1 Jiaxing University Master Degree Cultivation Base, Zhejiang Chinese Medical University, 310053 Hangzhou, Zhejiang, China
2 Department of Cardiology, The First Hospital of Jiaxing Affiliated Hospital of Jiaxing University, 314001 Jiaxing, Zhejiang, China
Abstract
Hypertension is a major risk factor for cardiovascular diseases (CVDs) and is closely related to metabolic abnormalities. The cardiometabolic index (CMI) integrates lipid profiles and anthropometric indicators, reflecting overall cardiometabolic health. However, the CMI and blood pressure (BP) relationship is poorly understood. Therefore, this study aimed to investigate the correlation between CMI and clinical BP and evaluate the potential of using this correlation as a cardiovascular risk indicator.
National Health and Nutrition Examination Survey (NHANES) data from 2015 to 2018 were used to calculate the CMI based on the triglycerides to high-density lipoprotein cholesterol ratio and the waist-to-height ratio. The relationship between CMI and systolic blood pressure (SBP)/diastolic blood pressure (DBP) was analyzed using multivariate regression, threshold effect analysis, and subgroup analysis.
In this study cohort of 4240 participants, CMI positively correlated with SBP and DBP. After adjusting for age, gender, and race, the partial correlation for SBP was 0.56 (95% CI: 0.19–0.93; p < 0.01), while for DBP, it was 1.15 (95% CI: 0.60–1.71; p < 0.001). The threshold effect analysis revealed a positive association with SBP when the CMI was below 6.83 (β = 1.44, 95% CI: 0.64–2.24; p < 0.001) and a negative association when the CMI was above 6.83 (β = –1.52, 95% CI: –2.77– –0.28; p = 0.0123). For the DBP, a positive correlation was found when the CMI was below 2.81 (β = 1.45, 95% CI: 0.10–2.79; p = 0.0345), and a negative correlation when the CMI was above 2.81 (β = –1.92, 95% CI: –3.08– –0.77; p = 0.0012). A strong interaction was observed between the CMI and gender for the SBP (p = 0.0054) and a trend for the interaction between CMI and age for the DBP (p = 0.1667).
This study found a significant positive correlation between the CMI and BP, with threshold effects supporting a non-linear relationship. The strong interaction between the CMI and gender for SBP suggests that the influence of the CMI on BP may be gender-dependent. These results highlight the importance of utilizing CMI in personalized cardiovascular risk stratification and underscore the relevance of considering patient factors such as gender in managing hypertension.
Keywords
- cardiometabolic index (CMI)
- clinic blood pressure
- cardiovascular risk
- NHANES
- multivariate regression analysis
Hypertension is one of the most important risk factors for cardiovascular disease (CVD) worldwide, and as such it constitutes a serious threat to cardiovascular health [1]. Hypertension’s complications, including heart disease, stroke, and renal disease, were estimated by the WHO to account for over 9 million deaths annually [2]. As well as increasing cardiovascular events, hypertension has also been associated with dementia and cognitive decline [3]. Early identification and treatment of hypertension are therefore critical for the prevention of CVD and its reduction in mortality.
The cardiometabolic index (CMI) is a comprehensive indicator that is strongly associated with metabolic diseases such as diabetes and is also related to an increased risk of cardiovascular and cerebrovascular events, including stroke [4, 5]. The CMI offers a quantitative approach to the assessment of cardiometabolic risk by incorporating the ratios of waist circumference to height, and triglycerides to high-density lipoprotein cholesterol. A higher value of CMI indicates greater cardiometabolic risk, and detects normal-weight but metabolically abnormal individuals, i.e., metabolically obese normal weight (MONW) individuals [6, 7]. CMI provides useful data in the primary screening of high-risk patients, and in the clinical setting is mainly used to ascertain risk for metabolic syndrome, diabetes, and CVD [4, 8, 9].
Although previous studies have explored the relationship between cardiovascular risk and CMI [10, 11], the relationship between CMI and clinic blood pressure (BP) is uncertain. Clarification of this relationship should provide significant insights into the metabolic processes involved in the determination of BP, as well as new strategies for its management. The predictive value of CMI for cardiovascular events could also create new avenues for the prevention and treatment of CVD. In the present study, we therefore utilized the National Health and Nutrition Examination Survey (NHANES) database to investigate the correlation between CMI and clinical BP. Our aim was to provide a scientific foundation for the improvement of CVD prevention and control strategies.
This cross-sectional analysis was conducted using data from the NHANES, a national, cross-sectional survey of the Centers for Disease Control and Prevention (CDC) to assess the health and nutritional status of adults and children in the United States. The research data were provided on the following website: https://wwwn.cdc.gov/nchs/nhanes/default.aspx. Consent was initially requested for all the anthropometric measurements and blood draws, and medical history was requested from all the participants.
The initial population involved in this research included 19,226 participants of the NHANES database enrolled in 2015–2018. The following exclusion criteria were applied to attain the reliability and validity of our outcome:
① Excluding participants
After applying the exclusion criteria, the final population of the study comprised 4240 subjects. (Fig. 1).
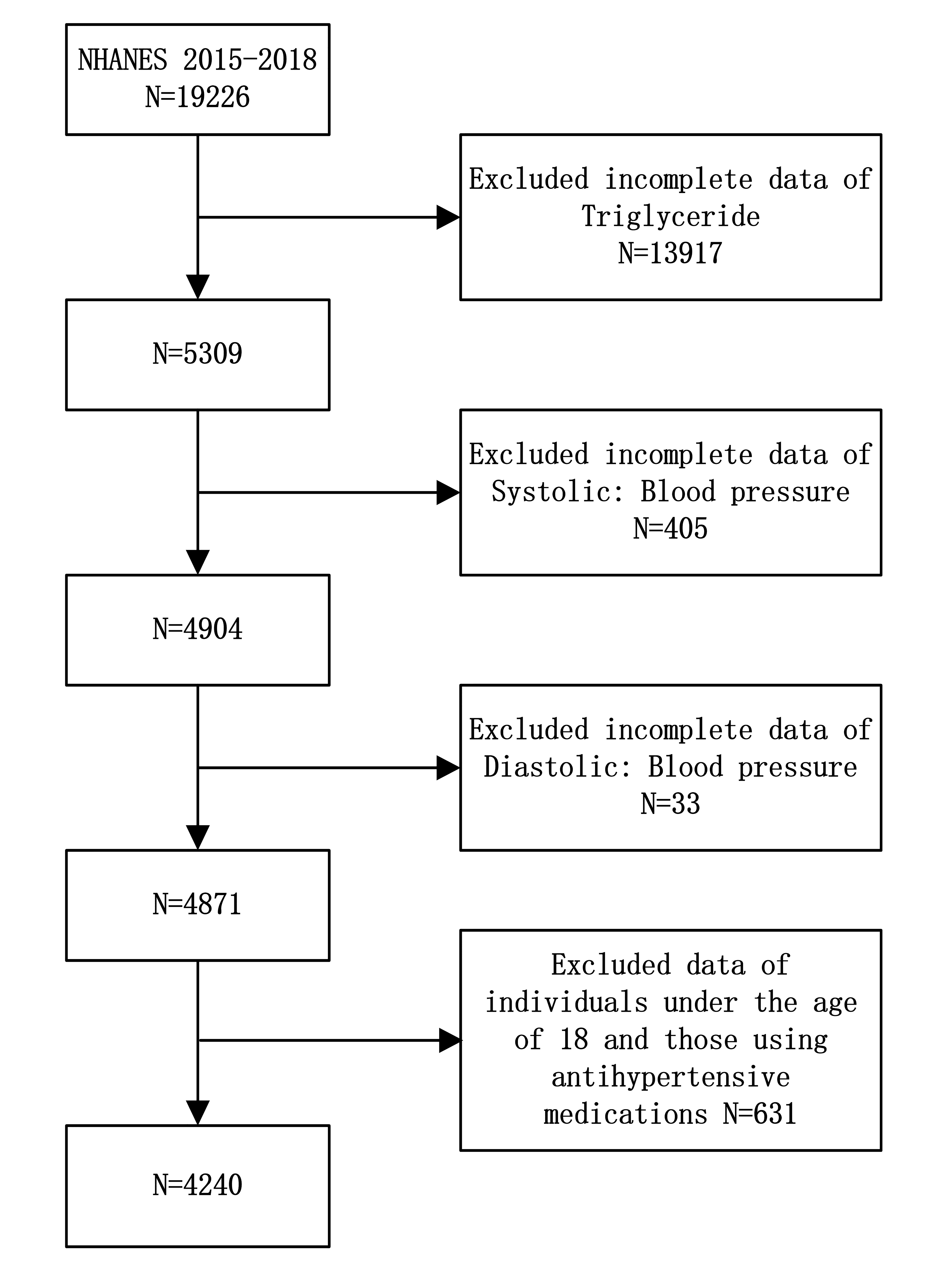 Fig. 1.
Fig. 1.
Study flow chart. NHANES, National Health and Nutrition Examination Survey.
The CMI is an overall measure of metabolic risk as it includes measures of
lipids and anthropometric markers of an individual’s cardiometabolic risk [4].
The four measures used in the current study to estimate CMI were triglycerides
(TG), HDL-C, waist circumference, and
height. With the high-density lipoprotein cholesterol-to-triglyceride ratio, CMI
could be determined from the following formula: CMI = WHtR
The clinic BP measurements in the NHANES database conform to American Heart Association and American Society of Hypertension guidelines. The BP measurements are made in a quiet, temperature-controlled room, with the participants resting quietly for at least 5 minutes before measurement. SBP and DBP are measured using calibrated automated sphygmomanometers, with the results averaged over three measures.
The covariates analysed included age, gender, race, family income-to-poverty ratio, body mass index (BMI), C-reactive protein levels, alcohol and smoking habits, diabetes status, and sleep disorders that may influence BP and the CMI. Data on these covariates were obtained from questionnaires, physical examinations, and laboratory tests.
Data were subjected to severe cleaning and quality control, including processing of missing data, screening and elimination of outliers, and checking measurement for errors. Poverty income ratio (PIR) data was missing for 467 cases, CRP data for 17 cases, alcohol use data for 754 cases, and smoking data for 2441 cases. To account for missing data, multiple imputation based on 5 replications and a chained equation approach method was adopted in the R MI procedure.
Linear regression was used to assess the relationship between CMI and clinic BP.
Modeling was performed under three different levels: non-adjusted model, Adjusted
I model, and Adjusted II model. The Adjusted I model controlled for basic
covariates such as age, gender, and race, while the Adjusted II model controlled
for additional potential confounders, including alcohol and smoking habits,
C-reactive protein (CRP) levels, diabetes status, and sleep disorders.
Additionally, threshold effect analysis and interaction tests were conducted to
explore the relationship between CMI and BP in different subgroups. Continuous
variables are presented as mean
This study included a total of 4240 participants, with 47.8% being male and
52.2% being female. The average age was 48.85
| Variable | Low CMI | Middle CMI | High CMI | p-value | |
| (0.19 |
(0.46 |
(1.38 | |||
| AGE, years | 44.86 |
50.27 |
51.43 |
||
| FAMILY.PIR | 2.66 |
2.47 |
2.42 |
||
| BMI, kg/m2 | 25.58 |
29.96 |
32.60 |
||
| CRP, mg/L | 2.97 |
4.04 |
5.13 |
||
| SBP | 120.55 |
126.75 |
127.88 |
||
| DBP | 68.36 |
71.22 |
72.22 |
||
| GENDER | |||||
| Male | 580 (41.05%) | 674 (47.70%) | 830 (58.70%) | ||
| Female | 833 (58.95%) | 739 (52.30%) | 584 (41.30%) | ||
| ALCOHOL.USE | 0.507 | ||||
| Yes | 216 (15.29%) | 245 (17.34%) | 245 (17.33%) | ||
| No | 1197 (84.71%) | 1168 (82.66%) | 1169 (82.67%) | ||
| SMOKING | 0.925 | ||||
| Every day | 430 (30.43%) | 421 (29.79%) | 444 (31.40%) | ||
| Some days | 143 (10.12%) | 144 (10.19%) | 143 (10.11%) | ||
| Not at all | 840 (59.45%) | 848 (60.01%) | 827 (58.49%) | ||
| RACE | |||||
| Mexican American | 143 (10.12%) | 258 (18.26%) | 284 (20.08%) | ||
| Other Hispanic | 116 (8.21%) | 169 (11.96%) | 212 (14.99%) | ||
| Non-Hispanic White | 463 (32.77%) | 453 (32.06%) | 521 (36.85%) | ||
| Non-Hispanic Black | 422 (29.87%) | 318 (22.51%) | 155 (10.96%) | ||
| Non-Hispanic Asian | 214 (15.15%) | 146 (10.33%) | 173 (12.23%) | ||
| Other Race-Including Multi-Racial | 55 (3.89%) | 69 (4.88%) | 69 (4.88%) | ||
| DIABETES | |||||
| Yes | 88 (6.23%) | 208 (14.72%) | 335 (23.69%) | ||
| No | 1295 (91.65%) | 1168 (82.66%) | 1029 (72.77%) | ||
| Borderline | 30 (2.12%) | 37 (2.62%) | 50 (3.54%) | ||
| SLEEP.DISORDERS | |||||
| Yes | 309 (21.87%) | 397 (28.10%) | 452 (31.97%) | ||
| No | 1104 (78.13%) | 1016 (71.90%) | 962 (68.03%) | ||
Mean
Table 2 shows the results of multivariate regression analysis of the
relationship between CMI and BP. In the unadjusted model, CMI was positively
correlated with both SBP (
| Non-adjusted | Adjusted I | Adjusted II | ||
| Y = SBP | ||||
| CMI | 1.07 (0.68, 1.46) c | 0.56 (0.19, 0.93) b | 0.51 (0.13, 0.89) b | |
| CMI Tertile | ||||
| Low | 0 | 0 | 0 | |
| Middle | 2.86 (1.99, 3.73) c | 2.31 (1.48, 3.14) c | 2.24 (1.39, 3.09) c | |
| High | 3.85 (2.99, 4.72) c | 2.65 (1.79, 3.50) c | 2.65 (1.73, 3.56) c | |
| Y = DBP | ||||
| CMI | 1.63 (1.01, 2.25) c | 1.15 (0.60, 1.71) c | 0.83 (0.27, 1.40) b | |
| CMI Tertile | ||||
| Low | 0 | 0 | 0 | |
| Middle | 6.20 (4.82, 7.58) c | 4.03 (2.79, 5.28) c | 3.52 (2.26, 4.79) c | |
| High | 7.34 (5.96, 8.72) c | 5.23 (3.94, 6.52) c | 4.44 (3.09, 5.79) c | |
bp
Model 1: no covariates were adjusted. Model 2: age, gender, and race were adjusted. Model 3: age, gender, race, FAMILY.PIR, BMI, CRP, smoking, alcohol use, diabetes, sleep disorders were adjusted. BP, blood pressure.
Fig. 2 and Table 3 present the results of the threshold effect analysis on the
relationship between CMI and BP. A distinct inflection point (K = 6.83) was
observed for SBP, below which CMI was positively correlated with SBP (
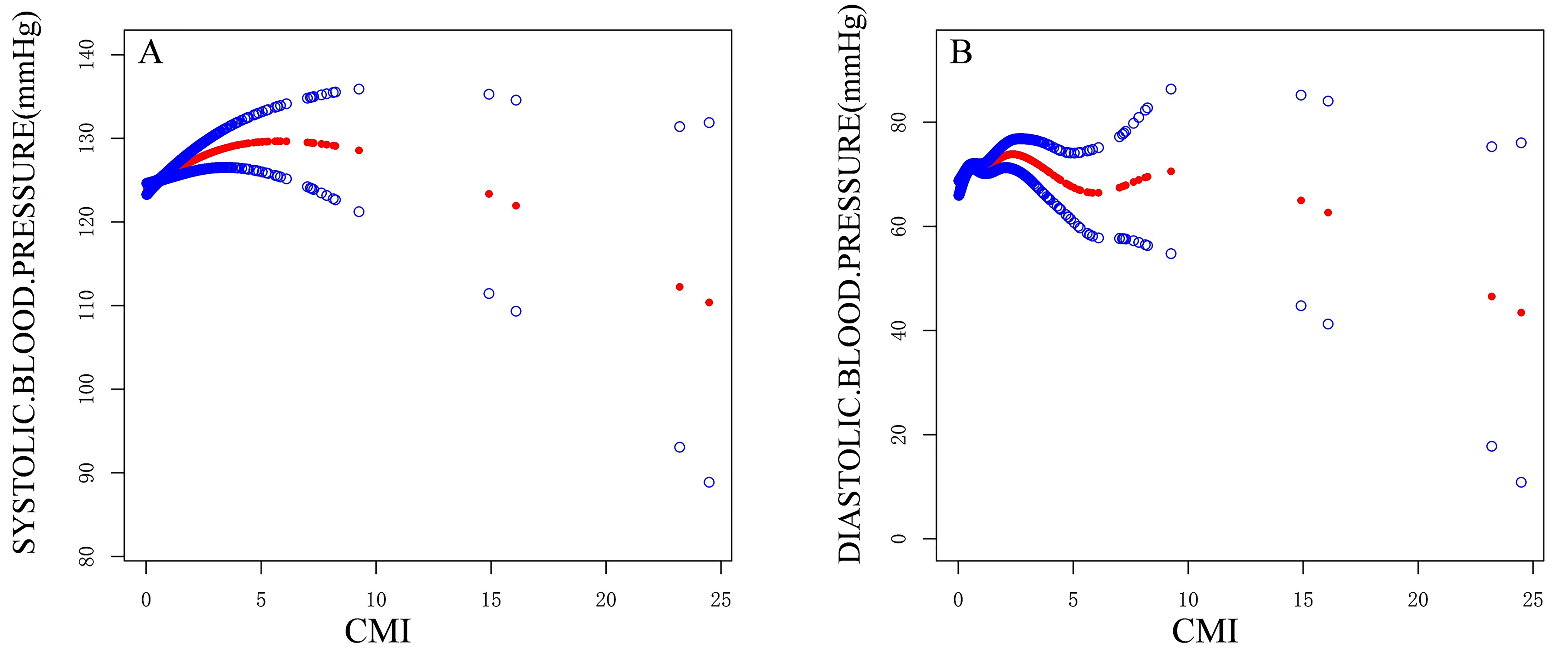 Fig. 2.
Fig. 2.
Fitted curve of CMI and clinic BP. (A) Fitted Curve of CMI and SBP. (B) Fitted Curve of CMI and DBP.
| Outcome | SBP | DBP | |
| Model I | |||
| Linear effect | 0.44 (–0.13, 1.01) | –0.99 (–2.11, 0.14) | |
| Model II | |||
| Inflection point (K) | 6.83 | 2.81 | |
| Effect below K (1) | 1.44 (0.64, 2.24) c | 1.45 (0.10, 2.79) a | |
| Effect above K (2) | –1.52 (–2.77, –0.28) a | –1.92 (–3.08, –0.77) b | |
| Difference between effects 2 and 1 | –2.96 (–4.64, –1.29) c | –3.37 (–4.39, –2.35) c | |
| Predicted value at inflection point | 145.43 (140.23, 150.63) | 76.85 (75.38, 78.31) | |
| Log–likelihood ratio test | |||
ap
Table 4 presents the results of the sensitivity analysis, revealing that the
results from the complete case analysis were consistent with those from the
multiple imputation analysis. In the Adjusted II model, the complete case
analysis showed a significant positive association between CMI and SBP (
| Outcome | Model | Complete Case Analysis (n = 1799) | Multiple Imputation (n = 4240) |
| SBP | Unadjusted | ||
| Adjusted I | |||
| Adjusted II | |||
| DBP | Unadjusted | ||
| Adjusted I | |||
| Adjusted II |
ap
Table 5 presents results of the interaction tests between CMI and factors such
as gender and age. A significant interaction was observed between CMI and gender
in SBP (p = 0.0054), suggesting the association between CMI and SBP
differs between males and females. Specifically, the effect of CMI on SBP was
stronger in females (
| Variables | N | SBP | p interaction | DBP | p interaction | |
| GENDER | 0.0054 | 0.0936 | ||||
| Male | 2084 | 0.51 (–0.13, 1.14) | 0.75 (0.31, 1.19) c | |||
| Female | 2156 | 5.74 (4.17, 7.31) c | 1.56 (0.66, 2.45) c | |||
| AGE, years | 0.8865 | 0.1667 | ||||
| 1153 | 3.03 (2.02, 4.04) c | 2.63 (1.68, 3.58) c | ||||
| 1663 | 1.16 (0.39, 1.93) b | 0.74 (0.25, 1.24) b | ||||
| 1424 | 0.23 (–0.99, 1.45) | –0.15 (–0.87, 0.57) | ||||
| CRP, mg/L | 0.4026 | 0.2285 | ||||
| 3887 | 1.58 (0.95, 2.21) c | 1.12 (0.73, 1.51) c | ||||
| 353 | 2.23 (–0.92, 5.39) | –0.09 (–2.15, 1.98) | ||||
| SLEEP.DISORDERS | 0.9652 | 0.9177 | ||||
| Yes | 1158 | 1.22 (0.01, 2.42) a | 0.91 (0.12, 1.70) a | |||
| No | 3082 | 1.71 (0.99, 2.43) c | 1.10 (0.66, 1.55) c | |||
| DIABETES | 0.9369 | 0.3462 | ||||
| Yes | 631 | –0.77 (–2.16, 0.62) | 0.46 (–0.39, 1.32) | |||
| No | 3492 | 1.68 (1.00, 2.37) c | 1.30 (0.86, 1.74) c | |||
| Borderline | 117 | –1.73 (–10.05, 6.59) | –0.73 (–5.20, 3.74) | |||
ap
This study used the NHANES database to explore the correlation between CMI and clinic BP. Multivariate regression analysis found that CMI was positively correlated with both SBP and DBP. Moreover, a consistent relationship remained across different age, gender, and race subgroups. The significant association observed between CMI and BP possibly reflects common metabolic pathways and biological mechanisms.
Threshold effect analysis revealed a non-linear relationship between CMI and BP, with critical thresholds identified for CMI. For SBP, an inflection point was observed at a CMI value of 6.83, indicating that CMI increases when SBP is below this threshold, but decreases above it. For DBP, an inflection point was observed at a CMI value of 2.81, and a similar non-linear relationship was observed between CMI and DBP. The results suggest that biological thresholds may play a role in modulating the relationship between CMI and BP. This is likely to be related to the complex mechanisms of BP regulation, including changes in sensitivity to metabolic factors across different metabolic states.
The non-linear relationship observed here may be attributed to several
underlying mechanisms. First, when CMI is at a lower level, the metabolic
abnormalities reflected by CMI (e.g., elevated triglycerides, low HDL-C, and
increased waist-to-height ratio) primarily influence BP through inflammation [17]
and endothelial dysfunction [18], resulting in a positive correlation between CMI
and BP. Specifically, these metabolic abnormalities trigger systemic inflammatory
responses that increase pro-inflammatory cytokines (e.g., tumor necrosis factor (TNF)-
However, when CMI exceeds certain thresholds, the relationship between CMI and BP changes significantly. The present study found distinct inflection points of 6.83 for SBP and 2.81 for DBP. This phenomenon may be related to the complex mechanisms of BP regulation. When CMI exceeds 6.83, it becomes negatively correlated with SBP, possibly because under extreme metabolic abnormalities (e.g., severe obesity or insulin resistance), the body activates a series of counter-mechanisms that prevent further increases in BP. For example, the renin-angiotensin-aldosterone system (RAAS) may be activated, but as metabolic abnormalities worsen, the feedback regulation mechanisms for RAAS may change, thereby weakening its regulatory effect on BP and even leading to paradoxical responses [21]. Additionally, the sympathetic nervous system (SNS) is often overactivated in metabolic syndrome, which may lead to receptor fatigue or desensitization when metabolic abnormalities reach a certain level [22], thus reducing its pressor effects.
When CMI exceeds 2.81, it also becomes negatively correlated with DBP. This may be related to further deterioration of endothelial function. When CMI is low, endothelial dysfunction is mainly manifested as reduced NO bioavailability, leading to decreased vasodilation. However, as CMI increases, endothelial dysfunction may worsen [18], reducing the responsiveness of blood vessels to vasodilatory signals and even offsetting the effects of vasoconstriction. Additionally, as metabolic abnormalities worsen, adipose tissue may secrete more anti-inflammatory factors (e.g., adiponectin) [23], which may improve endothelial function and reduce BP by reducing oxidative stress.
The different responses of SBP and DBP to changes in CMI may also be due to their different physiological mechanisms in relation to the formation and regulation of BP. SBP mainly reflects the peak BP during cardiac contraction, whereas DBP mainly reflects the maintenance of BP during cardiac relaxation. Therefore, SBP and DBP may have different sensitivities to metabolic abnormalities, leading to different correlations when CMI exceeds certain thresholds.
Interaction analysis revealed a significant interaction between CMI and gender
for SBP (p = 0.0054), indicating the association between CMI and SBP
differs between males and females. Specifically, the effect of CMI on SBP was
stronger in females (
Several studies have investigated the relationship between CMI and BP across
different regions and populations. A study comprising 11,400 adults in China
reported a significant positive association between CMI and hypertension [11].
Moreover, for each one standard deviation increase in CMI, the risk of
hypertension increased by 35.6% in women and 31% in men. Another study of
15,453 participants in Japan revealed a nonlinear relationship between CMI and
the risk of hypertension and other metabolic diseases [30]. The risk was
significantly increased when CMI was low (
As a comprehensive indicator of metabolic health, CMI may be related to BP through various biological pathways. Firstly, the ratio of triglycerides to high-density lipoprotein cholesterol in CMI reflects lipid abnormalities, which have been associated with endothelial dysfunction and vascular inflammation [31]. Endothelial dysfunction leads to a decrease in the vascular dilation capacity, thereby increasing the risk of cardiovascular events. Secondly, the waist-to-height ratio in CMI indicates abdominal obesity, a key component of metabolic syndrome, and is associated with insulin resistance and chronic inflammatory states [32, 33]. Furthermore, CMI is closely associated with the progression of vascular atherosclerosis. Studies have shown that the increase in CMI not only reflects metabolic abnormalities but may also accelerate the progression of atherosclerosis by promoting endothelial dysfunction [34] and inflammatory responses [35]. Wakabayashi et al. [6] further confirmed that CMI is significantly associated with the progression of atherosclerosis in patients with peripheral arterial disease. Therefore, CMI may serve as a potential indicator for assessing the risk of atherosclerosis. These pathophysiological processes collectively promote an elevated BP and increased cardiovascular risk.
The strengths of this study lie in its large sample size and national representativeness associated with the NHANES database, thereby enhancing the generalizability of our findings. Additionally, we employed multivariate regression analysis to control for several potential confounding factors, adding strength to the reliability of the results. However, there are also some limitations to this study. First, due to the cross-sectional nature of the NHANES data, causality from CMI to BP is not possible to determine. Second, although we controlled for different covariates, there could still be some unmeasured confounders such as genetic factors and psychosocial traits that could potentially affect the relationship between CMI and BP. Last but not least, although the computation formula for CMI is simple and easy to implement, application of it in diverse populations still needs further validation. It is also noteworthy that 2441 had missing information on smoking history. This may have introduced some bias and limited the generalizability of our findings, as smoking is a confirmed risk factor for hypertension and cardiovascular diseases. Future studies need to be geared towards addressing this shortcoming through the gathering of more detailed data on smoking practices.
Finally, this study reports novel evidence for the relationship between CMI and clinic BP and the potential of CMI for cardiovascular risk stratification. Even though our observations are limited by their nature, they provide the foundation for subsequent studies that might have important clinical implications for managing hypertension and other related disorders.
The CMI was found to be highly and positively correlated with clinic BP in the general United States population, which indicates its potential utility as a valuable predictor of cardiovascular risk. The correlation is particularly valuable in the identification of high-risk patients for hypertension and related cardiovascular complications. CMI follow-up can further elucidate how the BP of an individual is related to his or her metabolic health.
The threshold effects revealed in this research indicated substantial non-linear correlations between CMI and BP, and the influence of CMI on BP based on whether CMI was greater or less than specific thresholds. These data suggest that the treatment of hypertension should be tailored to an individual’s CMI score. For instance, when CMI is subthreshold, it should be addressed to manage other cardiovascular risk factors, whereas when CMI is over the threshold, intensified BP surveillance and treatment are appropriate.
Significant gender interactions with CMI for SBP emphasize the contribution of individual factors to the modification of the CMI-BP relationship. These interactions indicate that the association between CMI and BP is not uniform across the population, and can be influenced by factors such as gender. Therefore, a multi-factorial comprehensive analysis involving CMI and demographic and clinical factors is essential for identifying high-risk subgroups accurately and guiding targeted interventions.
CMI, cardiometabolic index; NHANES, National Health and Nutrition Examination Survey; SBP, systolic blood pressure; DBP, diastolic blood pressure; CVDs, cardiovascular diseases; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; WHtR, waist-to-height ratio; BMI, body mass index; CRP, C-reactive protein; PIR, poverty income ratio; ROC, receiver operating characteristic; RAAS, renin-angiotensin-aldosterone system; SNS, sympathetic nervous system.
Publicly available datasets were analyzed in this study. These data can be found at: https://www.cdc.gov/nchs/nhanes/about/erb.html.
LH: Collected the data, conducted the investigation, provided resources, and wrote the original draft of the manuscript. LH also contributed to the review and editing of the manuscript. LS: Conducted formal analysis and contributed to the methodology. HP: Contributed to the conception of the research, developed the methodology, provided software support, and critically reviewed and edited the manuscript. CZ: Designed the research, contributed to the methodology, provided software support, wrote the original draft of the manuscript, and critically reviewed and edited the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study utilized secondary analyses of publicly available and de-identified data obtained from NHANES. The protocols of NHANES adhered to the ethical guide lines of the 1975 Declaration of Helsinki and received approval from the NCHS research ethics review board. All patients/participants or their families/legal guardians provided informed consent.
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
This research was funded by the Zhejiang Province Traditional Chinese Medicine Scientific Research Fund (2023ZL700), Clinical Key Specialty Construction Project of Zhejiang Province–Cardiovascular Medicine (2024-ZJZK-001).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


