused by AS may ultimately lead to structural changes in the LV, which indicates LV hypertrophy. The MPG offers the most value for assessing the degree of AS [61]. Patients with higher MPG at baseline may also represent those with chronic and severe cardiac muscle fibrosis. Patients with higher LVMi at baseline were more likely to have underlying myocardial fibrosis [62]. A higher baseline LVMi may indicate that patients have a poor contractile reserve and substantial chronic myocardial damage due to LV pressure overload. E.K. Sim et al. [63] concluded that the extent of LVM regression may differ among individuals. The extent to which LVM regresses is affected by sex, age, the prosthetic valve size, and the prosthesis-patient mismatch. Hypertrophy is usually associated with increased fibrosis and decreased structural reversibility due to long-term overload [64]. Myocardial fibrosis commonly occurs in response to myocyte apoptosis, replacement fibrosis and expansion of the extracellular space in most patients with severe AS [65]. The early phase of LVM tends to regress because of the relief of LV pressure overload. The early phase of regression of myocardial edema only lasts several months, but it takes years for the late phase of LVM regression because of remodeling of interstitial fibrosis.
Although our study found the outcomes of TAVR procedures are different between different gender, this study has some limitations. First, our research involved a single-center study and a small-sized registry study. All clinical event data were obtained via review of medical records and telephone interviews. However, Firth’s correction was used for revision in our study due to its small sample size. Therefore, we believe that our use of multivariable analysis is statistically justifiable and provides valid insights within the limitations of our cohort. A further study with multicenter, large-scale, long-term follow-up cohorts and clinical outcomes would be better for evaluating LVMi regression. Second, the patients in our study did not undergo routine CMR. CMR offers a more accurate measurement of LVH and cardiac muscle fibrosis than echocardiography. The CMR is the gold standard for evaluating LV remodeling. However, further studies via CMR data are needed to determine the relationships between myocardial fibrosis and changes in LVMi. Third, further studies concerning the complications of TAVR are needed to assess the sex-related differences in reverse LV remodeling. In addition, based on echocardiographic measurements of LVM and RWT, four patterns were defined: normal geometry (NG), concentric remodeling (CR), concentric hypertrophy (CH), and eccentric hypertrophy (EH). Yet, the effect of different LV geometry was not determined. Due to the small sample size, it is challenging to perform statistical analysis based on the four cardiac remodeling patterns (NG, CR, CH, EH). In future studies, further studies enrolling a large number of patients with different LV geometry of patients who underwent TAVR are warranted to facilitate a more comprehensive classification and analysis. Moreover, although data specific to the Chinese population are limited, the aging trend suggests that ATTR-CM may be under-recognized in elderly Chinese patients with aortic stenosis. Therefore, additional research is warranted to investigate its prevalence, clinical significance, and the potential influence of sex-related differences on management strategies and patient outcomes.
This consecutive cohort study evaluated the trends in LV remodeling in patients who underwent isolated TAVR according to sex in a Chinese Population. The following major findings were identified: (1) females had a smaller BSA, AVA, LVEDD, IVSD and a lower incidence of dyslipidemia and PVD at baseline; (2) men had a larger aortic root structures at baseline and a larger size of valve implantation during the procedure, although indexed AVA was not significantly different between the two groups; (3) sex-specific outcomes, especially AVA, LVEDD, AO, IVSD were significantly different during each follow-up, which indicated that females had greater improvements after TAVR in those echocardiographic characteristics; (4) LVEF only improved significantly at 1-month follow-up in females compared to males; (5) women had favorable mid-term LV reverse remodeling (
TAVR in both groups appeared to be safe, and feasible according to our study in a Chinese Population. Multivariable linear-regression analysis showed only that female patients, higher IVSD, and higher baseline LVMi were independently associated with greater mid-term LVMi regression post-TAVR, indicating that the female sex is an independent predictor for favorable mid-term LV remodeling after TAVR.
The data sets analyzed during the current study are not publicly available due to restrictions apply to the availability of these data but are available from the corresponding authors on reasonable request.
JZ and CC designed the study and interpreted the results; YW and YS were responsible for the data analyses and manuscript writing; LC, QM and SZ contributed to the results interpretation and discussion; JZ and CC helped with data collection and pre-processing; EJ and JL approved the manuscript submission. All authors contributed to the conception and editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The studies involving human were reviewed and approved by the Ethics Committee of Affiliated Zhongshan Hospital of Dalian University (No. 2021062). The written informed consent was provided by the patients/participants in this study, and the study conformed to the Declaration of Helsinki.
We thank all study participants and all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/RCM39581.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



