1 Cardiovascular Department, Xiyuan Hospital of China Academy of Chinese Medical Sciences, 100091 Beijing, China
2 Geriatrics Department, The People's Hospital Medical Group of Xiangzhou, 519000 Zhuhai, Guangdong, China
3 National Clinical Research for Chinese Medicine Cardiology, 100091 Beijing, China
†These authors contributed equally.
Abstract
While the invasive index of microcirculation resistance (IMR) remains the gold standard for diagnosing coronary microvascular dysfunction (CMD), its clinical adoption is limited by procedural complexity and cost. Angiography-based IMR (Angio-IMR), a computational angiography-based method, offers a promising alternative. This study evaluates the diagnostic efficacy of Angio-IMR for CMD detection in angina pectoris (AP).
A comprehensive literature search was conducted across PubMed, Embase, Scopus, and the Cochrane Library to identify studies assessing Angio-IMR's diagnostic performance for CMD in AP populations. Primary outcomes included pooled sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the receiver operating characteristic (ROC) curve (AUC).
11 studies involving 927 patients were included. Angio-IMR demonstrated robust diagnostic performance: sensitivity 86% (95% CI: 0.83–0.90), specificity 90% (95% CI: 0.87–0.92), PPV 82% (95% CI: 0.78–0.86), NPV 91% (95% CI: 0.88–0.94), and AUC 0.91 (95% CI: 0.89–0.94), with low heterogeneity. Subgroup analyses revealed no significant differences in diagnostic accuracy between obstructive (stenosis ≥50%) and non-obstructive coronary artery disease. Hyperemic Angio-IMR measurements (adenosine-induced) showed superior sensitivity (89% vs. 86%) and specificity (94% vs. 91%) compared to resting-state assessments by AccuFFR system. Additionally, the sensitivity (88% vs. 82%), specificity (92% vs. 86%), PPV (82% vs. 78%) and NPV (91% vs. 88%) calculated based on AccuFFR were higher than that of quantitative flow ratio (QFR).
Angio-IMR is a reliable, non-invasive tool for CMD identification in angina patients, particularly under hyperemic conditions. Its diagnostic consistency across stenosis severity subgroups supports broad clinical applicability.
Keywords
- angina pectoris
- coronary artery disease
- angiography-based index of microcirculation resistance
- index of microcirculation resistance
- coronary microvascular dysfunction
Research indicates that ischemic heart disease (IHD) is a critical cause of cardiac death [1, 2]. The incidence of myocardial infarction in women is closely associated with the progressive rise in fatal IHD rates with advancing age [3]. As of 2018, the IHD mortality rate among non-Hispanic white women reached 64.9% per 100,000 population [3]. A U.S. study revealed that since 2000, there has been minimal improvement in IHD mortality among young women [4], which may be linked to insufficient risk communication by physicians. A survey showed that only 21% of women were informed about the potential adverse outcomes of IHD [5]. Although prior studies have exhaustively analyzed the poor prognosis of IHD, it remains a syndrome encompassing complex pathophysiology [6, 7, 8], with its conceptual scope extending beyond myocardial ischemia caused by atherosclerosis. Notably, the ORBITA (Optimal Medical Therapy of Angioplasty in Stable Angina) [9] and ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) [10] trials have challenged the traditional coronary stenosis-centric therapeutic paradigm for IHD. Furthermore, the SCOT-HEART (Scottish Computed Tomography of the Heart) study [11] demonstrated that most patients with coronary heart disease (CHD) lack epicardial stenosis, suggesting that angina symptoms and ischemic manifestations in non-obstructive CHD may stem from impaired microvascular regulation. Consequently, IHD caused by coronary microvascular dysfunction (CMD) has emerged as a pivotal factor in evaluating coronary revascularization and prognosis, making coronary microvascular disease a pressing public health challenge.
CMD is characterized by functional and structural abnormalities in the microvasculature (non-atherosclerotic stenosis) that disrupt coronary blood flow regulation, manifesting as enhanced microvascular constriction, impaired endothelium-dependent/independent vasodilation, and elevated microcirculation resistance [12, 13]. Diagnosis of CMD relies on imaging and functional assessments, with the pressure wire-derived index of microcirculation resistance (IMR) currently serving as a key tool for evaluating coronary microvascular disease (CMVD) [14, 15, 16, 17]. However, this invasive approach requires pharmacologically induced maximal coronary hyperemia to obtain measurements, often causing adverse effects such as chest tightness and bradycardia. Recent research has focused on angiography-based IMR (Angio-IMR), a non-invasive method that indirectly assesses coronary functional parameters without additional procedures. This study aims to evaluate the diagnostic efficiency of Angio-IMR for identifying CMD in populations suffering from angina pectoris.
We performed a systematic literature search across PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (http://www.embase.com), Scopus (https://www.scopus.com), and the Cochrane Library (http://www.thecochranelibrary.com). To ensure comprehensive coverage, no population restrictions were imposed, and all search terms related to Angio-IMR and its variants (including AMR, CaIMR, and AccuIMR) were systematically retrieved. The complete search strategy is detailed in Supplementary Data.
Two investigators (WW and YC) independently screened studies through a two-phase process: an initial review of titles and abstracts followed by full-text review. Discrepancies in eligibility assessment were resolved through adjudication by a third researcher (FHZ). Studies were retained if they were in accord with the inclusion criteria: (i) IMR was quantified using a pressure guide wire; (ii) Participants had angina pectoris, including chronic coronary syndrome (CCS), stable angina, or unstable angina; (iii) The study reported diagnostic performance metrics (e.g., sensitivity, specificity) of angiography-derived IMR (Angio-IMR) for detecting CMD. The main exclusion criteria included: (i) Absence of extractable diagnostic metrics including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV); (ii) Lack of Angio-IMR reporting; (iii) IMR measurements in patients with cardiomyopathy, valvular heart disease, coronary artery bypass grafts (CABG), or cardiac transplants; (iv) Duplicate literature, animal experiments, or non-research articles.
Two investigators independently conducted data extraction with subsequent cross-validation to ensure accuracy. The collected variables comprised author information, publication year, research design, and baseline demographic characteristics. For methodological quality evaluation, the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool [18] was employed to assess four critical dimensions: patient selection criteria, index test methodology, reference standard validity, and temporal consistency in testing procedures [18].
Diagnostic accuracy metrics—including sensitivity, specificity, PPV and NPV, and their 95% confidence intervals (95% CI)—were extracted from the included studies. Statistical analyses were performed using Stata 15.0 (StataCorp LLC, College Station, TX, USA) to calculate pooled estimates of sensitivity, specificity, PPV, NPV, and the area under the receiver operating characteristic (ROC) curve (AUC). Study quality was appraised with Review Manager 5.4 (The Cochrane Collaboration, Oxford, UK). A random-effects model was employed for meta-analysis, with forest plots generated to visualize effect sizes across studies. Heterogeneity was assessed using Higgins’ I2 statistic (
The systematic search protocol identified 4997 potentially relevant records across four biomedical databases: PubMed (n = 1446), Embase (n = 1095), Scopus (n = 1400), and Cochrane Library (n = 1056). Following rigorous screening procedures, 11 eligible studies [17, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29] met the inclusion criteria, with the complete selection workflow visually delineated in Fig. 1.
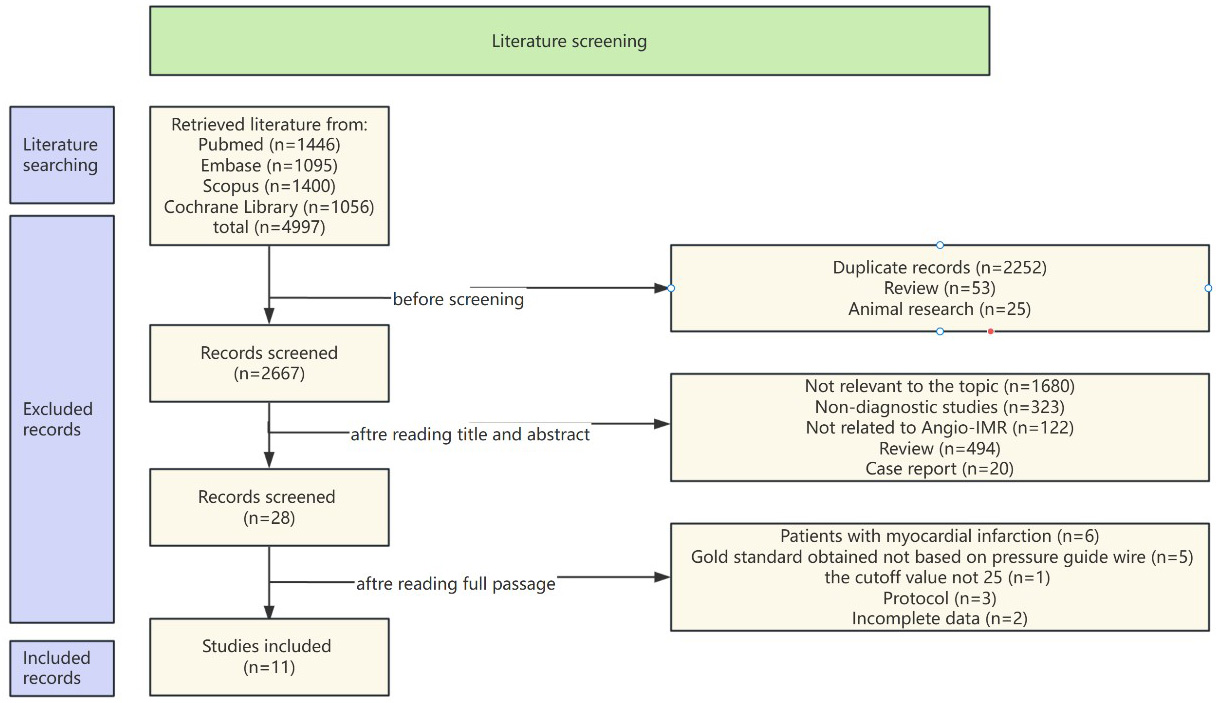 Fig. 1.
Fig. 1. Flow chart. Angio-IMR, angiography-based instantaneous wave-free ratio.
The study included first author, year, country, number of cases in the included studies, baseline characteristics, Angio-IMR cut-off value, study population, and study design, as detailed in Table 1 (Ref. [17, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29]).
| First Author | Year | Country | Study design | Disease | Number of patients | AGE (years) | Male (%) | Number of vessels | Cutoff value | Angiography‐based FFR |
| Zhongjue Qiu [20] | 2024 | China | single‐center | CCS | 75 | 54.30 | 36 (48%) | 79 | 2.6 mmHg·s/cm | QFR |
| Beibei Gao [21] | 2024 | China | single‐center | CCS | 66 | 67.74 | 37 (56%) | 103 | 2.66 mmHg·s/cm | QFR |
| Chenguang Li [22] | 2023 | China | single‐center | CCS | 101 | 61 | 78 (77%) | 101 | unknown | AccuFFR |
| Yongzhen Fan [23] | 2023 | China | single‐center | CCS | 61 | unknown | unknown | unknown | unknown | AccuFFR |
| Dong Huang [17] | 2023 | China | multi-center | INOCA | 116 | 62.9 | 64 (55%) | 113 | unknown | caFFR |
| Jun Jiang [24] | 2022 | China | multicenter | CCS | 203 | 64 | 140 (69%) | 203 | unknown | AccuFFR |
| Hernan Mejia-Renteria [25] | 2021 | UK | multicenter | CCS | 104 | 64.2 | 79 (76%) | 115 | unknown | QFR |
| Matteo Tebaldi [26] | 2020 | Italy | single‐center | CCS | 44 | 70 | 36 (82%) | 44 | 25 U | QFR |
| Hu Ai [27] | 2020 | China | multicenter | INOCA | 56 | 61.9 | 30 (54%) | 57 | 25 U | AccuFFR |
| Roberto Scarsini [28] | 2021 | UK | single‐center | CCS | 36 | 67 | 24 (67%) | 52 | 25 U | QFR |
| Yongzhen Fan [29] | 2025 | China | single‐center | INOCA | 65 | 64 | unknown | unknown | 25 U | unknown |
CCS, chronic coronary syndrome; INOCA, ischemia with non-obstructive coronary arteries; UK, The United Kingdom of Great Britain and Northern Ireland; IMR, index of microcirculation resistance; FFR, fractional flow reserve; U, unit; QFR, quantitative flow ratio; AccuFFR, accelerated fractional flow reserve; CaFFR, coronary angiography-derived fractional flow reserve.
Methodological appraisal conducted via QUADAS-2 (Fig. 2) revealed inherent validity concerns. All studies prospectively enrolled consecutive patients with rigorously defined exclusion criteria; thus, none employed a case-control design. However, potential bias may arise from the lack of predefined diagnostic cutoff value for Angio-IMR in these prospective studies. Notably, most trials adopted a blinded design to minimize observer bias, and the diagnostic criteria for CMD based on the gold standard (IMR) followed consensus-derived cutoffs [30].
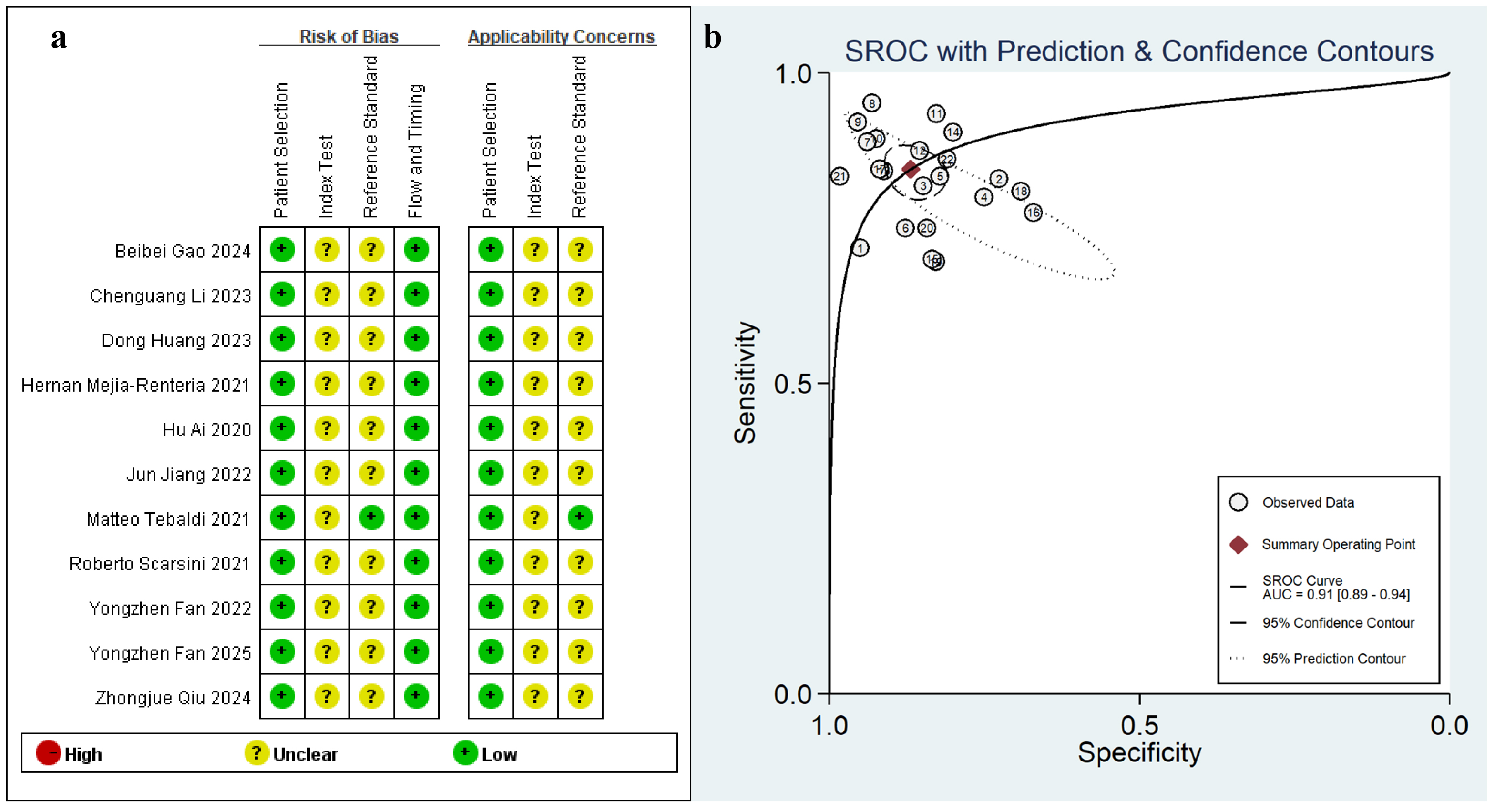 Fig. 2.
Fig. 2. Cumulative traffic light plots and weighted bar plots of the risk of bias (a), the summary receiver operating characteristic curve (SROC) (b). AUC, area under the curve.
11 eligible studies involving 927 participants were analyzed in this meta-analysis. Diagnostic performance evaluation demonstrated Angio-IMR exhibited 86% sensitivity (95% CI: 0.83–0.90; I2 = 13.3%, p
 Fig. 3.
Fig. 3. Pooled Overall Results. Forest plots showing the pooled sensitivity (a), specificity (b), positive predictive value (c) and negative predictive value (d).
3.3.2.1 Obstructive CAD and non-obstructive CAD
Subgroup analyses were stratified based on clinical characteristics. Three studies focused on ischemia with non-obstructive coronary arteries (INOCA) cohorts, while five [17, 20, 21, 23, 28] evaluated Angio-IMR in target vessels post-percutaneous coronary intervention (PCI), collectively representing eight studies involving patients with non-obstructive coronary artery disease. The aggregate sensitivity remained robust at 86% (95% CI: 0.81–0.91; p
 Fig. 4.
Fig. 4. Description of cumulative results in non-obstructive CAD. Forest plots showing the pooled sensitivity (a), specificity (b), positive predictive value (c) and negative predictive value (d).
Four studies specifically investigated obstructive CAD populations, defined by coronary stenosis
 Fig. 5.
Fig. 5. Description of cumulative results in obstructive CAD. Forest plots showing the pooled sensitivity (a), specificity (b), positive predictive value (c) and negative predictive value (d).
3.3.2.2 Angiography-based fractional flow reserve (FFR)
Four independent studies utilizing the Accelerated Fractional Flow Reserve (AccuFFR) computational platform for angio-IMR quantification were analyzed. This subgroup demonstrated exceptional diagnostic consistency, with aggregated sensitivity and specificity reaching 88% (95% CI: 0.84–0.92; p
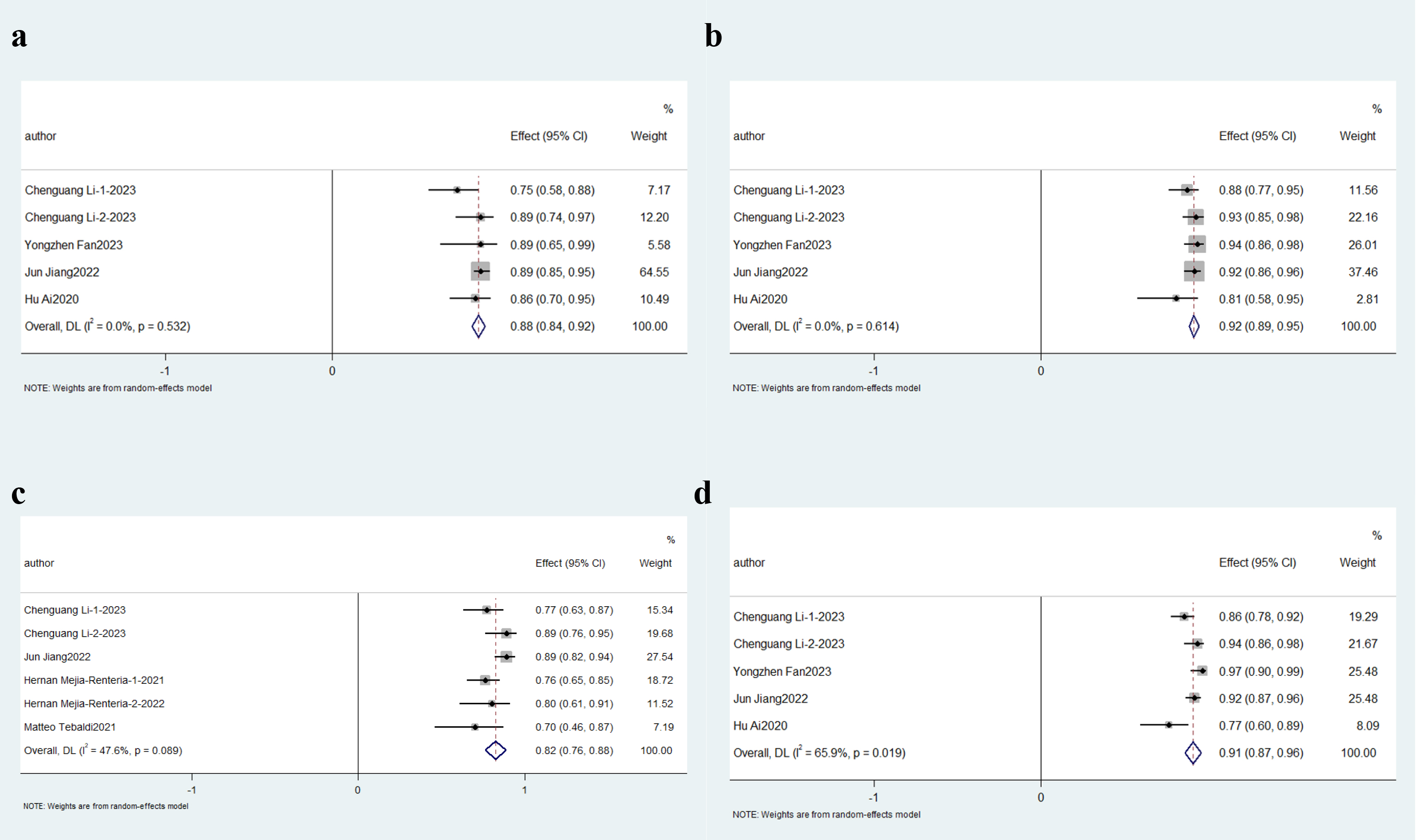 Fig. 6.
Fig. 6. Description of cumulative results based on AccuFFR. Forest plots showing the pooled sensitivity (a), specificity (b), positive predictive value (c) and negative predictive value (d).
Five investigations employing quantitative flow ratio (QFR) assessment methodologies were included in this sub-analysis. Diagnostic accuracy metrics demonstrated 82% aggregate sensitivity (95% CI: 0.76–0.87; p
 Fig. 7.
Fig. 7. Description of cumulative results in based on QFR. Forest plots showing the pooled sensitivity (a), specificity (b), positive predictive value (PPV)(c) and negative predictive value (NPV)(d).
3.3.2.3 The impact of vasodilators (adenosine) based on AccuFFRangio system
In-depth physiological state stratification revealed distinct diagnostic performance profiles. Within the AccuFFRangio platform, hyperemic state assessments (n = 2 studies) achieved 89% aggregate diagnostic sensitivity (95% CI: 0.79–0.99; p
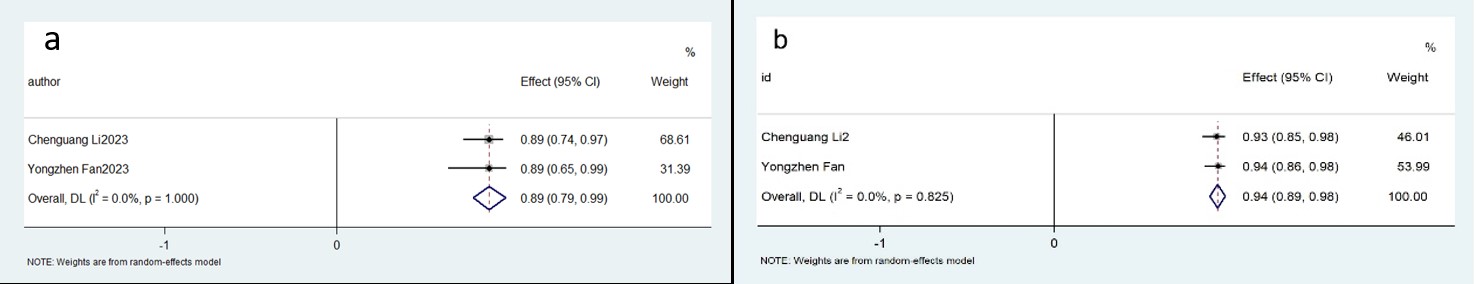 Fig. 8.
Fig. 8. Description of cumulative results using the AccuFFRangio software in the hyperemic state. Forest plots showing the pooled sensitivity (a) and specificity (b).
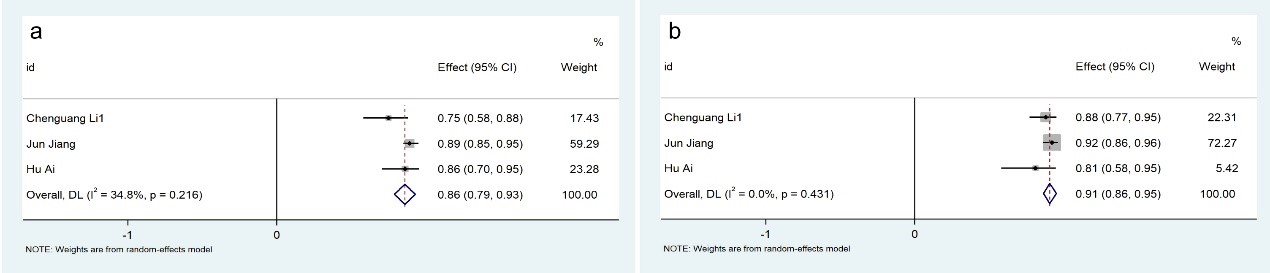 Fig. 9.
Fig. 9. Description of cumulative results using the AccuFFRangio software in the rest state. Forest plots showing the pooled sensitivity (a) and specificity (b).
Our study demonstrates that Angio-IMR exhibits high diagnostic concordance with invasive pressure wire-derived IMR for detecting CMD. Pooled estimates revealed clinically robust performance indicators, involving sensitivity, specificity, PPV, and NPV. The superior discriminative capacity of Angio-IMR was further evidenced by AUC
Patients with angina attributable to CMD constitute a clinically significant subset of those with chronic coronary syndrome (CCS). Although CMD is traditionally classified as a non-atherosclerotic disorder, the Women’s Ischemia Syndrome Evaluation (WISE) substudy employing intravascular ultrasound (IVUS) identified female patients with non-obstructive CAD and revealed that CMD-associated cardiovascular risk correlates strongly with atherosclerosis-related risk factors [31, 32, 33]. Current epidemiological data estimate the prevalence of coronary microvascular disease (CMVD) at 40–60% [34, 35], though heterogeneity persists due to inconsistent diagnostic criteria for CMD. Furthermore, the reliance on invasive pressure wire-derived IMR has limited the detection rate of CMD in clinical practice. These limitations have driven the development of Angio-IMR, a non-invasive computational framework rooted in the hemodynamic principles of invasive IMR. Specifically, invasive IMR is characterized as the product of distal coronary pressure (Pd) and mean transit time (Tmn) when reaching the maximal hyperemic state [36]. Current Angio-IMR algorithms primarily derive from this foundational formula [2, 17, 20]:
Pd: distal coronary pressure, QFR: quantitative flow ratio, Pa: proximal coronary pressure, L: length of blood vessels, Velocityhyp: flow velocity in the hyperemic status.
The derivation of angiography-based IMR fundamentally involves simulating distal coronary pressure (Pd) via FFR calculations [37], followed by multiplying Pd by aortic pressure (Pa) and contrast transit time (Tmn). This approach hinges on angiography-derived FFR computations, with microvascular resistance (MR) subsequently quantified through computational fluid dynamics (CFD). Large-scale trials have demonstrated non-inferiority of angiography-based FFR-guided percutaneous coronary intervention (PCI) compared to wire-based FFR strategies [38], validating its equivalence in assessing stenotic epicardial vessel function. However, there is limited evidence regarding the correlation between pressure wire-based IMR and Angio-IMR. Furthermore, prior meta-analyses exhibited significant methodological bias due to heterogeneous cohorts (e.g., combining CCS and acute coronary syndrome populations) [39] and inconsistent diagnostic thresholds for the gold-standard IMR across studies. To address these limitations, this meta-analysis exclusively focused on angina populations (excluding myocardial infarction) to minimize confounding variables.
Based on angiographic stenosis severity, enrolled patients were stratified into two cohorts: obstructive CAD (stenosis
Given the inclusion of four studies [22, 24, 25, 26] utilizing the AccuFFRangio system to quantify Angio-IMR, we performed additional subgroup analyses. These revealed that hyperemia-induced Angio-IMR measurements (achieved via adenosine-mediated vasodilation) exhibit superior reliability compared to resting-state assessments. This is consistent with the findings of Scarsini et al. [28], who revealed no significant correlation between non-hyperemic Angio-IMR and invasive IMR in CCS cohorts. Collectively, these observations underscore the necessity of hyperemic conditions for optimizing Angio-IMR’s diagnostic utility in CMD.
While the limited number of included studies and heterogeneity in computational platforms introduce potential confounding biases, subgroup analyses reinforced the robustness of the primary findings. The meta-analysis conclusively demonstrates that Angio-IMR achieves high diagnostic performance (AUC: 0.91, 95% CI: 0.89–0.94), indicating its clinical applicability as a non-invasive alternative.
This comprehensive meta-analytical synthesis establishes angio-IMR as a diagnostically robust modality, demonstrating superior discriminative capacity for detecting CMD in angina pectoris cohorts. The concordance with invasive wire-based IMR measurements collectively confirms its clinical validity, thereby positioning this modality as a viable non-invasive surrogate for traditional intracoronary physiological assessment.
The original data for this study is available from the corresponding author.
WW, YC, and YQZ collected the data and wrote the manuscript; MWL, BLX, MJG and LLJ analyzed the data; FHZ and KJC reviewed the manuscript, designed this work, and guided the methodology. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This work was supported by Hospital capability enhancement project of Xiyuan Hospital, CACMS (No.CI2021A00901), Beijing Clinical Research Ward (BCRW202108) and Beijing Traditional Chinese Medicine Technology Development Fund Project (BJZYZD-2023-11).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/RCM25764.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.









