1 Center for Coronary Artery Disease, Division of Cardiology, Beijing Anzhen Hospital, Capital Medical University, 100029 Beijing, China
2 Department of Cardiology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, 100730 Beijing, China
3 Cardiometabolic Medicine Center, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, 100037 Beijing, China
†These authors contributed equally.
Abstract
Excessive daytime sleepiness (EDS) is a commonly observed symptom in people with obstructive sleep apnea (OSA). However, the impact of EDS on the outcome of patients with acute coronary syndrome (ACS) and OSA is not known. Therefore, this study aimed to investigate the association between OSA and cardiovascular events in ACS patients with or without EDS.
This cohort study prospectively enrolled eligible ACS patients who underwent cardiorespiratory polygraphy during hospitalization between June 2015 and January 2020. We defined OSA as an apnea–hypopnea index (AHI) ≥15 events per h. EDS was described as having an Epworth Sleepiness Scale score ≥10. Major adverse cerebrovascular and cardiovascular events (MACCEs) were the primary outcome and included cardiovascular death, stroke, myocardial infarction, ischemia-driven revascularization, or hospitalization for heart failure or unstable angina.
The final study cohort comprised 1154 participants, of whom 398 (34.5%) had EDS, and 607 (52.6%) had OSA. During the median follow-up period of 2.9 years (interquartile range 1.5, 3.6), OSA was associated with a significantly increased risk of MACCEs in patients without EDS (adjusted hazard ratio (HR) = 1.42, 95% CI: 1.01–2.02, p = 0.046), but not in patients with EDS (adjusted hazard ratio HR = 1.05, 95% CI: 0.67–1.66, p = 0.84).
OSA was associated with an elevated risk of MACCEs In ACS patients without EDS but not those with EDS. Therefore, screening for OSA should be performed in ACS patients without EDS, and future trials should prioritize such high-risk patients.
NCT03362385, https://clinicaltrials.gov/study/NCT03362385.
Keywords
- obstructive sleep apnea
- acute coronary syndrome
- excessive daytime sleepiness
- cardiovascular events
Obstructive sleep apnea (OSA) is a prevalent sleep disorder that is characterized by repeat occurrences of partial or complete obstruction of the upper airway during sleep. Recent studies have highlighted the substantial public health cost associated with OSA, with the global prevalence in 30–69 year old adults estimated to be 940 million [1]. The incidence of OSA in patients with acute coronary syndrome (ACS) was found to be 36–63% in different races [2]. Several different studies have reported that OSA has prognostic significance in ACS patients [3, 4]. However, there may be specific subsets (e.g., with different symptoms or comorbidities) that are more likely to be affected by OSA [5, 6, 7] and have yet to be identified.
Patients with OSA are commonly accompanied by sleep disruption, which frequently results in excessive daytime sleepiness (EDS). EDS may adversely impact daily performance, emotional states, and various facets of overall well-being [8, 9, 10]. Neuroimaging also reveals changes in the white matter and gray matter within the cerebral structure of individuals presenting with both OSA and EDS. A higher mean diffusivity and significant gray matter concentration deficits were detected in the EDS group by whole-brain analysis [11, 12]. Recent work has also proposed an association between EDS and major adverse cerebrovascular and cardiovascular events (MACCEs) after myocardial infarction (MI) [13]. Currently, therapy with continuous positive airway pressure (CPAP) or pharmacological treatments are usually offered to OSA patients with EDS. However, it is still unclear if the effect of OSA on cardiovascular outcome differs according to the EDS status. In the present study we therefore investigated whether OSA was associated with cardiovascular events in ACS patients who did or did not have EDS. To achieve this, we conducted post-hoc analyses of an earlier OSA-ACS study.
The OSA-ACS project (NCT03362385) was a large, prospective cohort study that
that has been reported previously [5]. The study enrolled eligible ACS patients
aged 18 to 85 years at the Beijing Anzhen Hospital, Capital Medical University
with the aim of investigating possible associations between OSA and
cardiovascular outcomes. Data collection occurred between June 2015 to January
2020, with the participants followed until December 2020. The exclusion criteria
were: individuals with cardiogenic shock, cardiac arrest,
cancer, patients with unsuccessful sleep monitoring due to unsatisfactory or
inadequate signal recording, and patients who received regular CPAP treatment.
Also excluded were patients who had predominantly central sleep
apnea (
MI diagnosis followed the universal definition of myocardial infarction, requiring both clinical evidence of acute myocardial ischemia and cardiac biomarker changes [14, 15]. Unstable angina (UA) diagnosis was based on the guideline, primarily emphasizing ischemic symptoms without mandatory biomarker elevation [16].
This investigation followed the principles of the Declaration of Helsinki, and was approved by the Ethics Committee, Beijing Anzhen Hospital, Capital Medical University (2013025). Written informed consent was obtained from all participants.
Following clinical stabilization in the hospital, all eligible
participants underwent an overnight sleep study with a Type III
portable cardiorespiratory polygraphy
instrument (ApneaLink Air; ResMed, San Diego, California, USA). The mean time
interval from admission to sleep study was 2
Sleepiness was self-reported using the Epworth Sleepiness Scale (ESS). Patients subjectively assessed their daytime sleepiness by completing the ESS questionnaire [22]. This scale includes eight questions, each relating to the risk of falling asleep in different situations over the past month. A categorization of EDS was assigned to patients who scored at least 10 out of a possible total of 24.
All eligible patients were monitored until December 2020. Follow-up assessments were performed 1, 3 and 6 months post-discharge, then subsequently each year. Adverse clinical events were registered through clinic visits, review of medical records, and phone interviews by researchers who were blinded to sleep data for each patient. The primary endpoint (MACCEs) included cardiovascular death, stroke, ischemia-driven revascularization, MI, and hospitalization due to UA or heart failure (HF). As detailed previously [23], secondary endpoints were single components of the primary endpoint, all-cause death, all repeat revascularization, and an amalgam of all events. Endpoints were established using definitions outlined by the Standardized Data Collection for Cardiovascular Trials Initiative [24]. Only the first occurrence from baseline was counted when the patient experienced multiple events. Events and source documents were assessed independently by researchers who were blind to the sleep study results.
Quantitative data was presented as mean
Cox proportional hazards regression analysis was used to assess whether OSA was an independent prognostic factor for the observed events, with stratification for EDS status. Adjustments were made in the multivariable models for confounders that were clinically related to the outcomes, or showed an association with these endpoints in univariate analysis. The first model was constructed without any adjustments, while age and sex were covariates in the second model. The third model included variables from the second model, as well as body mass index (BMI), history of hypertension, hyperlipidemia, diabetes, current smoking, prior stroke, prior MI, and the clinical presentation including acute MI versus UA. All analyses were conducted by using SPSS, version 26.0 (IBM SPSS, Armonk, New York, NY, USA).
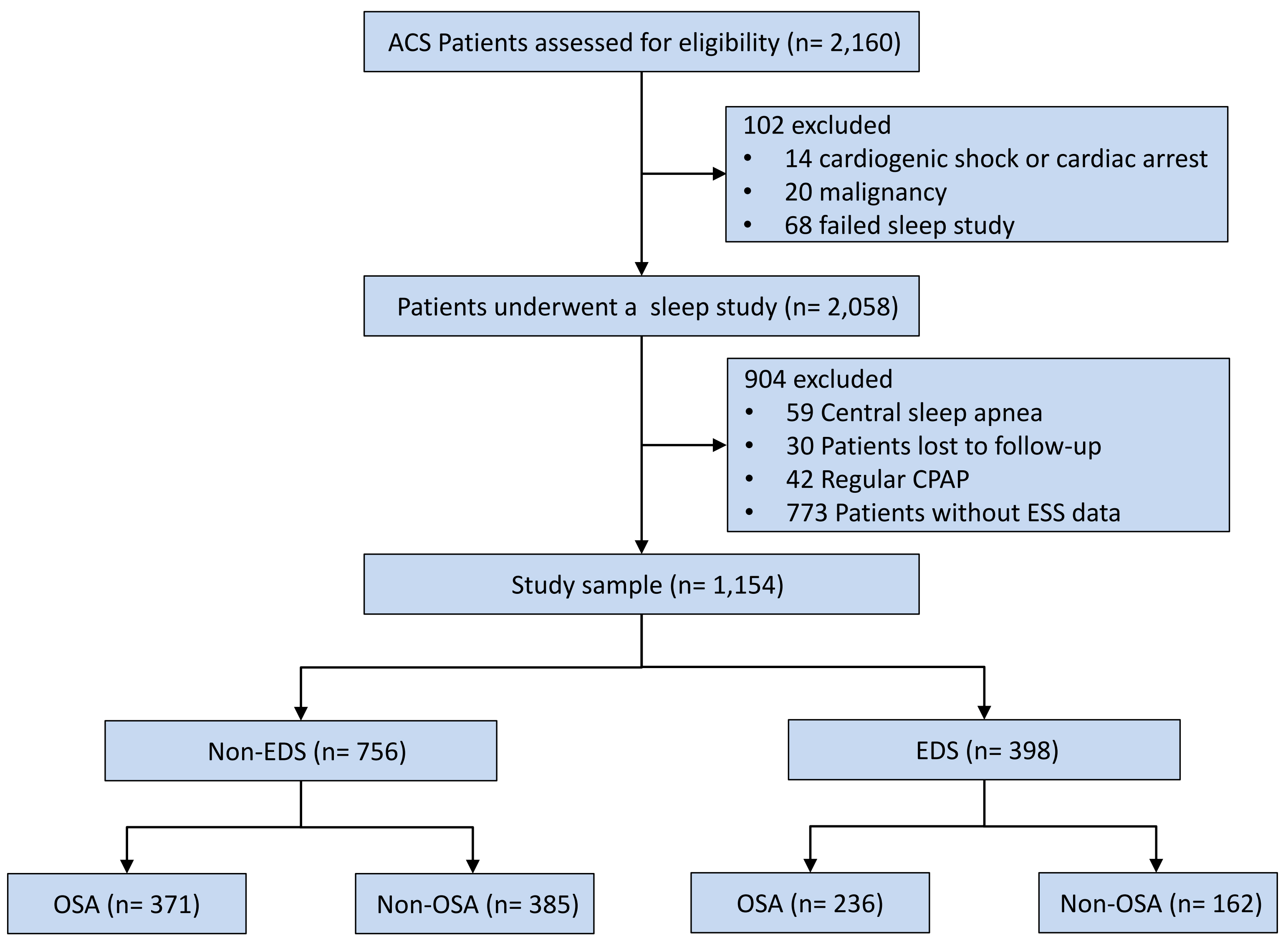
The final analysis included 1154 patients (Fig. 1), of which 398 subjects
(34.5%) had EDS and 756 (65.5%) did not have EDS. The EDS group had a greater
percentage of males compared to the non-EDS group. EDS patients exhibited higher
measures of obesity (BMI, waist and hip circumferences, ratio of waist/hip, neck
circumference) and OSA (AHI, oxygen desaturation index [ODI],
time with SaO2
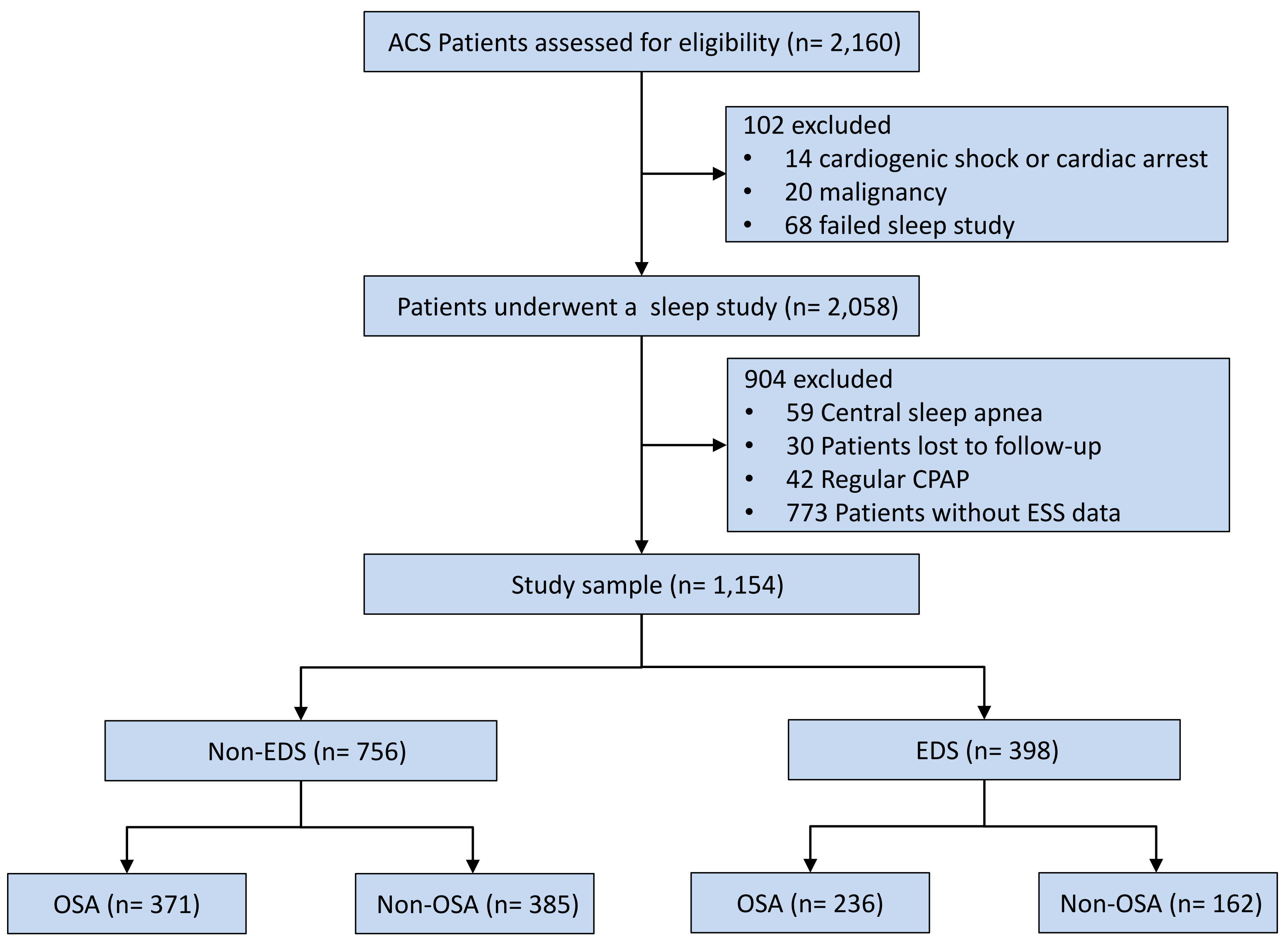 Fig. 1.
Fig. 1.
Study flowchart. Abbreviations: ACS, acute coronary syndrome; CPAP, continuous positive airway pressure; EDS, excessive daytime sleepiness; ESS, epworth sleepiness scale; OSA, obstructive sleep apnea.
The measures of obesity and high-sensitivity C-reactive protein (hs-CRP) levels were elevated in the OSA group, both for EDS and non-EDS patient groups. No other differences were found in EDS patients. In non-EDS patients, those with OSA had a greater prevalence of hypertension, prior percutaneous coronary intervention (PCI), current drinking, a higher level of triglyceride, higher proportion of PCI, and larger number of stents placed compared to those without OSA (Table 1). Additionally, there is no difference in the primary baseline data and outcomes (Log rank p = 0.200) between excluded patients (n = 773) and included patients (n = 1154) (Supplementary Table 4, Supplementary Fig. 2).
| Variables | ESS |
ESS | |||||
| OSA (n = 236) | Non-OSA (n = 162) | p-value | OSA (n = 371) | Non-OSA (n = 385) | p-value | ||
| Demographics | |||||||
| Age, years | 55.25 |
56.1 |
0.565 | 56.89 |
56.66 |
0.766 | |
| Male | 210 (89) | 141 (87) | 0.555 | 316 (85.2) | 302 (78.4) | 0.017 | |
| BMI, kg/m2 | 28.52 |
26.15 |
27.72 |
25.74 |
|||
| Waist circumference, cm | 103.7 |
96.77 |
101.81 |
95.52 |
|||
| Neck circumference, cm | 42.05 |
40.31 |
41.04 |
39.27 |
|||
| Hip circumference, cm | 103.6 |
99.81 |
102.35 |
99.21 |
|||
| Waist/hip ratio | 1.00 (0.96–1.03) | 0.97 (0.94–1.01) | 0.001 | 0.99 (0.95–1.02) | 0.97 (0.93–1.00) | ||
| Medical history | |||||||
| Hypertension | 158 (66.9) | 103 (63.6) | 0.487 | 252 (67.9) | 224 (58.2) | 0.006 | |
| Hyperlipidemia | 73 (30.9) | 60 (37) | 0.205 | 120 (32.3) | 114 (29.6) | 0.416 | |
| Diabetes | 76 (32.2) | 55 (34) | 0.716 | 107 (28.8) | 107 (27.8) | 0.949 | |
| Prior PCI | 44 (18.6) | 36 (23.5) | 0.244 | 95 (25.6) | 63 (16.4) | 0.002 | |
| Prior stroke | 31 (13.1) | 18 (11.1) | 0.546 | 40 (10.8) | 38 (9.9) | 0.680 | |
| Prior MI | 32 (13.6) | 31 (19.1) | 0.134 | 72 (19.4) | 60 (15.6) | 0.166 | |
| Current smoking | 119 (50.4) | 78 (48.1) | 0.656 | 176 (47.4) | 157 (40.8) | 0.065 | |
| Current drinking | 84 (35.6) | 55 (34) | 0.736 | 130 (35) | 108 (28.1) | 0.039 | |
| Laboratory data | |||||||
| Creatinine, µmol/L | 74.7 (65.8–84.0) | 73.3 (64.0–81.5) | 0.222 | 73.7 (65.4–83.0) | 70.8 (61.3–81.3) | 0.019 | |
| Hs-CRP, mg/L | 2.64 (1.16–8.14) | 1.50 (0.51–5.56) | 0.001 | 2.44 (0.87–6.79) | 1.41 (0.62–4.13) | ||
| LVEF, % | 61 (56–65) | 62 (56–66) | 0.416 | 60 (55–65) | 62 (58–65) | 0.085 | |
| LDL-C, mmol/L | 2.55 (2.01–3.09) | 2.36 (1.81–3.24) | 0.358 | 2.4 (1.88–3.01) | 2.43 (1.85–3.12) | 0.644 | |
| HDL-C, mmol/L | 0.97 (0.86–1.11) | 0.96 (0.85–1.16) | 0.797 | 0.98 (0.84–1.14) | 1.03 (0.88–1.22) | 0.005 | |
| TC, mmol/L | 4.26 (3.62–4.88) | 4.01 (3.35–5.19) | 0.422 | 4.05 (3.45–4.81) | 4.13 (3.42–4.98) | 0.246 | |
| TG, mmol/L | 1.76 (1.26–2.40) | 1.50 (1.15–2.32) | 0.066 | 1.52 (1.12–2.14) | 1.40 (1.04–2.02) | 0.031 | |
| HbA1c, % | 6.2 (5.6–7.4) | 5.9 (5.5–6.9) | 0.089 | 6.1 (5.6–7.0) | 6.0 (5.6–6.8) | 0.113 | |
| Systolic BP, mmHg | 126 (119–138) | 124 (112–135) | 0.071 | 126 (117–139) | 127 (118–139) | 0.876 | |
| Diastolic BP, mmHg | 76 (70–86) | 75 (67–80) | 0.045 | 77 (70–85) | 75 (69–83) | 0.079 | |
| Sleep information | |||||||
| AHI, events per hr | 33.45 (22.35–48.97) | 7.95 (4.1–11.2) | 28.2 (20.1–41.6) | 7.6 (4.2–10.3) | |||
| ODI, events per hr | 30.6 (21.7–46.9) | 8.8 (5.0–12.0) | 26.5 (19–38.5) | 8.25 (4.92–11.60) | |||
| Minimum SaO2, % | 82 (75–86) | 87 (85–89) | 83 (77–86) | 88 (85–90) | |||
| Mean SaO2, % | 94 (93–95) | 93 (92–94) | 93 (92–94) | 94.3 (93–95) | |||
| Percentage of time with SaO2 |
8.0 (2.0–21.0) | 1.0 (0.15–3.0) | 12.91 (2.0–17) | 5.42 (0.10–3.15) | |||
| Epworth Sleepiness Scale | 13.43 |
12.88 |
0.06 | 5.09 |
4.42 |
0.002 | |
| Diagnosis | 0.448 | 0.273 | |||||
| STEMI | 60 (25.4) | 38 (23.5) | 85 (22.9) | 70 (18.2) | |||
| NSTEMI | 52 (22) | 29 (17.9) | 67 (18.1) | 73 (19.0) | |||
| Unstable angina | 124 (52.5) | 95 (58.6) | 219 (59.0) | 242 (62.9) | |||
| Non-obstructive CAD | |||||||
| MINOCA | 6/112 (5.4) | 3/67 (4.5) | 0.781 | 5/152 (3.3) | 11/143 (7.7) | 0.092 | |
| INOCA | 12/124 (9.7) | 9/95 (9.5) | 0.963 | 19/219 (8.7) | 19/242 (7.9) | 0.775 | |
| Procedures | |||||||
| Coronary angiography | 228 (96.6) | 157 (96.9) | 0.867 | 366 (98.7) | 375 (97.4) | 0.218 | |
| PCI | 137 (58.1) | 83 (51.2) | 0.449 | 211 (56.9) | 187 (48.6) | 0.164 | |
| PTCA | 21 (8.9) | 13 (8.0) | 31 (8.4) | 35 (9.1) | |||
| CABG | 12 (5.1) | 15 (9.3) | 23 (6.2) | 36 (9.4) | |||
| Multivessel disease | 153 (64.8) | 107 (66) | 0.968 | 242 (65.2) | 221 (57.4) | 0.082 | |
| Number of stents | 1 (0–1) | 1 (0–1) | 0.227 | 1 (0–2) | 0 (0–1) | 0.009 | |
| Medications on discharge | |||||||
| Aspirin | 229 (97.0) | 154 (95.1) | 0.310 | 361 (97.3) | 377 (97.9) | 0.578 | |
| P2Y12 inhibitor | 219 (92.8) | 149 (92.0) | 0.760 | 342 (92.2) | 347 (90.1) | 0.321 | |
| ACEI or ARB | 153 (64.8) | 95 (58.6) | 0.211 | 236 (63.6) | 216 (56.1) | 0.035 | |
| CCB | 56 (23.7) | 30 (18.5) | 0.215 | 80 (21.6) | 67 (17.4) | 0.148 | |
| 185 (78.4) | 124 (76.5) | 0.664 | 298 (80.3) | 286 (74.3) | 0.048 | ||
| Statins | 234 (99.2) | 157 (96.9) | 0.095 | 363 (97.8) | 381 (99.0) | 0.219 | |
Abbreviations: ACEI, angiotensin-converting enzymes inhibitor; AHI,
apnea-hypopnea index; ARB, angiotensin receptor blocker; BMI, body mass index;
BP, blood pressure; CABG, coronary artery bypass grafting; CAD, coronary artery
disease; CCB, calcium channel blockers; ESS, epworth sleepiness scale; HDL-C, high-density lipoprotein cholesterol; Hs-CRP,
high-sensitivity C-reactive protein; INOCA, ischemia with non-obstructive
coronary artery disease [defined as angina with non-obstructive CAD (
MACCEs occurred in 223 patients during the median follow-up period of 2.9 years (interquartile range: 1.5–3.6), accounting for 19.3% of the total cohort. The incidence of MACCEs was not significantly different between patients who did or did not have EDS (adjusted hazard ratio (HR) = 1.16, 95% CI: 0.89–1.52, p = 0.28) (Supplementary Fig. 1). For the secondary endpoints, cardiovascular death was observed in 22 patients (1.9%), MI in 31 patients (2.7%), stroke in 24 patients (2.1%), ischemia-driven revascularization in 133 patients (11.5%), hospitalization for UA in 156 patients (13.5%), and hospitalization for HF in 7 patients (0.6%). For the other individual cardiovascular events, no significant differences were observed between EDS and non-EDS patients (Supplementary Table 3).
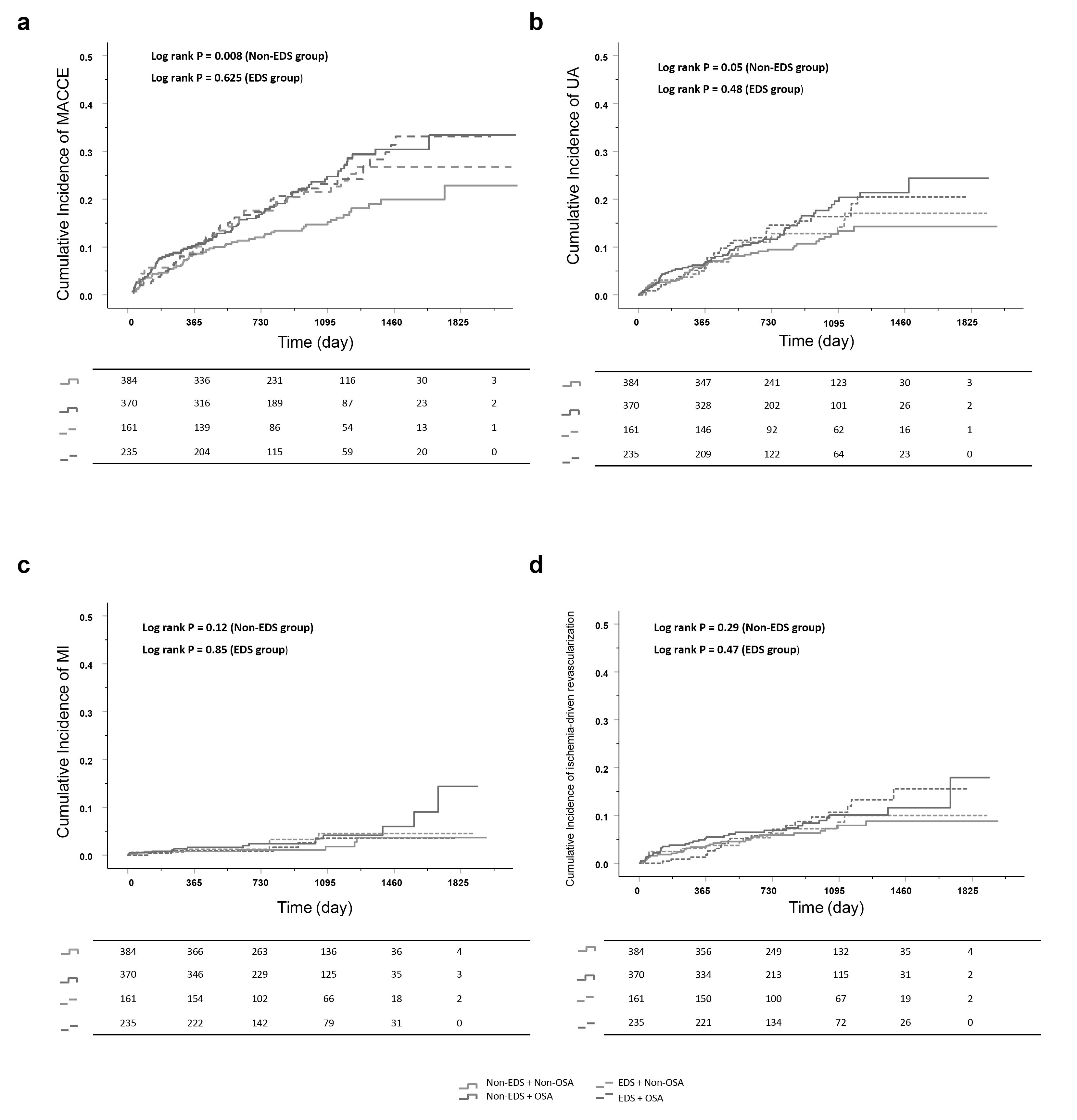
We next categorized EDS patients into those with or without OSA, and non-EDS patients into those with or without OSA. For EDS patients, no significant difference in MACCEs was found between OSA and non-OSA groups (HR = 1.12, 95% CI: 0.72–1.72, p = 0.63). Moreover, the outcomes did not change after adjusting for clinical confounders (adjusted HR = 1.05, 95% CI: 0.67–1.66, p = 0.84). Similarly, in patients with EDS, no significant differences were found between OSA and non-OSA groups for any secondary endpoint. For patients without EDS, a significantly higher risk of MACCEs was observed in OSA patients compared to non-OSA patients (HR = 1.57, 95% CI: 1.12–2.20, p = 0.008). Following adjustment for clinical confounders, multivariable analysis revealed OSA was an independent predictor for MACCEs in patients without EDS (adjusted HR = 1.42, 95% CI: 1.01–2.02, p = 0.046). No significant interactions between EDS and OSA were observed for MACCEs (interaction p = 0.23). Notably, in patients without EDS, a difference in hospitalization for unstable angina was observed in model 1 (HR = 1.32, 95% CI: 0.99–2.22, p = 0.05). However, this difference was not significant in model 2 and model 3. No differences were observed in other secondary endpoints (Table 2, Fig. 2).
| Variables | ESS |
ESS | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| MACCE | |||||
| Model 1 | 1.12 (0.72–1.72) | 0.63 | 1.57 (1.12–2.20) | 0.008 | |
| Model 2 | 1.13 (0.73–1.75) | 0.60 | 1.52 (1.09–2.14) | 0.015 | |
| Model 3 | 1.05 (0.67–1.66) | 0.84 | 1.42 (1.01–2.02) | 0.046 | |
| Cardiovascular death | |||||
| Model 1 | 0.87 (0.23–3.24) | 0.84 | 1.72 (0.56–5.24) | 0.34 | |
| Model 2 | 0.90 (0.24–3.35) | 0.87 | 1.62 (0.53–4.97) | 0.40 | |
| Model 3 | 0.71 (0.19–2.70) | 0.62 | 1.68 (0.53–5.31) | 0.38 | |
| Myocardial infarction | |||||
| Model 1 | 0.89 (0.27–2.91) | 0.85 | 2.08 (0.84–5.15) | 0.12 | |
| Model 2 | 0.89 (0.27–2.93) | 0.85 | 1.87 (0.75–4.67) | 0.18 | |
| Model 3 | 0.91 (0.26–3.20) | 0.89 | 1.43 (0.56–3.67) | 0.45 | |
| Stroke | |||||
| Model 1 | 0.86 (0.26–2.83) | 0.81 | 0.92 (0.31–2.73) | 0.88 | |
| Model 2 | 0.88 (0.27–2.88) | 0.83 | 0.90 (0.30–2.69) | 0.85 | |
| Model 3 | 0.94 (0.27–3.25) | 0.93 | 0.84 (0.27–2.58) | 0.76 | |
| Ischemia-driven revascularization | |||||
| Model 1 | 1.30 (0.64–2.65) | 0.47 | 1.32 (0.79–2.22) | 0.29 | |
| Model 2 | 1.32 (0.65–2.70) | 0.44 | 1.26 (0.75–2.11) | 0.39 | |
| Model 3 | 1.20 (0.57–2.52) | 0.64 | 1.13 (0.66–1.93) | 0.66 | |
| Hospitalization for unstable angina | |||||
| Model 1 | 1.22 (0.71–2.09) | 0.48 | 1.47 (0.99–2.18) | 0.05 | |
| Model 2 | 1.23 (0.72–2.11) | 0.45 | 1.43 (0.96–2.13) | 0.08 | |
| Model 3 | 1.13 (0.64–1.99) | 0.67 | 1.37 (0.91–2.06) | 0.14 | |
| Hospitalization for heart failure | |||||
| Model 1 | 0.75 (0.05–12.1) | 0.84 | 1.58 (0.27–9.48) | 0.61 | |
| Model 2 | 0.68 (0.04–11.3) | 0.79 | 1.38 (0.23–8.25) | 0.73 | |
| Model 3 | 0.23 (0.01–5.67) | 0.37 | 1.18 (0.19–7.51) | 0.86 | |
Abbreviations: CI, confidence interval; ESS, epworth sleepiness scale; HR, hazard ratio; MACCE, major adverse cardiovascular and cerebrovascular event. Model 1: Unadjusted model; Model 2: Adjusted for age, sex; Model 3: Adjusted for age, sex, body mass index, current smoking, history of hypertension, diabetes, dyslipidemia, prior myocardial infarction, prior stroke, and clinical presentation (acute myocardial infarction vs unstable angina).
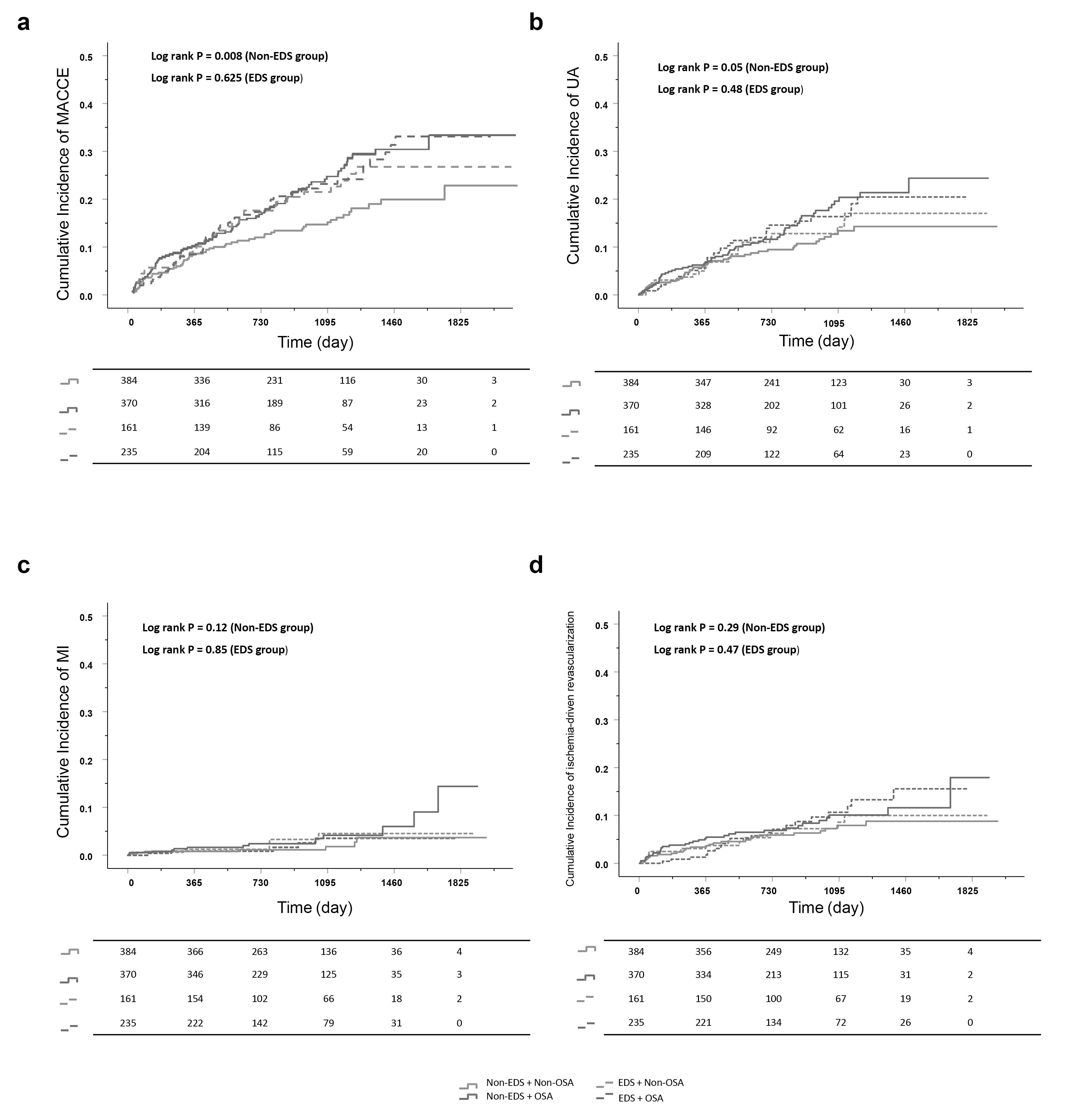 Fig. 2.
Fig. 2.
Kaplan-Meier curves in OSA vs non-OSA groups stratified by EDS categories. (a) MACCE, (b) hospitalization for unstable angina, (c) myocardial infarction, and (d) ischemia-driven revascularization. Abbreviations: MACCE, major adverse cardiovascular and cerebrovascular event; EDS, Excessive daytime sleepiness; MI, myocardial infarction; OSA, obstructive sleep apnea; UA, unstable angina.
Finally, we performed sensitivity analysis to assess stability of the effect on the primary endpoint in patients without EDS. The observed association between OSA and MACCE showed no influence from confounding factors (Table 3).
| Subgroups | HR (95% CI) | p-value | p-value for interaction | |
| Old age | 0.257 | |||
| Yes | 2.199 (1.108–4.363) | 0.024 | ||
| No | 1.389 (0.939–2.055) | 0.100 | ||
| Sex | 0.897 | |||
| Male | 1.522 (1.053–2.201) | 0.026 | ||
| Female | 1.675 (0.709–3.956) | 0.239 | ||
| Obesity | 0.448 | |||
| Yes | 1.931 (0.982–3.797) | 0.057 | ||
| No | 1.408 (0.936–2.12) | 0.101 | ||
| Diabetes | 0.120 | |||
| Yes | 2.297 (1.282–4.116) | 0.005 | ||
| No | 1.287 (0.846–1.957) | 0.239 | ||
| Dyslipidemia | 0.306 | |||
| Yes | 2.123 (1.116–4.036) | 0.022 | ||
| No | 1.393 (0.934–2.079) | 0.104 | ||
| Hypertension | 0.714 | |||
| Yes | 1.621 (1.069–2.459) | 0.023 | ||
| No | 1.402 (0.781–2.516) | 0.257 | ||
| Diagnosis | 0.743 | |||
| STEMI | 1.568 (0.793–3.098) | 0.196 | ||
| NSTEMI | 1.145 (0.566–2.320) | 0.706 | ||
| UA | 1.765 (1.102–2.828) | 0.018 | ||
Abbreviations: HR, hazard ratio; NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; UA, unstable angina. Data are presented as median (first quartile, third quartile).
For ACS patients without EDS, OSA was found to be associated with subsequent adverse cardiovascular events. However, this association was not observed for patients with EDS. Additionally, subgroup analyses revealed no differences in the non-EDS OSA group, indicating that OSA was a key predictor of MACCEs in patients with no EDS. These results advance our understanding in this field, as previous research suggested that EDS was associated with poor prognosis. Furthermore, our findings suggest that more attention should be given to OSA patients without EDS, as they may have an elevated risk of adverse events that has been overlooked in the past.
An earlier study indicated that EDS was associated with a higher incidence of MACE during a 4-year follow-up period in post-MI patients with moderate to severe OSA [13]. This relationship has been consistently observed in a wider cohort, including non-MI patients [25, 26, 27]. It suggests there may be an underlying pathophysiological mechanism that connects sleep disorder with cardiovascular risks, such as intermittent hypoxia, systemic inflammation, and other metabolic disorders [28]. However, some studies have reported different findings. Longitudinal analysis of participants in the Nurses’ Health Study II found that daytime sleepiness was not independently associated with the risk of cardiovascular disease [29]. Another recent study based on the UK Biobank database revealed that EDS was not a significant risk factor for incident MI or stroke, regardless of sleep duration [30]. The focus of our study was patients with established ACS. An increased risk of subsequent MACCEs was observed in OSA patients with no EDS, but not in those with EDS. However, the small size of each group and low frequency of events reduced the statistical power of the study. Hence, caution is required in interpreting the results, and additional trials with large cohorts are needed to validate our results.
To minimize the potential bias, patients with regular CPAP were excluded from
this analysis. However, from an ethical perspective, OSA patients with severe
daytime sleepiness should be treated with CPAP.
Notably,
previous randomized controlled trials on patients with
cardiovascular disease did not demonstrate a benefit from CPAP on the incidence
of cardiovascular events [31, 32]. Post hoc analysis indicated that patients who
adhered to CPAP therapy (
Potential associations between OSA and clinical outcomes in patients without EDS may be better understood by analyzing the unique characteristics of OSA symptoms, pathophysiological mechanisms, and their consequences. Exploring endocrine dysfunction, inflammation, sympathetic hyperactivity, and oxidative stress in sleep-deprived individuals may clarify the mechanisms linking non-EDS and cardiovascular outcomes in ACS patients. EDS is closely associated with established factors for cardiovascular risk, including obesity, uncontrolled hypertension, diabetes, and a sedentary lifestyle [36, 37]. Notably, while our findings indicated that OSA had a more pronounced impact on the prognosis of non-sleepy patients compared to those of sleepy patients, EDS per se still had an adverse trend (although not statistically significant) on the prognosis. This observation aligns with the study focused on the symptom subtypes, which reported that patients with the excessively sleepy subtype were at increased risk of cardiovascular disease compared to patients with minimally or moderately sleepy [27].
OSA correlates with a higher prevalence of hypertension, HF, diabetes, and atrial fibrillation. Even in patients without EDS, those with OSA tend to have a poor prognosis. The obstructive respiratory episodes characteristic of OSA may induce circadian dysregulation, primarily mediated by sleep disorder and intermittent hypoxia. This disruption triggers inflammatory responses and disturbs neural and hormonal balance, ultimately leading to dysregulation of the molecular circadian clock and associated biological pathways [38]. Prolonged exposure to hypoxia is implicated in the development of disorders commonly associated with circadian rhythm disturbances, including cardiovascular and respiratory diseases, dementia, cancer, and metabolic disorders [39, 40]. Several studies have also demonstrated that OSA patients exhibit significantly higher sympathetic nerve activity in postganglionic muscles during wakefulness and normal breathing patterns [41, 42]. Additionally, patients with OSA have increased basal sympathetic tone during wakefulness, and experience acute cyclical sympathetic excitation during sleep [43]. We hypothesize that sympathetic excitation in OSA patients without EDS may further contribute to poor prognosis. Additional research is needed to investigate such mechanisms.
The present study has a number of limitations. Firstly, Type III portable polygraphy was used to diagnose OSA. This could underestimate the severity of OSA, as the total sleep time cannot be calculated accurately. Nevertheless, portable polygraphy is a viable alternative to polysomnography for diagnosing OSA [44]. Second, OSA severity in the acute setting of ACS may have been overestimated [45]. To minimize this potential bias, clinical stabilization was achieved prior to conducting the sleep study during hospitalization. Third, because of its subjectivity the assessment of daytime sleepiness was limited to a one-time questionnaire. Daytime sleepiness is variable and may not be accurately captured by a single measurement. Fourth, about 40% of patients were excluded due to unavailable ESS, which may lead to selection bias. But there was no difference in the primary baseline data and outcomes between excluded patients and included patients, which may allay some of the concerns of selection bias. Fifth, our study was that the majority of participants were of Asian population. This demographic characteristic may limit the generalizability of our findings to other ethnic populations. Sixth, this cohort showed a high proportion of UA and a low proportion of revascularization, suggesting that enrolled ACS patients were relatively at lower risk. This may introduce a potential bias and the results need to be further validated in high-risk populations.
A higher risk of MACCEs was observed in ACS patients with OSA but with no EDS. This finding highlights the necessity for comprehensive OSA screening of all ACS patients, regardless of their EDS status. Moreover, this study also underlines the importance of intervention in OSA patients without EDS.
ACEI, angiotensin-converting enzymes inhibitor; ACS, acute coronary syndrome; AHI, apnea-hypopnea index; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCB, calcium channel blockers; CPAP, continuous positive airway pressure; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; HR, hazard ratio; Hs-CRP, high-sensitivity C-reactive protein; INOCA, ischemia with non-obstructive coronary artery disease [defined as angina with non-obstructive CAD (
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Study concept and design: XW, SPN, YYQ and ZXL. Acquisition, analysis, or interpretation of data: WH, XCL, QG, YYG, BQ, WG, WZ. Drafting of the manuscript: YYQ and WH. Critical revision of the manuscript for important intellectual content: All authors. Obtained funding: XW, SPN, WH. Administrative, technical, or material support: BQ, WG, WZ,LX. XW, SPN, YYQ, ZXL, WH had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The research protocol was carried out in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Beijing Anzhen Hospital, Capital Medical University (Ethic Approval Number: 2013025). All of the participants provided signed informed consent.
We gratefully acknowledge the assistance from MD Yang Xu, Hui Xu and Yushi Zhang for collecting study data (Center for Coronary Artery Disease, Division of Cardiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China).
This study was funded by grants from Beijing Natural Science Foundation (JQ24039), National Key R&D Program of China (2022YFC2505600), National Natural Science Foundation of China (82470339, 82270258, 82200495).
Dr. Shaoping Nie: research grants to the institution from Boston Scientific, Abbott, Jiangsu Hengrui Pharmaceuticals, China Resources Sanjiu Medical & Pharmaceuticals, East China Pharmaceuticals. The rest of the authors have no relevant relationships to disclose.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/RCM33439.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.