1 Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA
2 HeartDrug Research LLC, West Friendship, MD 21294, USA
3 R&D Group, Vice Presidency for Scientific Research and Innovation, Imam Abdulrahman bin Faisal University (IAU), 31441 Dammam, Saudi Arabia
4 UNAM-UABJO Research Center, Universidad Autónoma Benito Juárez de Oaxaca, 68120 Oaxaca, Mexico
5 Department of Pharmacology, University of Maryland, College Park, MD 20742, USA
6 Physician, retired from FDA, Alexandria, VA 22314, USA
Abstract
This study aimed to determine the impact of central adjudication of site-reported events in patients with acute coronary syndromes treated with ticagrelor or clopidogrel in addition to aspirin within the frame of indication-seeking The PLATelet Inhibition and Clinical Outcomes (PLATO) trial. Adjudication in randomized outcome-driven trials is supposed to maintain integrity by applying uniform rules for the quality assessment of clinical events. Some preliminary data suggest an imbalance between central and site diagnoses in PLATO. We gained access to the Food and Drug Administration (FDA)-issued adjudication dataset and analyzed the evidence.
Death, myocardial infarction (MI), stroke/ transient ischemic attack (TIA), bleeding, arterial thrombotic events, and cardiac ischemic events underwent central adjudication. We assessed geography, timing, impact of disagreements, and primary endpoint composition.
Among 18,624 trial enrollees, 10,704 central adjudications occurred across 7171 patients in 43 countries. There were 938 deaths, 2751 cases of MI, 359 strokes/TIAs, 2680 cardiac events, 130 thrombotic events, and 3782 bleeding events. The match occurred for 5451 events, while mismatches favoring clopidogrel (n = 2535) or ticagrelor (n = 2706) (p = 0.79) were common for major (n = 1797), moderate (n = 942), or minor (n = 735) disagreements. The central decision prevailed in 2945 cases. There was a significant (HR = 0.84; 95% confidence intervals (CI): 0.75–0.95; p = 0.004) adjudication delay in the 2007–2008 events but finalized in 2009. Ticagrelor was significantly less favored in 2009 than in 2007–2008 (HR = 1.19; 95% CI: 1.05–1.34; p = 0.005). There was a remarkably consistent match for bleeding adjudication (HR = 1.02; 95% CI: 0.83–1.25; p = 0.859) between treatment arms. The primary endpoint in the PLATO trial exhibited highly significant disagreement favoring ticagrelor for vascular death (HR = 2.02; 95% CI: 1.1–3.64; p = 0.019); MI (HR = 2.31; 95% CI: 2.79–43.94; p = 0.034); stroke (HR = 1.37; 95% CI: 2.66–63.28; p = 0.036); total events (HR = 2.51; 95% CI: 1.86–3.39; p = 0.01).
Central adjudication in the PLATO trial was delayed and impacted the primary endpoint by inflating the ticagrelor benefit, resulting in drug approval. The regulatory authorities should consider independent audits when unblinding is suspected in the indication-seeking clinical trials.
Keywords
- event adjudication
- clinical trial
- primary endpoint
- bleeding
- death
- myocardial infarction
- stroke
Central event adjudication in randomized controlled trials is common for multicenter international outcome-driven studies. Delegating such a critical mission for the final assessment of the site-reported events should fix the variability of definitions, contribute to comprehending and resolving complicated clinical scenarios, remove “noise” and potential bias from the totality of evidence [1, 2, 3]. However, whether or not the uniform use of central adjudication is always justified and unbiased is unclear. The PLATelet Inhibition and Clinical Outcomes (PLATO) trial enrolled 18,624 patients with unstable angina or myocardial infarction (MI) treated coronary stenting or managed medically. The patients were randomized to receive ticagrelor 180 mg loading followed by 90 mg twice daily or clopidogrel 300–600 mg loading followed by 75 mg once daily, for up to one year [4]. The primary endpoint was defined as a combination of vascular death including bleeding fatalities, MI, and stroke. These events occurred in 11.7% of patients from clopidogrel arm, compared to 9.8% of ticagrelor treated patients (HR = 0.84; 95% confidence intervals (CI): 0.77–0.92; p
Lately, the alleged clinical benefit of ticagrelor has been challenged further by mortality timing, causes, and vanished fatalities [7, 8], “friendly” pool of central adjudicators and International Central Adjudication Committee (ICAC) leadership [9], and involvement of sponsor representatives in the adjudicator selection [5, 6, 10]. Moreover, ticagrelor benefit based on site-reporting was not significant in PLATO [5, 6], while the disagreement in MI counts favoring ticagrelor was concerning [11, 12]. The latest comprehensive BMJ investigation confirmed the adjudication bias in PLATO [13]. However, several critical pieces with regard to the timing of final adjudication and primary endpoint data analyses were still unclear. We gained access to the FDA-issued adjudication dataset and analyzed the evidence focusing on geography, timing of final adjudication, disagreements, and trends for drug favoritisms in crosstabulation including primary trial endpoint components.
We filed a legal complaint in the US Federal Court (case 1:21-CV 01572 BAH), reached a Joined Status Report Order with the FDA and Department of Justice based on the Freedom of Information Act law. The FDA issued over 800 pages of evidence, and among other documents we were provided with the entire PLATO adjudicated event listings submitted to the FDA by the ticagrelor New Drug Application (NDA) 22-433 sponsor.
Study participants and procedures are described in details elsewhere [4, 5, 6]. Patients were enrolled if presented with recent (no more than 24 hours) acute coronary syndrome (ACS) onset. Among major exclusions were contraindication against clopidogrel, fibrinolytics, oral anticoagulants, the bradycardia risk, or concomitant use of a strong cytochrome P-450 3A inhibitor or inducer. Overall, 18,624 patients were enrolled, about a quarter were diabetics, over 60% underwent stent implantation, 10% underwent heart surgery and 46% received prehospital clopidogrel. The follow-up duration was restricted to 1 year. However, 23% of participants stop taking the study drug before the end of follow-up most frequently due to repeated bleedings.
Most adjudicated events such as death, MI, stroke, and bleeding have been defined and described in detail [4, 5, 6]. Briefly, each event was characterized by an adjudication code. The ICAC evaluated data of every patient designated by a local investigator as a possible event and also all patients who underwent heart surgery during the study. The ICAC determined that some events reported by Investigators did not qualify. On occasion, the ICAC identified additional unreported events and directed the sponsor or clinical research organization to query a site to register the event for official adjudication. If the local Investigator agreed, the event was registered and processed by the ICAC. Ultimately, each event was characterized by a unique adjudication code.
The FDA-issued database spreadsheet contains 10,704 events. Each event is marked by trial identification unique number, country, enrolling site, patient age, gender, treatment assignments, discontinuations, outcome codes, precise dates, and causes of trial entry and exit. In addition, enrollment codes, event tracking numbers, and patient participation in Holter, pharmacokinetics, and pulmonary function sub studies were also provided. Final adjudicated event results (CADJRES) were coded as 1 – death; 2 – myocardial infarction; 3 – stroke; 4 – recurrent ischemia; 5 – severe recurrent ischemia; 6 – fatal/life-threatening bleed; 7 – major bleed; 8 – minor bleed; 9 – minimal bleed; 10 – no event; 11 – transient ischemic attack; 12 – arterial thrombotic event; and 99 – withdrawal of consent. Exact classifications and subtypes of death, myocardial infarction, and stroke were also provided for each entry.
These were identified by the mismatch between event classification from the local site and central adjudication. The FDA provided full disclosure of such mismatches including event details from the site, final adjudication results, disagreement dates, resolution by reviewers or committee (if any), and disagreement severity (minor, moderate, or major), type, and details. We summarize such upgrades and downgrades in Table 1.
| Adjudicated event | Upgrade | Downgrade |
| Cardiac ischemic | Angina to MI | No event |
| Arterial thrombotic | Confirmed | No event |
| Stroke/TIA | TIA to stroke | No event, or stroke to TIA |
| Bleeding* | More severe | Less severe or no event |
| Myocardial infarction | Confirmed | No event, or angina |
| Death | Nonvascular or unknown to vascular cause | Vascular to nonvascular or unknown cause |
*, per original PLATO classification (minimal, minor, major, life-threatening or fatal). PLATO, the PLATelet Inhibition and Clinical Outcomes; MI, myocardial infarction; TIA, transient ischemic events.
The significant differences were defined when a two-sided alpha value was less than 0.05 uncorrected for multiple comparisons. Categorical data were assessed by frequency and percentage statistics. Chi-square calculations were conducted to test for interplay between various bleeding types (per PLATO novel bleeding scale) and endpoint components (vascular death, MI, and stroke). Unadjusted odds ratios (OR) with 95% CI were calculated and interpreted for all chi-square tests. Unpaired t-test with Welch’s correction has been applied to establish the disagreement differences. A chi-square test was conducted to evaluate the significance of the observed shift towards ticagrelor “benefit”. The null hypothesis (H0) posits that the observed shift is due to random chance alone (sporadic), while the alternative hypothesis (H1) suggests that the observed shift is not solely attributable to random chance (non-sporadic). All analyses were performed using SPSS Version 28 (IBM Corp., Armonk, NY, USA), with the exception of the forest plot, which was constructed in GraphPad Prism Version 10.3.1 (GraphPad Software, San Diego, CA, USA).
The FDA-issued database spreadsheet contains 10,704 events that occurred across 7171 patients. There were 938 deaths, 2751 MI’s, 359 strokes/TIAs, 2680 cardiac ischemia’s, 130 arterial thromboses, and 3782 bleeds.
Geography: The comparison of the FDA-reported and their matches with the site-reported events in different countries is shown in Table 2.
| Country | Patients enrolled | *Events reported | Match | Favors clopidogrel | Favors ticagrelor | Minor disagreement | Moderate disagreement | Major Disagreement |
| Argentina | 410 | 284 | 165 | 49 | 70 | 12 | 31 | 58 |
| (69.3%) | (58.1%) | (17.3%) | (24.6%) | (2.9%) | (7.6%) | (14.1%) | ||
| Australia | 83 | 70 | 43 | 15 | 12 | 4 | 4 | 15 |
| (84.3%) | (61.4%) | (21.4%) | (17.1%) | (4.8%) | (4.8%) | (18.1%) | ||
| Austria | 143 | 62 | 25 | 20 | 17 | 5 | 2 | 8 |
| (43.4%) | (40.3%) | (32.3%) | (27.4%) | (3.5%) | (1.4%) | (5.6%) | ||
| Belgium | 170 | 124 | 60 | 27 | 37 | 8 | 7 | 11 |
| (72.9%) | (48.4%) | (21.8%) | (29.8%) | (4.7%) | (4.1%) | (6.5%) | ||
| Brazil | 590 | 476 | 272 | 103 | 101 | 21 | 43 | 62 |
| (80.7%) | (57.1%) | (21.6%) | (21.2%) | (3.6%) | (7.3%) | (10.5%) | ||
| Bulgaria | 451 | 162 | 88 | 34 | 40 | 7 | 17 | 28 |
| (35.9%) | (54.3%) | (21.0%) | (24.7%) | (1.6%) | (3.8%) | (6.2%) | ||
| Canada | 401 | 247 | 120 | 61 | 66 | 21 | 17 | 48 |
| (61.6%) | (48.6%) | (24.7%) | (26.7%) | (5.2%) | (4.2%) | (12.0%) | ||
| China | 416 | 220 | 105 | 66 | 49 | 18 | 22 | 44 |
| (52.9%) | (47.7%) | (30.0%) | (22.3%) | (4.3%) | (5.3%) | (10.6%) | ||
| Czech Republic | 1021 | 588 | 251 | 161 | 176 | 48 | 53 | 76 |
| (57.6%) | (42.7%) | (27.4%) | (29.9%) | (4.7%) | (5.2%) | (7.4%) | ||
| Denmark | 382 | 329 | 196 | 73 | 60 | 18 | 19 | 62 |
| (86.1%) | (59.6%) | (22.2%) | (18.2%) | (4.7%) | (5.0%) | (16.2%) | ||
| Finland | 154 | 91 | 44 | 22 | 25 | 13 | 8 | 8 |
| (59.1%) | (48.4%) | (24.2%) | (27.5%) | (8.4%) | (5.2%) | (5.2%) | ||
| France | 422 | 215 | 93 | 65 | 57 | 8 | 21 | 30 |
| (50.9%) | (43.3%) | (30.2%) | (26.5%) | (1.9%) | (5.0%) | (7.1%) | ||
| Georgia | 519 | 109 | 44 | 28 | 37 | 6 | 13 | 21 |
| (21.0%) | (40.4%) | (25.7%) | (33.9%) | (1.2%) | (2.5%) | (4.0%) | ||
| Germany | 1156 | 665 | 349 | 156 | 160 | 59 | 56 | 103 |
| (57.5%) | (52.5%) | (23.5%) | (24.1%) | (5.1%) | (4.8%) | (8.9%) | ||
| Greece | 90 | 62 | 21 | 23 | 18 | 7 | 9 | 8 |
| (68.9%) | (33.9%) | (37.1%) | (29.0%) | (7.8%) | (10.0%) | (8.9%) | ||
| Hong Kong | 16 | 10 | 1 | 5 | 4 | 1 | 1 | 3 |
| (62.5%) | (10.0%) | (50.0%) | (40.0%) | (6.3%) | (6.3%) | (18.8%) | ||
| Hungary | 1267 | 593 | 336 | 121 | 136 | 32 | 60 | 103 |
| (46.8%) | (56.7%) | (20.4%) | (22.9%) | (2.5%) | (4.7%) | (8.1%) | ||
| India | 575 | 210 | 93 | 59 | 58 | 8 | 18 | 30 |
| (36.5%) | (44.3%) | (28.1%) | (27.6%) | (1.4%) | (3.1%) | (5.2%) | ||
| Indonesia | 62 | 34 | 19 | 8 | 7 | 1 | 3 | 8 |
| (54.8%) | (55.9%) | (23.5%) | (20.6%) | (1.6%) | (4.8%) | (12.9%) | ||
| Israel | 636 | 354 | 171 | 93 | 90 | 26 | 30 | 66 |
| (55.7%) | (48.3%) | (26.3%) | (25.4%) | (4.1%) | (4.7%) | (10.4%) | ||
| Italy | 625 | 275 | 116 | 80 | 79 | 16 | 15 | 42 |
| (44.0%) | (42.2%) | (29.1%) | (28.7%) | (2.6%) | (2.4%) | (6.7%) | ||
| Malaysia | 56 | 68 | 38 | 20 | 10 | 0 | 6 | 18 |
| (121.4%) | (55.9%) | (29.4%) | (14.7%) | (0.0%) | (10.7%) | (32.1%) | ||
| Mexico | 137 | 93 | 44 | 27 | 22 | 4 | 10 | 14 |
| (67.9%) | (47.3%) | (29.0%) | (23.7%) | (2.9%) | (7.3%) | (10.2%) | ||
| Netherlands | 913 | 594 | 269 | 142 | 183 | 54 | 46 | 109 |
| (65.1%) | (45.3%) | (23.9%) | (30.8%) | (5.9%) | (5.0%) | (11.9%) | ||
| Norway | 159 | 121 | 57 | 31 | 33 | 13 | 9 | 18 |
| (76.1%) | (47.1%) | (25.6%) | (27.3%) | (8.2%) | (5.7%) | (11.3%) | ||
| Philippines | 78 | 70 | 44 | 13 | 13 | 4 | 5 | 18 |
| (89.7%) | (62.9%) | (18.6%) | (18.6%) | (5.1%) | (6.4%) | (23.1%) | ||
| Poland | 2666 | 1381 | 669 | 333 | 379 | 76 | 130 | 206 |
| (51.8%) | (48.4%) | (24.1%) | (27.4%) | (2.9%) | (4.9%) | (7.7%) | ||
| Portugal | 152 | 120 | 62 | 31 | 27 | 4 | 10 | 13 |
| (78.9%) | (51.7%) | (25.8%) | (22.5%) | (2.6%) | (6.6%) | (8.6%) | ||
| Romania | 397 | 163 | 105 | 30 | 28 | 6 | 12 | 31 |
| (41.1%) | (64.4%) | (18.4%) | (17.2%) | (1.5%) | (3.0%) | (7.8%) | ||
| Russia | 678 | 381 | 200 | 83 | 98 | 14 | 41 | 68 |
| (56.2%) | (52.5%) | (21.8%) | (25.7%) | (2.1%) | (6.0%) | (10.0%) | ||
| South Korea | 120 | 72 | 22 | 24 | 26 | 4 | 3 | 12 |
| (60.0%) | (30.6%) | (33.3%) | (36.1%) | (3.3%) | (2.5%) | (10.0%) | ||
| Singapore | 64 | 30 | 14 | 9 | 7 | 2 | 2 | 8 |
| (46.9%) | (46.7%) | (30.0%) | (23.3%) | (3.1%) | (3.1%) | (12.5%) | ||
| Slovakia | 336 | 184 | 118 | 25 | 41 | 14 | 24 | 30 |
| (54.8%) | (64.1%) | (13.6%) | (22.3%) | (4.2%) | (7.1%) | (8.9%) | ||
| South Africa | 149 | 129 | 75 | 29 | 25 | 9 | 13 | 24 |
| (86.6%) | (58.1%) | (22.5%) | (19.4%) | (6.0%) | (8.7%) | (16.1%) | ||
| Spain | 314 | 209 | 110 | 50 | 49 | 20 | 18 | 41 |
| (66.6%) | (52.6%) | (23.9%) | (23.4%) | (6.4%) | (5.7%) | (13.1%) | ||
| Sweden | 347 | 272 | 168 | 55 | 49 | 36 | 18 | 47 |
| (78.4%) | (61.8%) | (20.2%) | (18.0%) | (10.4%) | (5.2%) | (13.5%) | ||
| Switzerland | 211 | 134 | 53 | 46 | 35 | 13 | 8 | 22 |
| (63.5%) | (39.6%) | (34.3%) | (26.1%) | (6.2%) | (3.8%) | (10.4%) | ||
| Taiwan | 92 | 85 | 36 | 16 | 33 | 3 | 5 | 6 |
| (92.4%) | (42.4%) | (18.8%) | (38.8%) | (3.3%) | (5.4%) | (6.5%) | ||
| Thailand | 152 | 136 | 88 | 21 | 27 | 14 | 19 | 12 |
| (89.5%) | (64.7%) | (15.4%) | (19.9%) | (9.2%) | (12.5%) | (7.9%) | ||
| Turkey | 51 | 37 | 15 | 5 | 17 | 1 | 5 | 3 |
| (72.5%) | (40.5%) | (13.5%) | (45.9%) | (2.0%) | (9.8%) | (5.9%) | ||
| UK | 281 | 166 | 100 | 30 | 36 | 18 | 23 | 35 |
| (59.1%) | (60.2%) | (18.1%) | (21.7%) | (6.4%) | (8.2%) | (12.5%) | ||
| Ukraine | 169 | 76 | 42 | 18 | 16 | 8 | 18 | 26 |
| (45.0%) | (55.3%) | (23.7%) | (21.1%) | (4.7%) | (10.7%) | (15.4%) | ||
| USA | 1413 | 1003 | 510 | 234 | 259 | 79 | 68 | 202 |
| (71.0%) | (50.8%) | (23.3%) | (25.8%) | (5.6%) | (4.8%) | (14.3%) | ||
| Total | 18,624 | 10,704 | 5451 | 2535 | 2706 | 735 | 942 | 1797 |
* , as reported in the FDA-issued dataset, the numbers per country do not match since the same patient may experience several events with different adjudication results and level of potential disagreements. The site-reported events do not necessarily match the adjudication results since events may be added or rejected.
The geographical distribution of such frequencies and disagreements was remarkably consistent in PLATO. About half of the enrolled patients experienced events, with the matches and disagreements being consistent as well. Importantly such massive disagreements were well balanced between treatment arms. They were mostly due to the introduction of a novel PLATO bleeding classification with 4 categories and the controversial inclusion of “enzymatic” MI’s.
We conducted the analysis of events that occurred in 2007–2008 versus 2009 dependent on the time of final adjudication. The differences by arms crosstabulation are shown in Table 3.
| Arm | Total | ||||
| Clopidogrel | Ticagrelor | ||||
| Year | 2007–2008 | Count | 679 | 843 | 1522 |
| % within years | 44.6% | 55.4% | 100.0% | ||
| 2009 | Count | 1789 | 1867 | 3656 | |
| % within years | 48.9% | 51.1% | 100.0% | ||
| Total | Count | 2468 | 2710 | 5178 | |
| % within years | 47.7% | 52.3% | 100.0% | ||
Enrollment in PLATO started in October 2006 through July 2008. The follow-up period ended in February 2009. Hence, most events occurred during 2007–2008 especially considering follow-up restriction to 365 days. The evidence in Table 3 suggests that PLATO ICAC kept a huge pool of events to be adjudicated later in 2009 after the trial closure. There was a significant (HR = 0.84; CI: 0.75–0.95; p = 0.004) adjudication delay of 2007–2008 site-reported events which was finalized in 2009. Critically, ticagrelor was less favored in 2009 in comparison to 2007–2008 (HR = 1.19; CI: 1.05–1.34; p = 0.005).
There was a remarkably consistent bleeding event rate over time (HR = 1.02; CI = 0.83–1.25; p = 0.859) between treatment arms (Table 4).
| Favor | Total | ||||
| Tica | Clop | ||||
| Group year | 2007–2008 | Count | 373 | 337 | 710 |
| % within group year | 52.5% | 47.5% | 100.0% | ||
| 2009 | Count | 390 | 359 | 749 | |
| % within group year | 52.1% | 47.9% | 100.0% | ||
| Total | Count | 763 | 696 | 1459 | |
| % within group year | 52.3% | 47.7% | 100.0% | ||
The disagreements between centrally adjudicated and site reported deaths over time of enrollment in PLATO are shown in Table 5.
| Favor | Total | ||||
| Ticagrelor | Clopidogrel | ||||
| Group year | 2007–2008 | Count | 99 | 26 | 125 |
| % within group year | 79.2% | 20.8% | 100.0% | ||
| 2009 | Count | 62 | 33 | 95 | |
| % within group year | 65.3% | 34.7% | 100.0% | ||
| Total | Count | 161 | 59 | 220 | |
| % within group year | 73.2% | 26.8% | 100.0% | ||
There was no difference (HR = 0.85; 95% CI = 0.46–1.58; p = 0.628) in favoring in terms of MI between 2007–2008 versus 2009. However, overall, there was a consistent significant (p = 0.02) less MI adjudication in the ticagrelor arm (Table 6). There was a significant difference in favoring ticagrelor over clopidogrel in terms of mismatched death (HR = 2.02; 95% CI = 1.1–3.64; p = 0.0208). There was a significantly (p = 0.001) higher rate of centrally adjudicated deaths in 2009 than in 2007–2008.
| Favor | Total | ||||
| Ticagrelor | Clopidogrel | ||||
| Group year | 2007–2008 | Count | 32 | 28 | 60 |
| % within group year | 53.3% | 46.7% | 100.0% | ||
| 2009 | Count | 68 | 51 | 119 | |
| % within group year | 57.1% | 42.9% | 100.0% | ||
| Total | Count | 100 | 79 | 179 | |
| % within group year | 55.9% | 44.1% | 100.0% | ||
The severity of adjudication disagreements for 3 components of the trial primary endpoint is presented in Table 7.
| CEA result | Total | |||||
| Death-vascular | MI | Stroke | ||||
| Disagreement severity | Minor | Count | 21 | 29 | 34 | 84 |
| % within Adj result | 9.4% | 4.4% | 69.4% | 9.0% | ||
| Moderate | Count | 138 | 425 | 0 | 563 | |
| % within Adj result | 61.9% | 64.0% | 0.0% | 60.1% | ||
| Major | Count | 64 | 210 | 15 | 289 | |
| % within Adj result | 28.7% | 31.6% | 30.6% | 30.9% | ||
| Total | Count | 223 | 664 | 49 | 936 | |
| % within Adj result | 100.0% | 100.0% | 100.0% | 100.0% | ||
CEA, clinical endpoint adjudication; Adj, adjudication.
In contrast to stroke when most disagreements were minor (69.4%) the severity of mismatched for vascular death and MI was either moderate or major. In fact, the average of major disagreements hovered around 30% for all adjudicated results.
There were 864 events in the ticagrelor arm and 1014 events in the clopidogrel arm constituting PLATO’s main efficacy result. Table 8 indicates favors of central adjudication exclusively for the events constituting the trial primary endpoint.
| Endpoint | Total | |||||
| Death-vascular | MI | Stroke | ||||
| Group assignment | Ticagrelor | Count | 59 | 73 | 7 | 139 |
| % within adjudication | 72.0% | 61.9% | 63.6% | 65.9% | ||
| Clopidogrel | Count | 23 | 45 | 4 | 72 | |
| % within adjudication | 28.0% | 38.1% | 36.4% | 34.1% | ||
| Total | Count | 82 | 118 | 11 | 211 | |
| % within adjudication | 100.0% | 100.0% | 100.0% | 100.0% | ||
With regard to favoring, there was a consistent shift towards ticagrelor advantage for all 3 primary endpoint trial components. There was relatively equal dispersion of ticagrelor “favoring” for vascular death (72.0%), MI (61.9%), and stroke (63.6%). Overall, the central decisions differed from sites in only 211 out of 1878 primary endpoint events (11.2%). However, an extra 67 endpoint events (36 vascular deaths, 28 MI’s, and 3 strokes) were adjudicated in favor of ticagrelor. Hazard Ratios for the Primary Endpoint Adjudicated Results favoring Ticagrelor in PLATO are presented in Fig. 1.
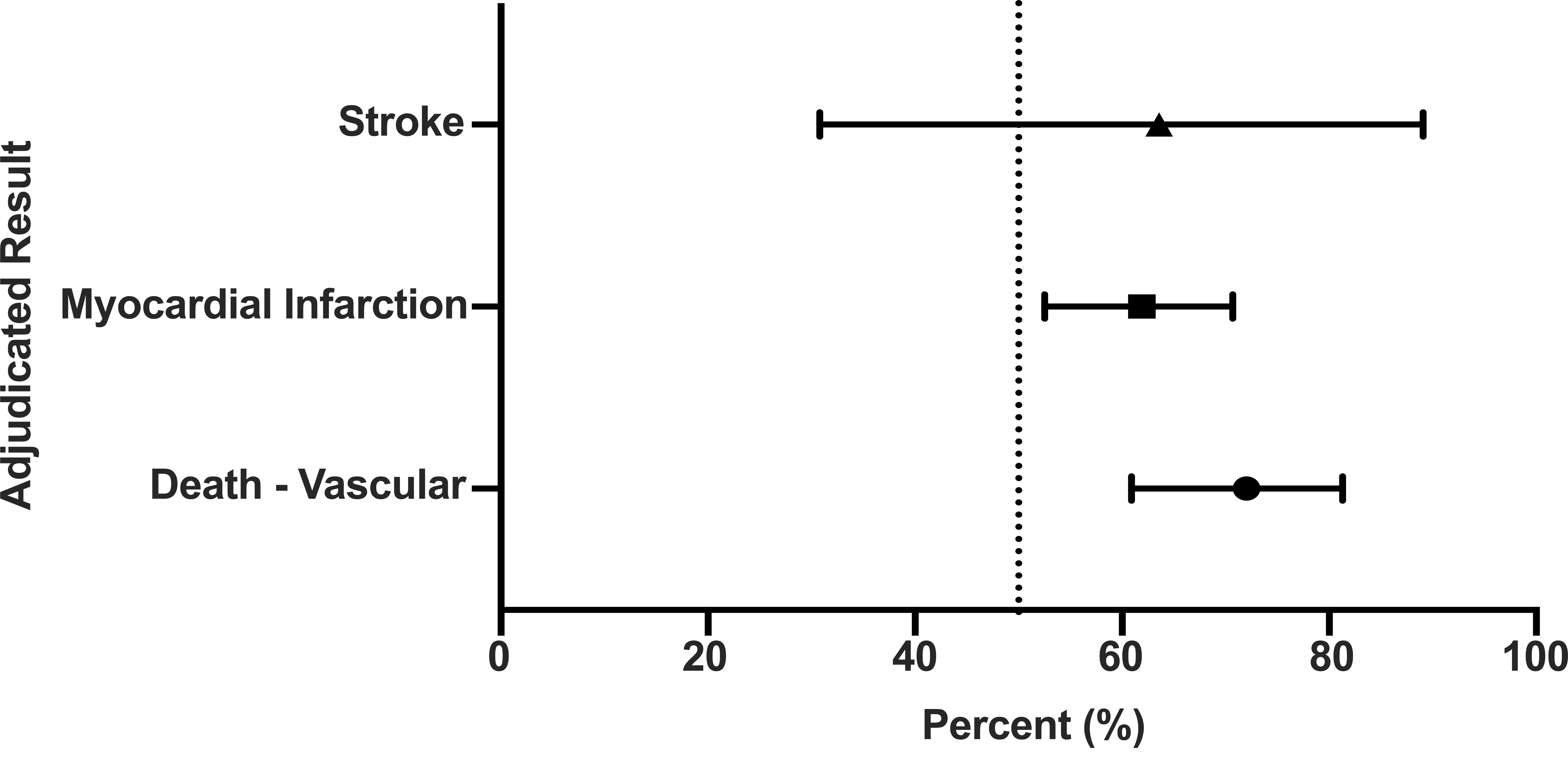 Fig. 1.
Fig. 1. Adjudicated primary end point results favor Ticagrelor in the PLATO study.
With regard to the exclusive primary endpoint events there was highly significant disagreement favoring ticagrelor for vascular death (HR = 2.02; 95% CI: 1.1–3.64; p = 0.019); MI (HR = 2.31 95% CI: 2.79–43.94; p = 0.034); stroke (HR = 1.37; 95% CI: 2.66–63.28; p = 0.036; and total events (HR = 2.51, 95% CI: 1.86–3.39; p = 0.01). Considering that 211/1878 Primary events were found to have disagreements, an extra 67/211 (31.8%) endpoint events were adjudicated in favor of ticagrelor. Given that there were 67 extra events this will happen by chance in 1 of approximately 485 trillion trials.
The clinical validity of central event adjudication has often been a challenge since adjudicated data usually match well with the local sites, but are substantially costly [1, 2, 3, 14, 15] and adding extra bureaucratic organizational burden [2, 15]. Historically, the balance of adjudication results and severity of disagreements were kept secretive and submitted only to the regulatory authorities, especially for the landmark indication-seeking trials. The current analyses became possible because we gained access to the full adjudication database after being awarded of the Joined Status Report by the US Federal Court Order agreement with the FDA and Department of Justice.
The main finding of this report suggests that central adjudication played a pivotal role in escalating ticagrelor benefits far beyond previously described misreported deaths [7, 8]. Without ICAC activities the benefit will remain non-significant [5, 6] making regulatory approval of ticagrelor impossible. In fairness, the ICAC in PLATO was extremely busy adjudicating over 10,000 events in a short-duration megatrial. Such massive tasks were substantiated by two main reasons: the introduction of the unique trial bleeding classification and questionable inclusion of enzymatic or “triggered” MIs. The exact definitions of these two categories were somewhat unclear to the local enrolling sites but subjected to the mandatory central adjudication accounted for about half of all adjudications and disagreements in PLATO. Also, most events were adjudicated fairly including bleedings, arterial thrombosis. and cardiac ischemic events. To make it crystal clear, most deaths, MIs and strokes were also adjudicated properly, but not all of them [7, 13]. For 1878 events constituting the primary endpoint the odds of favoring ticagrelor were 2.51 times higher (95% CI: 1.86–3.39) versus clopidogrel. Importantly, all 3 components of the primary endpoint were adjudicated favoring ticagrelor potentially challenging the treatment arm blindness of ICAC leadership. Importantly, issues with unfair adjudication rendered the PLATO results do not explain the rate of death from any cause which were reported in PLATO being lower after ticagrelor (4.5% vs. 5.9% with clopidogrel; p
The geographical distribution of the central adjudication patterns was remarkably consistent in PLATO. About half of the enrolled patients experienced an event, with the matches and disagreements among 43 enrolling countries being similar as well. Importantly, such massive double-digit disagreements were well-balanced between treatment arms. They were mostly due to the introduction of novel PLATO bleeding scale classifying hemorrhages into 4 categories and the controversial inclusion of “enzymatic” or “triggered” MIs into the equation. It seems most investigators were not well aware of such novelties causing massive disagreements easier to comprehend. In contrast to stroke when most disagreements were minor the severity of mismatched decisions for vascular death and MI was either moderate or major. In fact, the average of major disagreements hovered around 30% for all adjudicated results. There was a heavy selective bias in PLATO ICAC constitution and governing. Importantly, ICAC for PLATO was primarily pre-planned and was not created per the FDA suggestion [5, 6, 9]. In fact, sponsor was definitely informed and potentially involved in ICAC members/reviewers selection although the details on how such communications occurred was hard to comprehend, and the ICAC regulations charter was also ‘silent on the matter’ [6]. Importantly, the FDA inspector did not find any records that explain how the ICAC selections occurred. In short, the FDA’ confirmed that the ICAC constitution was approved by the PLATO study sponsor [5, 6] what is obviously concerning. It is unclear whether any specific procedures were in place during the PLATO trial to ensure consistency and fairness in central event adjudication. That particular issue was also the FDA reviewers concern since the sponsor refused to provide any communication records among ICAC leadership, members and sponsor. In fairness, despite the friendly pool of adjudicators, we have no proof that they were unblinded or biased. Also surprising that despite outcome disparities in the United States, Russia, Georgia, Israel, and part of Ukraine (harm with ticagrelor when Clinical Research Organization (CRO) monitoring) and the other 39 countries (extreme benefit with ticagrelor when sponsor monitoring), the FDA did not institute its own independent ICAC [6], which would be reasonable considering inversed outcomes within the same trial. What matters the most is the fact that, central adjudication changed the PLATO primary endpoint results. Without the ICAC activities, the combined primary endpoint benefit of ticagrelor versus clopidogrel in PLATO was not significant (HR = 0.92; p = 0.095) even by applying liberal log-rank statistics [5, 6]. Importantly, these findings obviously may be extrapolated or confined to the specific context of the PLATO trial and may not be generalizable to other clinical trials or patient populations.
There are a few strengths worth mentioning: This analysis was conducted within the framework of a governmental database that entailed mandatory event reporting. Independent specialists with an expertise on outcome data mining and advanced statistics were used to avoid any potential bias. The sample size for all events represents one of the largest single trial uniform datasets containing 10,704 site-reported entries, allowing us to make reasonable assessments of central adjudication patterns. In fact, we analyzed here a real “terra incognita” of clinical trials historically keeping adjudications and disagreements away from the public. Finally, the comprehensive in-dept independent review of primary evidence [13, 16] confirmed our concerns. There are also several limitations to this study. While various statistical methods were employed, the study did not use multivariate analysis to control for potential confounding factors, which may limit the precision and accuracy of the inferences drawn about the adjudication outcomes. As with any mega indication-seeking trial, the evidence did not contain any potential individual confounding variables making it impossible to analyze further. Applying a multivariable model that could control for baseline and follow-up variables would result in more precise and accurate inferences impacting how each individual event was adjudicated. Such analyses would have been conducted and reported if confounders and characteristics were available in the PLATO dataset. However, the FDA redacted the adjudication database, making it impossible to explore further. We also did not have any access to the ICAC communications, or most local hospital records. Finally, we have no definite proof that ICAC leadership had been unblinded by providing them with the randomization codes before the trial ended. Therefore, the heavy shift towards ticagrelor advantages may represent a play-of-chance. On the other hand, the ICAC could have been provided with biased data, e.g., incomplete for ticagrelor events but thorough for clopidogrel events. The investigation of patient-level factors that may affect outcomes is limited by the study’s lack of comprehensive individual patient data, such as baseline characteristics and treatment responses. However, the FDA provided us with the heavily redacted adjudication dataset making this task impossible to implement. In fairness, we have no definite proof that the central adjudication panel knew the randomization codes. There is a possibility that ICAC members or leadership somehow assume or guess the treatment assignment. However, the sponsor was aware of the adjudicator’s selection, and many of them had definite conflicts of interest [9]. Regardless, the consistent shift in the primary endpoint components suggests that the biases against clopidogrel were not random. This is especially true since the probability of the observed distribution may happen by chance in 1 of 485 trillion trials but is still possible.
Central event adjudication in PLATO was unaccountably delayed while the primary endpoint shifted towards inflated ticagrelor benefit resulting in drug approval. The regulatory authorities should consider independent audits when there is a major disagreement between event assessments changing the results of the indication-seeking clinical trials.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
VLS, TM — conception; VLS, WZ, HACF designed the research study; HACF, BP, IH performed the research; WZ, HACF, BP, IH analyzed and interpreted the data; VLS, TM drafted the paper; VLS, WZ, HACF, BP, IH, TM critically revised the manuscript for intellectual content. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The original PLATO adjudication dataset was provided by the FDA based on the Federal Court Order and Joined Status Report with the Department of Justice (Washington, DC USA). CONSORT checklist or ethical approval is not applicable since we do not report here the results of the clinical trial.
Special thanks for the independent statistical work to Dr. Eric Heidel from the Department of Surgery, Section of Biostatistics, University of Tennessee, Knoxville, TN, USA.
This study was funded by HeartDrug Research, which covered affiliated legal fees to obtain FDA evidence and conduct statistical analyses.
VLS, BP and IH are from HeartDrug Research LLC, but have no conflict of interest. The authors declare that HeartDrug Research LLC had no role in the decision-making and conduct of this study. VLS is listed as an inventor and received compensation for the U.S. Patent Application P-17232 “Method for treating vascular diseases with prasugrel” assigned to Lilly; and “Treating Cardiac Arrhythmias, heart failure, peripheral artery disease and stroke with CYCLOPENTYLTRIAZOLO- PYRIMIDINE or derivative thereof” (USN 61/253,829) assigned to HeartDrug™ Research. He received funding for research studies with prasugrel, and clopidogrel, and consultant fees from the clopidogrel and ticagrelor manufacturers. WZ receives funds from the NIH. TM was the Medical Team leader for the FDA PLATO clinical reviews. HeartDrug Research LLC (Wilmington, Delaware, USA) paid the legal fees to obtain the FDA-issued documents, covered travel and statistical expences. Dr. Serebruany is an solo owner of HeartDrug Rssearch. Prof. Victor L. Serebruany is serving as one of the Editorial Board members and Guest editors of this journal. We declare that Prof. Victor L. Serebruany had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Dr. Lihua Sun and Dr. Leonardo De Luca.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

