1 Department of Emergency Medicine, National Taiwan University Hospital, National Taiwan University Medical College, 100 Taipei
2 Department of Emergency Medicine, Taipei City Hospital, 111 Taipei
3 Department of Emergency Medicine, National Taiwan University Hospital Yun-Lin Branch, National Taiwan University Medical College, 640 Douliu
Abstract
Frailty is a physical condition characterized by increased vulnerability to external stressors. This study investigated the impact of premorbid frailty, as measured by the Clinical Frailty Scale (CFS), on neurological prognosis in patients with out-of-hospital cardiac arrest (OHCA).
This is a single-center retrospective study. Data from 2006 to 2020 were analyzed for 595 adult OHCA patients admitted to the intensive care unit of National Taiwan University Hospital following resuscitation. Variables included demographics, medical history, resuscitation details, post-resuscitation data, and frailty assessments based on CFS. The primary outcome was favorable neurological performance, defined as a cerebral performance category (CPC) score of 2 or less at discharge.
In total, 523 of the 595 patients were included in the analysis. Among these, 224 survived, and 173 exhibited favorable neurological outcomes. Patients with favorable outcomes had significantly lower CFS scores than those with poor outcomes (3.2 ± 1.5 vs. 4.5 ± 1.8, p < 0.0001). The proportion of favorable neurological outcomes declined as CFS scores increased. Multivariate logistic regression analysis identified several factors independently associated with worse neurological outcomes: CFS >4 (odds ratio (OR): 0.301, 95% confidence interval (CI): 0.163–0.540), age >70 years (OR: 0.969, 95% CI: 0.953–0.986), history of malignancy (OR: 0.421, 95% CI: 0.209–0.813), epinephrine >2 mg during resuscitation (OR: 0.776, 95% CI: 0.712–0.840), and arterial blood gas pH <7.1 (OR: 28.396, 95% CI: 6.487–129.350). The model demonstrated good performance, with an area under the curve (AUC) value of 0.853. No significant relationships were observed between CFS and other variables.
CFS values ≤4 were independently associated with favorable neurological outcomes following OHCA.
Keywords
- clinical frailty scale
- out-of-hospital cardiac arrest
- neurological outcomes
Out-of-hospital cardiac arrest (OHCA) is the leading cause of mortality worldwide, with an incidence rate of 30–60 per 100,000 person-years [1]. Despite efforts to improve outcomes, e.g., promoting bystander cardiopulmonary resuscitation (CPR), strengthening emergency medical services, and advancing post-resuscitation care, the survival-to-discharge rate of OHCA remains poor [2]. A proportion of cardiac arrest survivors experience neurological sequelae, including cognitive dysfunction or persistent vegetative state [3].
Resuscitation and post-resuscitation care are both costly and labor-intensive [4]. Therefore, accurately predicting the prognosis of OHCA can help guide critical decisions regarding the continuation of resuscitation and withdrawal of life-sustaining treatment [5]. Key outcome indicators in medical intensive care unit (ICU) patients include age, the severity of acute illness, and underlying comorbidities [6, 7, 8]. Notably, while older patients typically exhibit low rates of successful resuscitation and favorable neurological outcomes, a substantial number survive with good cerebral performance after discharge [8].
Several methods have been utilized to predict neurological outcomes, including clinical assessments, biomarkers, electrophysiological studies, and brain imaging. However, none of these methods are sufficient when used in isolation [9]. Existing models, such as the OHCA score, CaRdiac Arrest Survival Score (CRASS), and the Cardiac Arrest Hospital Prognosis (CAHP) score, integrate demographic information, CPR variables, and laboratory data, demonstrating strong predictive performance [10, 11, 12]. Nevertheless, these models are limited in applicability before the return of spontaneous circulation (ROSC). Additionally, their reliance on laboratory data and resuscitation variables complicates effective communication with the patient’s family during decision-making processes. The Clinical Frailty Scale (CFS), which relies solely on history-taking, offers a practical and comprehensive overview of the patient’s condition, aligning closely with clinical intuition. Moreover, the simplicity and alignment of the CFS make it a promising tool for objectifying “clinical judgment” in such scenarios.
In geriatric medicine, frailty describes an increased vulnerability to external stressors [9]. Beyond age, frailty independently predicts mortality [9]. Frailty is measured through various methods, including the frailty phenotype and frailty index [9]. The frailty phenotype encompasses physical conditions such as weight loss, self-reported exhaustion, slow gait speed, decreased muscle strength, and low physical activity; three or more conditions indicate frailty [9]. In contrast, the frailty index integrates deficits across multiple domains to quantify frailty levels, such as symptoms, abnormal laboratory values, diseases, and disabilities [9].
The CFS is a clinical judgment tool closely associated with the frailty index [9]. Widely used in critical care research, the CFS reliably predicts patient outcomes due to its convenience [9]. Further, the CFS considers medical and functional conditions, including activity levels, underlying medical conditions, dependence on daily activities, and dementia. Initially, the CFS employed a seven-point scale, ranging from one (very fit) to seven (severely frail), as introduced by the Canadian Study on Health and Aging [9]. In 2007, the scale was expanded to include two end-of-life points: CFS 8 (severe frailty) and CFS 9 (terminally ill). However, recent study indicated no significant differences in neurological outcomes among patients with CFS scores of 5 or higher [13]. Consequently, it is appropriate to group patients with CFS scores exceeding seven, consistent with the original seven-point scale.
This study aimed to determine the association between the degree of frailty and the neurological prognoses of OHCA survivors. To enhance understanding of the role of frailty in OHCA, this study incorporated a CFS assessment with other significant factors into a risk stratification model using multiple logistic regression.
This study involved a secondary database analysis containing records of prospectively recruited adult OHCA patients.
The inclusion criteria were as follows: adult OHCA patients aged over 20, patients who achieved a ROSC, patients who survived and were admitted to the ICU, and patients admitted between January 1, 2006, and December 31, 2020. Conversely, the exclusion criteria included traumatic OHCA patients and patients or family members who signed do-not-resuscitate (DNR) forms before or during CPR.
The study was conducted at the National Taiwan University Hospital, a leading tertiary medical center and teaching hospital in Taipei, Taiwan, equipped with 150 ICU beds. The emergency department handles approximately 100,000 patients annually. To optimize resuscitation, healthcare providers perform CPR using the advanced cardiac life support (ACLS) teamwork model, typically involving 4 to 7 personnel assigned specific roles such as airway management, chest compressions, defibrillation, medication administration, and leadership. For OHCA patients who do not achieve ROSC, CPR is performed for at least 30 minutes unless a do-not-resuscitate order has been documented. Based on ultrasonography findings and blood gas analysis, physicians treat reversible causes such as hemorrhage, cardiac tamponade, and metabolic acidosis. Extracorporeal membrane oxygenation CPR is employed for OHCA patients with refractory shockable rhythms.
Collected data included age, gender, and pre-existing systemic diseases categorized using Charlson’s comorbidity index [14]. Core resuscitation elements were registered using the Utstein style [15]. Information such as CPR duration, epinephrine dosage during CPR, post-ROSC vital signs, and laboratory values (including arterial blood gas, lactic acid, and white blood cell counts) was recorded upon ICU admission and analyzed.
Frailty was assessed by physicians using the seven-point CFS through a review of medical and nursing records. Guided utilizing the framework for retrospective chart reviews by Worster et al. [16], this evaluation involved 20 emergency medicine physicians anonymized to the study hypothesis and the patient’s outcomes at discharge. Before the study, physicians were trained on the CFS and the cerebral performance category based on its 2005 original version.
Due to the reliance of the CFS on clinical judgment of baseline cognitive function, mobility, and underlying medical conditions, admission notes incorporated baseline condition information. These data were gathered from caregivers or family members during history-taking and from patients’ comprehensive electronic medical records, including details from prior hospital admissions, outpatient visits, and diagnostic reports. Patients with incomplete histories or insufficient data to assess the CFS accurately were excluded from the study. This study did not conduct an inter-rater reliability assessment of the CFS, citing prior research indicating substantial reliability of the CFS, and the primary outcome was good cerebral performance at hospital discharge, defined as a cerebral performance category (CPC) in emergency department settings [15]. Mean values were used to interpolate missing laboratory or vital sign data, as missing data occurred in less than 5% of cases. Ethical considerations and data abstraction methods were approved by the Institutional Review Board (IRB) of the affiliated university. Data were anonymized, coded using identification numbers, and stored securely.
The primary outcome was good cerebral performance at hospital discharge, defined as a CPC score of 1 or 2. Neurological outcomes were categorized into five CPC levels ranging from 1 (conscious with normal function or slight disability, capable of work) to 5 (brain death state). Intermediate levels included CPC 2 (conscious with moderate disability, able to perform daily activities independently), CPC 3 (conscious with severe disability, dependent on others for daily activities), and CPC 4 (comatose or in a vegetative state). For this study, favorable neurological outcomes were defined as CPC scores of 1 or 2 [17].
Univariate analyses of patient characteristics, pre-existing conditions, resuscitation events, post-ROSC vital signs, and laboratory data were conducted using a student’s t-test and chi-square test. A generalized additive model (GAM) identified the CFS threshold. Factors with a value of p
A total of 595 patients were analyzed. After excluding patients with pre-arrest CPC values greater than two or missing CFS data, 523 patients (224 survivors and 299 non-survivors) were included in the analysis. Among these, favorable neurological outcomes were demonstrated in 173 survivors (Fig. 1).
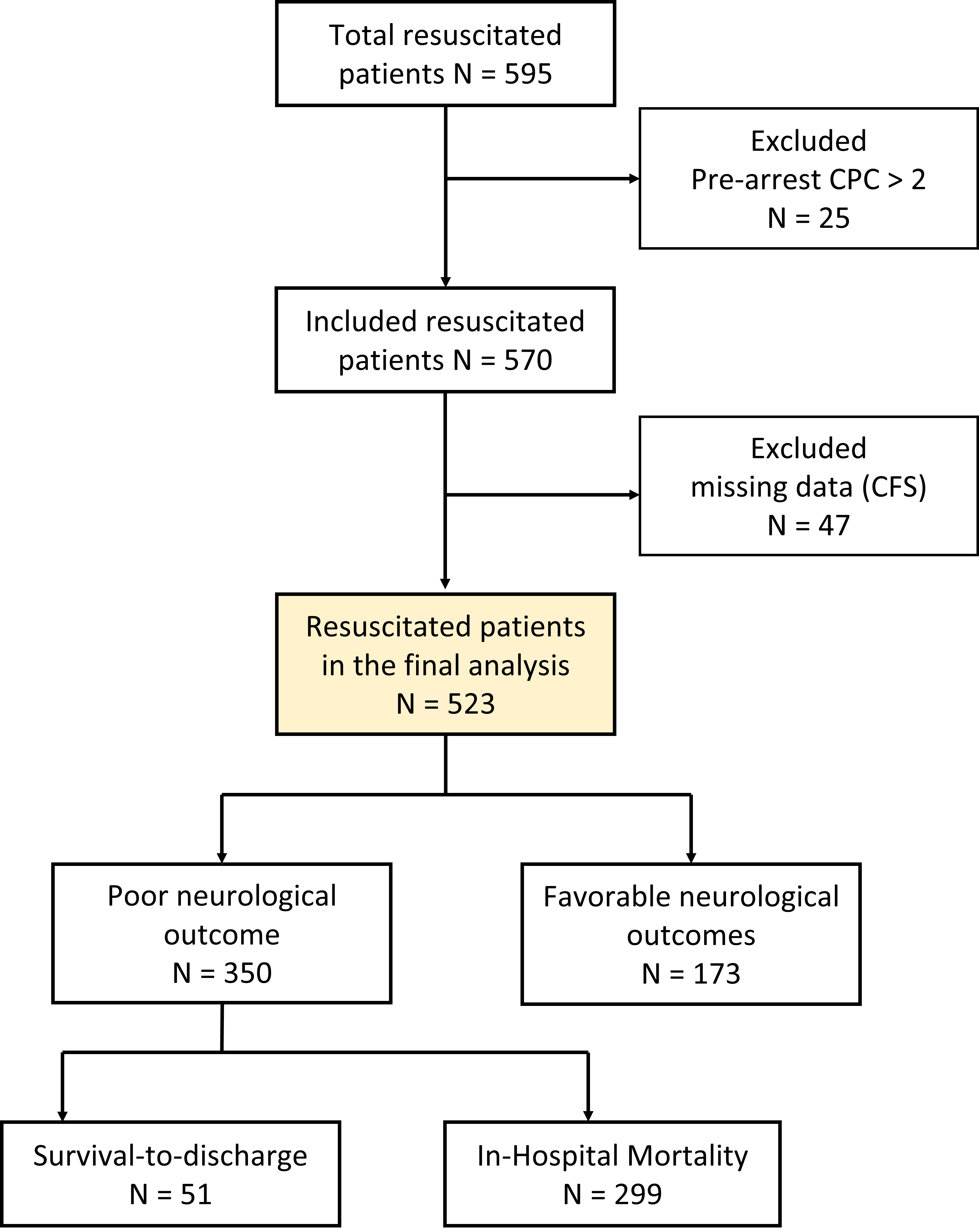 Fig. 1.
Fig. 1. Flowchart of patient inclusion and exclusion. This study initially included 595 resuscitated patients. After excluding those with pre-arrest cerebral performance category (CPC) values
Patients with favorable neurological outcomes were younger, predominantly male, had lower CFS values, and were more likely to have experienced a witnessed collapse. Compared with poor neurological outcomes, these patients had a lower rate of malignancy among pre-existing systemic diseases, received lower doses of epinephrine, and underwent shorter durations of CPR. Additionally, they exhibited significantly higher SBP and DBP values. Notably, the factors influencing survival and neurological prognosis were highly similar across groups, except for a considerably lower prevalence of chronic obstructive pulmonary disease (COPD) or asthma in the favorable neurological outcomes group, which was not observed in the survival group (Table 1). However, this difference was not statistically significant.
| Survivor | Non-survivor | p-value | Favorable neurological outcomes (n = 173) | Poor neurological outcomes (n = 350) | p-value | |
| (n = 224) | (n = 299) | |||||
| Age (SD) | 60.81 (15.82) | 68.56 (15.34) | 59.17 (15.40) | 68.24 (15.45) | ||
| Gender (male) (%) | 177 (79.0%) | 197 (65.9%) | 0.003 | 139 (80.3%) | 235 (67.1%) | 0.002 |
| Clinical Frailty Scale (SD) | 3.4 (1.6) | 4.6 (1.8) | 3.2 (1.5) | 4.5 (1.8) | ||
| Witnessed collapse (%) | 198 (88.4%) | 223 (74.6%) | 153 (88.4%) | 268 (76.6%) | 0.002 | |
| Prehospital cardiopulmonary resuscitation (%) | 191 (85.3%) | 256 (85.6%) | 1.000 | 148 (85.5%) | 299 (85.4%) | 1.000 |
| Malignancy (%) | 25 (11.2%) | 72 (24.1%) | 17 (9.8%) | 80 (22.9%) | 0.001 | |
| End-stage renal disease (%) | 16 (7.1%) | 28 (9.4%) | 0.455 | 11 (6.4%) | 33 (9.4%) | 0.307 |
| Renal disease (%) | 22 (9.8%) | 30 (10.0%) | 1.000 | 19 (11.0%) | 33 (9.4%) | 0.687 |
| Diabetes mellitus (%) | 58 (25.9%) | 96 (32.1%) | 0.148 | 44 (25.4%) | 110 (31.4%) | 0.189 |
| Chronic obstructive pulmonary Disease/asthma (%) | 13 (5.8%) | 28 (9.4%) | 0.182 | 8 (4.6%) | 33 (9.4%) | 0.080 |
| Heart failure (%) | 16 (7.1%) | 33 (11.0%) | 0.174 | 12 (6.9%) | 37 (10.6%) | 0.237 |
| Cerebral vascular accident (%) | 21 (9.4%) | 38 (12.7%) | 0.292 | 15 (8.7%) | 44 (12.6%) | 0.238 |
| Hypertension (%) | 122 (54.5%) | 1978 (59.5%) | 0.285 | 91 (52.6%) | 209 (59.7%) | 0.146 |
| Coronary artery disease (%) | 77 (34.4%) | 104 (34.8%) | 0.997 | 59 (34.1%) | 122 (34.9%) | 0.942 |
| Cardiopulmonary resuscitation duration (min) (SD) | 27.29 (19.69) | 36.11 (20.07) | 24.61 (19.27) | 36.15 (19.82) | ||
| Epinephrine (mg) (SD) | 2.959 (3.278) | 4.634 (4.051) | 2.451 (3.013) | 4.584 (3.987) | ||
| Heart rate (bpm) (SD) | 101.1 (29.4) | 104.6 (32.8) | 0.210 | 101.5 (29.6) | 103.9 (32.3) | 0.400 |
| Systolic blood pressure (mmHg) (SD) | 130.6 (45.3) | 114.7 (43.0) | 131.9 (45.3) | 116.4 (43.5) | ||
| Diastolic blood pressure (mmHg) (SD) | 77.1 (26.2) | 67.0 (25.3) | 79.7 (26.1) | 67.1 (25.2) | ||
| pH (SD) | 7.139 (0.165) | 7.047 (0.161) | 7.161 (0.151) | 7.050 (0.165) | ||
| Lactic acid (mmol/L) (SD) | 9.435 (4.325) | 11.265 (4.049) | 9.316 (4.466) | 11.062 (4.043) | ||
| White blood cell (count/µL) (SD) | 13,250 (5469) | 14,286 (21,284) | 0.420 | 13,032 (5447) | 14,246 (19,832) | 0.290 |
Comparisons were made across the survivors (n = 224), non-survivors (n = 299), patients with favorable neurological outcomes (n = 173), and those with poor outcomes (n = 350). Key parameters include age, gender, health conditions, and clinical measures. Continuous variables are presented with the standard deviation (SD), and categorical data are indicated as percentages. A p-value
A total of 134 patients were classified with a CFS score of 3, while 119, 87, 55, 50, 39, and 39 were categorized with a CFS score of 4, 7, 2, 6, 1, and 5, respectively (Fig. 2A). The percentages of patients with favorable neurological outcomes by CFS scores are shown in Fig. 2B.
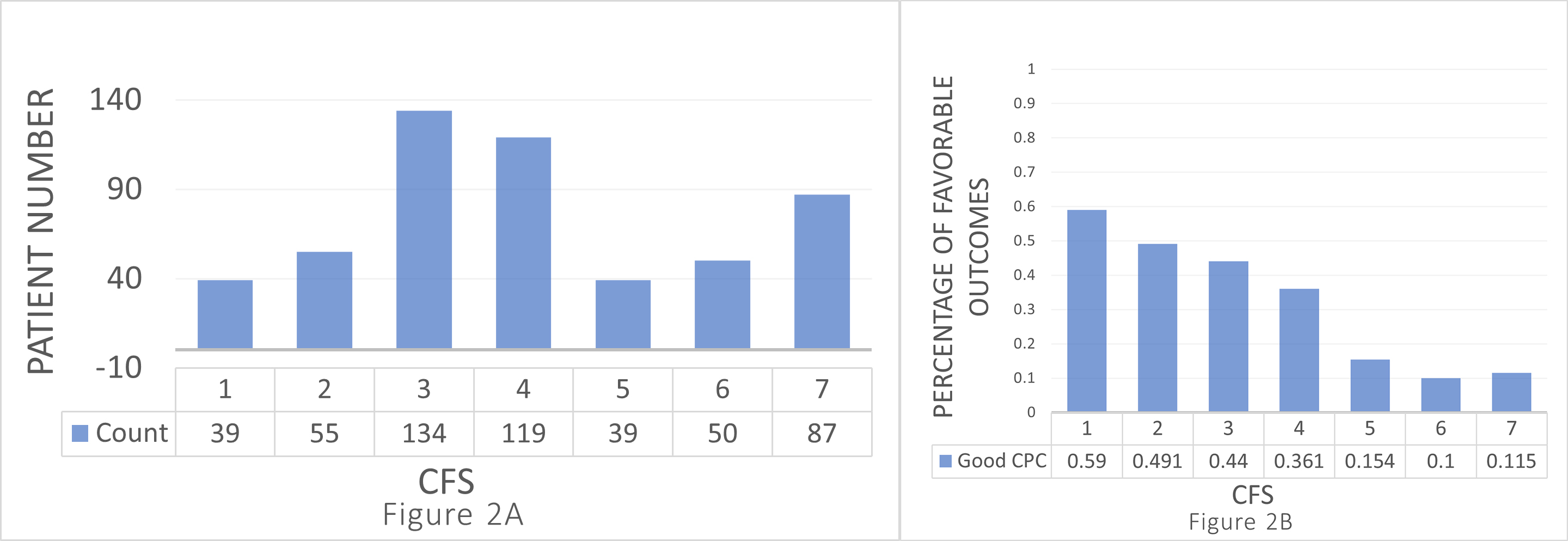 Fig. 2.
Fig. 2. Distribution and outcomes by Clinical Frailty Scale (CFS) scores. (A) Number of patients for each CFS score. The distribution of individuals across different CFS categories ranges from 1 to 7. The distribution is as follows: CFS 1 (very fit), n = 39; CFS 2 (well), n = 55; CFS 3 (managing well), n = 134; CFS 4 (vulnerable), n = 119; CFS 5 (mildly frail), n = 39; CFS 6 (moderately frail), n = 50; CFS 7 (severely frail), n = 87. (B) Percentage of patients with good cerebral performance categories (CPC) for each CFS score. The percentage for good CPC (
Patients with CFS 1 had the highest proportion of favorable neurological outcomes (59%, 23/39), followed by CFS 2 (49.1%, 27/55). In contrast, CFS 6 (10%, 5/50) and CFS 7 (11.5%, 10/87) presented the lowest percentages. The remaining 44% (59/134), 36.1% (43/119), and 15.4% (6/39) of patients with favorable neurological outcomes had a CFS score of 3, 4, and 5, respectively. Overall, the proportion of favorable outcomes decreased as the CFS scores increased.
The GAM plot in Fig. 3A shows the relationship between favorable neurological outcomes and the CFS.
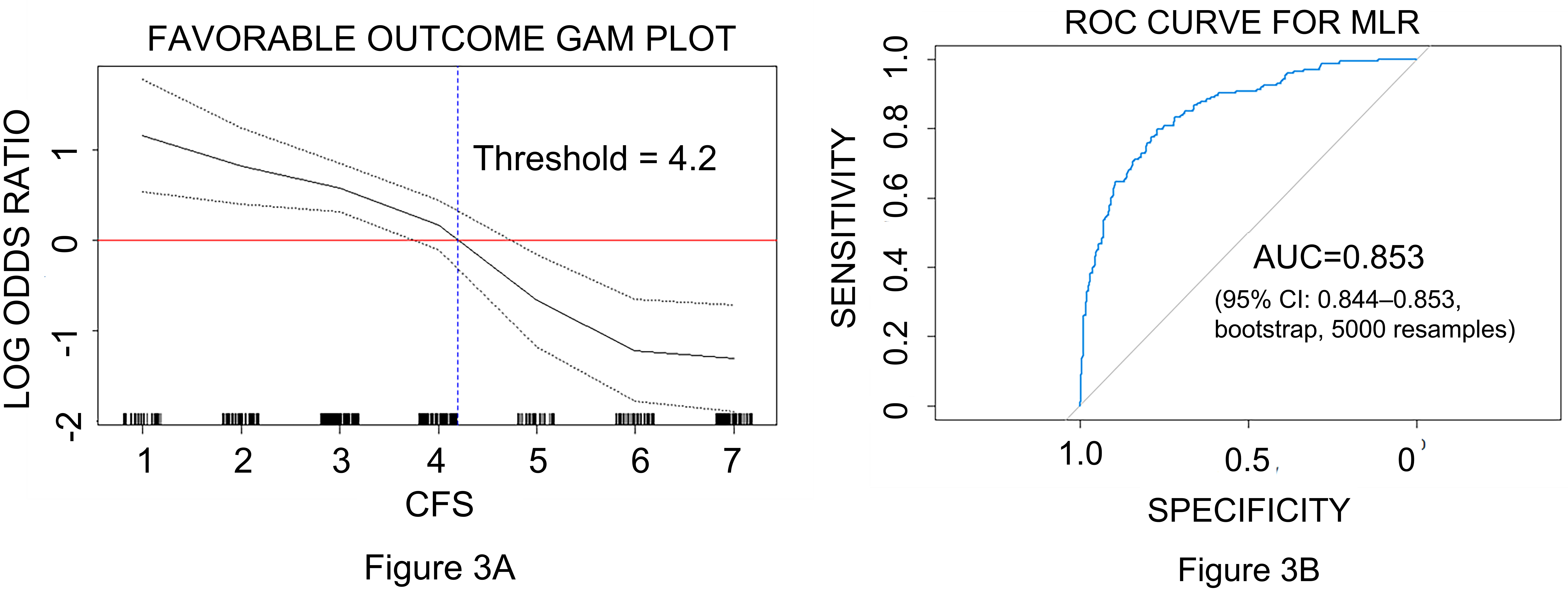 Fig. 3.
Fig. 3. Threshold analysis and model performance for predicting neurological outcomes. (A) Generalized additive model for determining the threshold of the CFS. The generalized additive model (GAM) plot illustrates the association between CFS and favorable neurological outcomes. The inflection point at CFS = 4.2 corresponds to a log OR of 0. (B) Receiver operating characteristic curve (ROC) of the MLR model for association with good neurological outcomes. The ROC curve derived from the MLR model includes age, gender, CFS, witnessed collapse, pre-existing malignancy, underlying COPD or asthma, epinephrine dose during CPR, arterial blood gas pH, and DBP. The AUC of the model was 0.853, with a 95% confidence interval (CI) of 0.844–0.853, calculated using 5000 bootstraps resamples. CFS, Clinical Frailty Scale; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; AUC, area under the curve; MLR, multiple logistic regression; CPR, cardiopulmonary resuscitation; OR, odds ratio.
At a CFS threshold of 4.2, a log odds ratio (OR) of 0 indicated no association between the CFS score and the likelihood of a favorable neurological outcome. Given the ordinal nature of the CFS, we identified that patients with favorable neurological outcomes had CFS scores
The MLR model included factors such as age
| Variables | Odds ratio | 95% CI | p-value |
| Age | 0.969 | 0.953–0.986 | 0.0003 |
| Gender (male) | 1.638 | 0.958–2.842 | 0.0746 |
| Clinical Frailty Scale score | 0.301 | 0.163–0.540 | |
| Witnessed collapse | 1.903 | 1.034–3.624 | 0.0434 |
| Malignancy | 0.421 | 0.209–0.813 | 0.0122 |
| Chronic obstructive pulmonary disease/asthma | 0.431 | 0.164–1.038 | 0.0712 |
| Epinephrine | 0.776 | 0.712–0.840 | |
| pH | 28.396 | 6.487–129.350 | |
| Diastolic blood pressure | 1.015 | 1.006–1.024 | 0.0015 |
This analysis evaluated factors influencing favorable neurological outcomes. Odds ratio (OR)
The MLR analysis identified CFS scores of
Representative cutoff points were selected across different sensitivity levels and their corresponding specificity and Youden index values, as summarized in Table 3. The clinical balance between sensitivity and specificity is optimized at a cutoff of 302, with a sensitivity of 0.8, a specificity of 0.771, and a maximum Youden index of 0.569. These findings provide a reference for selecting appropriate cutoff points to optimize diagnostic performance.
| Cutoff | Sensitivity | Specificity | Youden index |
| 40 | 1.000 | 0.114 | 0.114 |
| 220 | 0.902 | 0.589 | 0.490 |
| 302* | 0.798 | 0.771 | 0.569 |
| 344 | 0.699 | 0.843 | 0.542 |
| 384 | 0.601 | 0.909 | 0.510 |
| 408 | 0.503 | 0.929 | 0.431 |
| 436 | 0.399 | 0.957 | 0.356 |
| 462 | 0.301 | 0.983 | 0.283 |
| 481 | 0.202 | 0.989 | 0.191 |
| 501 | 0.098 | 0.994 | 0.093 |
| 519 | 0.000 | 1.000 | 0.000 |
Cutoff points represent different sensitivity levels corresponding to their cutoff values and specificity. The cutoff point marked with * indicates the maximum Youden index.
A forest plot (Fig. 4) examined potential interactions between the CFS and significant variables identified in the MLR model. This analysis assessed whether the relationship between lower CFS scores and improved neurological outcomes was influenced by factors such as epinephrine dosage during CPR (
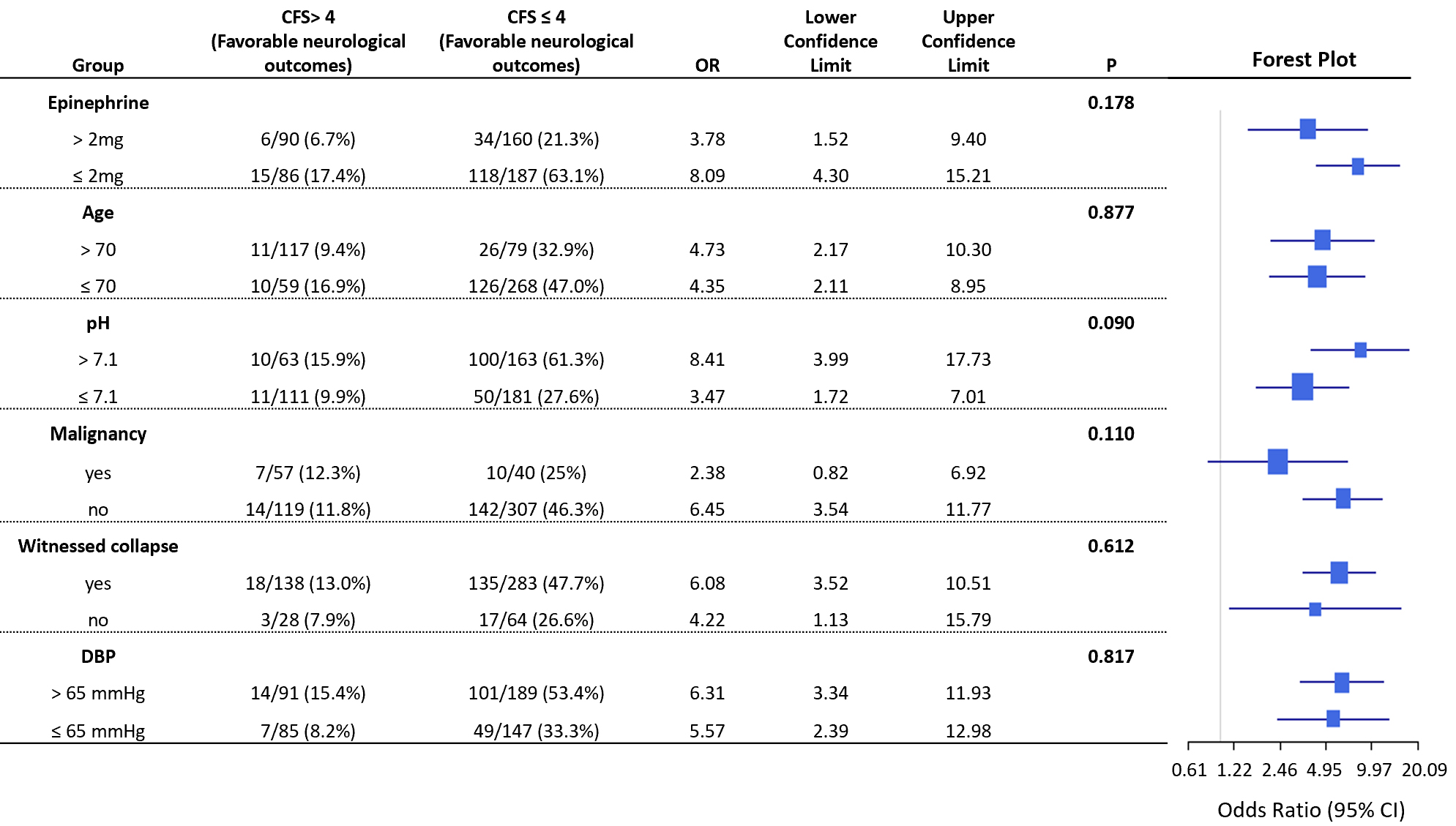 Fig. 4.
Fig. 4. Forest plot for analyzing the interaction between the CFS and other variables. The forest plot illustrates potential interactions between the Cerebral Function Score (CFS) and significant variables in the multiple logistic regression (MLR) model. The plot investigates the interplay between CFS and factors such as epinephrine dose
The analysis found no statistically significant interactions between the CFS and these variables. Specifically, no interaction was observed for an epinephrine dose of
Fig. 5 illustrates the distribution of age and its relationship with CFS. The histogram (Fig. 5A) shows age distribution with a kernel density estimation (KDE) curve. The Q–Q plot (Fig. 5B) compares age quantiles to a theoretical normal distribution, with the Kolmogorov–Smirnov test suggesting approximate normality (p = 0.0749). A scatter plot (Fig. 5C) reveals a moderate positive correlation between age and CFS (Spearman’s coefficient = 0.512, p
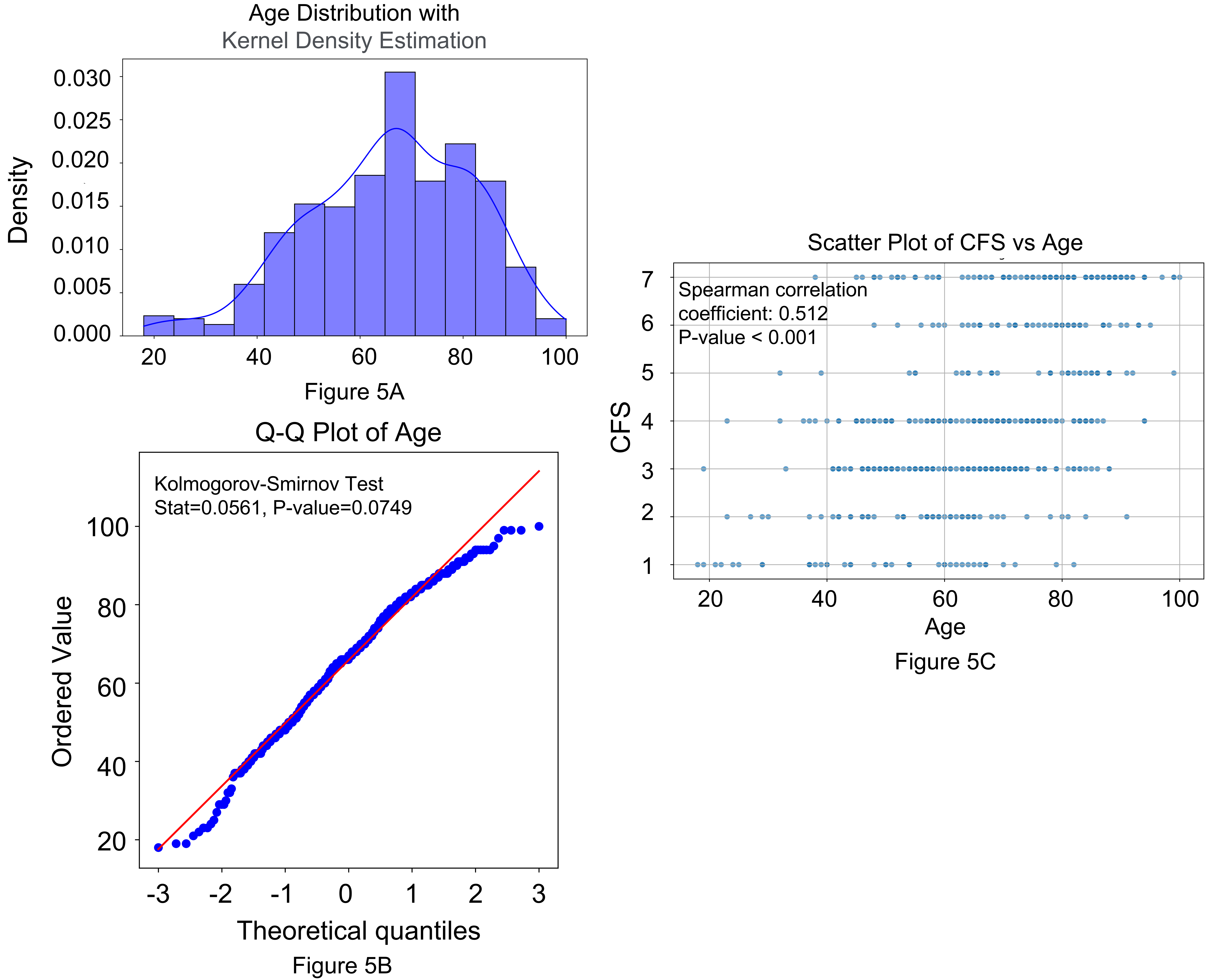 Fig. 5.
Fig. 5. Analysis of age distribution and its relationship with frailty. (A) Age distribution with kernel density estimation (KDE). The histogram illustrates the distribution of age values in the dataset, while the KDE curve represents the smoothed probability density function. The x-axis shows age, and the y-axis shows density. (B) Q–Q plot of age. This Q–Q plot compares the quantiles of observed age data to a theoretical normal distribution. The red diagonal line represents the expected normal distribution, while the blue dots are the observed quantiles. The Kolmogorov–Smirnov test result (Stat = 0.0561, p = 0.0749) suggests that the age distribution follows approximate normality (p
This study demonstrated that higher CFS scores are significantly associated with worse neurological outcomes in OHCA patients and identified CFS scores
The CFS has garnered increasing attention among resuscitation researchers for its effectiveness in predicting patient outcomes. A 2023 study by Yamamoto et al. [18] investigated the impact of frailty on neurological outcomes in ROSC patients using a nine-point CFS. While this study aligns with our focus, it differs in two key aspects: it included a larger cohort and used a nine-point scale instead of the seven-point scale employed in our study. Yamamoto et al. [18] predefined CFS scores
A study by Mowbray et al. [19], focusing on cardiac arrest survivors discharged to home care, found that severe frailty (CFS
Similarly, McPherson et al. [13] observed that lower CFS scores were associated with improved survival and a higher prevalence of shockable rhythms in cardiac arrest patients. Shockable rhythm, indicative of cardiac-origin arrests, is also associated with better outcomes [20]. While our study excluded variables such as shockable rhythm and post-resuscitation care interventions (e.g., extracorporeal membrane oxygenation), further research is needed to explore whether optimal CFS cutoffs vary by cardiac arrest etiology and population demographics.
In our cohort, most ROSC patients had mid-range CFS scores, with a CFS score of 3 being the most frequent, followed by a CFS score of 4. This distribution reflects a specific subset of OHCA patients who survived resuscitation efforts. However, it may not represent the broader OHCA population, particularly given that CFS has not been validated in younger populations (
The GAM plot suggested a CFS score of 4 as the threshold for poor neurological outcomes. This finding supports the hypothesis that lower frailty levels are associated with better post-cardiac arrest outcomes. However, interpreting the CFS as an ordinal scale within a GAM introduces challenges, as the scale does not assume linear intervals. For example, the difference between a CFS score of 1 and 2 may not equate to that between a CFS score of 2 and 3; thus, a CFS score threshold of 4 should be viewed as a transitional zone rather than an exact cutoff.
Epinephrine dosage during CPR, reflecting resuscitation duration, significantly correlated with neurological outcomes. Our analysis selected epinephrine dosage as a representative variable. Both SBP and DBP also exhibited strong correlations with favorable outcomes. Consistent with prior findings, DBP outperformed SBP in predicting favorable neurological outcomes, with a lower cutoff value (
Previous prediction models, such as the OHCA score (AUC 0.82–0.88), CAHP score (AUC 0.91–0.93), and CRASS score (AUC 0.88), have demonstrated robust performances in large cohorts [10, 11, 12]. While our study comprised a smaller sample size (n = 523), our model incorporating the CFS achieved a competitive AUC of 0.853. Notably, CFS scores
Frailty arises from multifactorial mechanisms, including impaired metabolism, cellular senescence, inflammation, hormone dysregulation, and immune dysfunction [23]. These processes overlap with those underlying brain recovery, which is equally multifaceted. Following OHCA, hypoxic-ischemic encephalopathy is the leading cause of poor neurological outcomes, characterized by hypoxia-induced cerebral dysfunction, inflammatory responses, apoptosis, cytotoxic edema, excitotoxicity, and seizures. These processes culminate in cell death and brain remodeling, reducing neuronal excitability [24]. Frail patients with pre-existing physiological dysregulation may face additional challenges in recovery; thus, further research into the shared mechanisms of frailty and brain recovery is essential.
Frailty predictably correlates with age, as evidenced by a moderate positive correlation (Spearman’s coefficient = 0.512). However, our MLR analysis revealed that age and CFS independently influence neurological outcomes, underscoring their distinct prognostic roles. Biologically, frailty arises from an interplay between aging and disease mechanisms, contributing to disability, dependency, and mortality [23]. Therefore, incorporating age and frailty into clinical assessments provides a more comprehensive evaluation of patient prognosis.
This study has several limitations. First, the single-center, retrospective design, conducted at a metropolitan tertiary center with advanced interventions such as targeted temperature management and extracorporeal membrane oxygenation, may limit generalizability. Our model was also not externally validated. Second, the retrospective determination of CFS through medical record review introduces potential bias. Third, the relatively small sample size affects statistical power. Fourth, while the GAM identified a CFS threshold, its linearity assumptions are challenged by the ordinal nature of the CFS. Fifth, excluding patients with DNR orders, who are typically frailer, may underestimate the broader impact of frailty. Finally, our study did not explore the relationship between cardiac arrest etiology and premorbid CFS, which could influence results.
Future studies should adopt multicenter, prospective designs with larger, diverse populations to enhance generalizability. Investigating the biological mechanisms linking frailty and neurological outcomes and incorporating biomarkers or imaging techniques could improve prognostic accuracy. Studies examining frail patients with DNR orders would further elucidate the impact of frailty in broader care contexts.
This study identified higher CFS scores as independently associated with worse neurological outcomes in OHCA patients with ROSC. Prospective multicenter studies are needed to validate the prognostic utility of the CFS.
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy protection. However, they are available from the corresponding author on reasonable request.
HH, CKW, CYC, MST, and CHH were responsible for the conception and design of the study. HH, CKW, CYC, MST, and CHH contributed to data acquisition, analysis, and interpretation. HH, CKW, CYC, MST, and CHH drafted the manuscript and critically revised it for intellectual content. HH, CKW, CYC, MST, and CHH made the final revisions to the submitted version. All authors contributed substantially to the study and approved the final manuscript. All authors agree to take responsibility for the integrity and accuracy of the work, addressing any questions that may arise.
This retrospective study followed the principles of the Declaration of Helsinki. Ethical approval for this study was granted by the National Taiwan University Hospital Ethics Committee (Reference No. 202202082RIND). Informed consent was obtained from all participants. Patient data were accessed through an authorized account within the hospital’s secure electronic medical records (EMR) system. Extracted data were anonymized and stored on a password-protected computer within the hospital, accessible only to authorized research personnel. These measures ensured patient confidentiality and compliance with institutional and legal data protection policies.
We want to thank everyone who contributed to this study, including the participants, colleagues, and institutions that provided support and resources.
This research received no external funding.
The authors declare no conflict of interest. Chien-Hua Huang is serving as Guest Editor of this journal. We declare that Chien-Hua Huang had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Ying Su.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





