1 Department of Cardiac Surgery Intensive Care Unit, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, 510080 Guangzhou, Guangdong, China
Abstract
The plasma uric acid/albumin ratio (UAR) has emerged as a novel inflammatory biomarker for predicting the development of acute kidney injury (AKI) following percutaneous coronary intervention. However, the potential of the UAR to serve as a predictive marker for AKI in patients undergoing isolated tricuspid valve (TV) surgery remains unknown. This study aimed to explore the association between the UAR and AKI and to assess whether the UAR can predict AKI in these patients.
We conducted a retrospective analysis of patients who underwent isolated TV surgery between January 2018 and June 2019. The patients were divided into three groups based on the tertiles of the UAR. We utilized multivariate logistic regression and restricted cubic spline analysis to examine the association between the UAR and AKI. Additionally, we used the receiver operating characteristic (ROC) curve analysis to assess the predictive accuracy of the UAR for AKI.
A total of 224 patients were enrolled in this study, of whom 41 developed AKI. The incidence of AKI across the three UAR tertiles was 3.8%, 22.2%, and 29.7%, with a significant difference between the group (p < 0.001). In the multivariate analysis, UAR ≥8.5 was associated with a 7-fold increased risk of AKI (odds ratio (OR): 7.73, 95% confidence interval (CI): 1.61–37.14), while a UAR ≥10.8 was a linked to a 9-fold increased risk (OR: 9.34, 95% CI: 1.96–44.60). The restricted cubic spline model showed a linear association between the UAR and AKI development. The area under the curve (AUC) value for the UAR was 0.713 (95% CI: 0.633–0.793; p < 0.001) with a cutoff value of 8.89.
An increased UAR was significantly associated with a higher risk of AKI in patients undergoing isolated TV surgery; however, while the UAR could serve as a marker to predict AKI, it was not superior to uric acid alone.
Keywords
- uric acid/albumin ratio
- acute kidney injury
- tricuspid valve surgery
Acute kidney injury (AKI) is a common significant complication following cardiac surgery [1]. The development of AKI is associated with prolonged hospital stays, an increased need for renal replacement therapy, and higher mortality rates both in the short and long term [2]. The incidence of postoperative AKI in patients receiving tricuspid valve (TV) surgery and cardiac valve replacement is estimated to be 30% and 46.8%, respectively [3, 4]. Patients demonstrated a 1.58-fold increased risk of AKI after tricuspid valve replacement [3]. Although some novel biomarkers, such as urinary liver fatty acid binding protein (L-FABP), urinary neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule 1 (KIM-1) have been studied for AKI stratification and prediction [5], the cost-effectiveness of using these in clinical practice remains unclear [6]. Therefore, predicting AKI with readily calculable markers before tricuspid valve surgery is essential for identifying high-risk patients and implementing preventative strategies.
Inflammation and oxidative stress are key pathophysiologies in cardiac surgery-related AKI [7]. Uric acid enhances proinflammatory mediators, leading to glomerulosclerosis and tubular damage [8]. The redox state of serum albumin, indicative of systemic antioxidant capacity, is associated with reduced renal function [9]. Both serum uric acid and albumin serve as an independent risk marker for AKI [10, 11]. However, conditions such as malnutrition, sepsis, inflammation, congestive heart failure, and chronic kidney disease can influence their levels [12, 13, 14]. The serum uric acid/albumin ratio (UAR), comprising serum uric acid and serum albumin levels, has emerged as a novel inflammatory biomarker for predicting the onset of AKI [14] and is associated with short-term mortality in patients with acute kidney injury [15]. This marker has been evaluated for its predictive value in post-contrast acute kidney injury following percutaneous coronary intervention (PCI) [16]. However, the clinical features and underlying pathophysiological processes differ between contrast-induced acute kidney injury after PCI and acute kidney injury associated with cardiac surgery. There is a notable absence of research validating the predictive value of this marker in patients following tricuspid valve surgery. Consequently, this study aimed to determine whether the UAR can predict the risk of AKI in those who have undergone such surgery.
This study included 224 patients and was conducted in the thirty-bed cardiac surgery intensive care unit (ICU) at Guangdong Provincial People’s Hospital in Guangzhou, China, from January 2018 to June 2019. Eligible participants were 18 years of age or older and had undergone isolated tricuspid valve repair or replacement surgery with cardiopulmonary bypass support. Initially, the study had 273 participants, but 49 were excluded due to the lack of cardiopulmonary bypass support, preoperative laboratory data, or serum creatinine levels exceeding 120 µmol/L (Fig. 1). After ranking the UAR values from the lowest to the highest, the UAR values at the 1/3 and 2/3 quantiles were established as the cutoff points for stratifying patients into low, medium, and high UAR groups. These patients were then stratified again into three tertiles based on their UAR levels: UAR
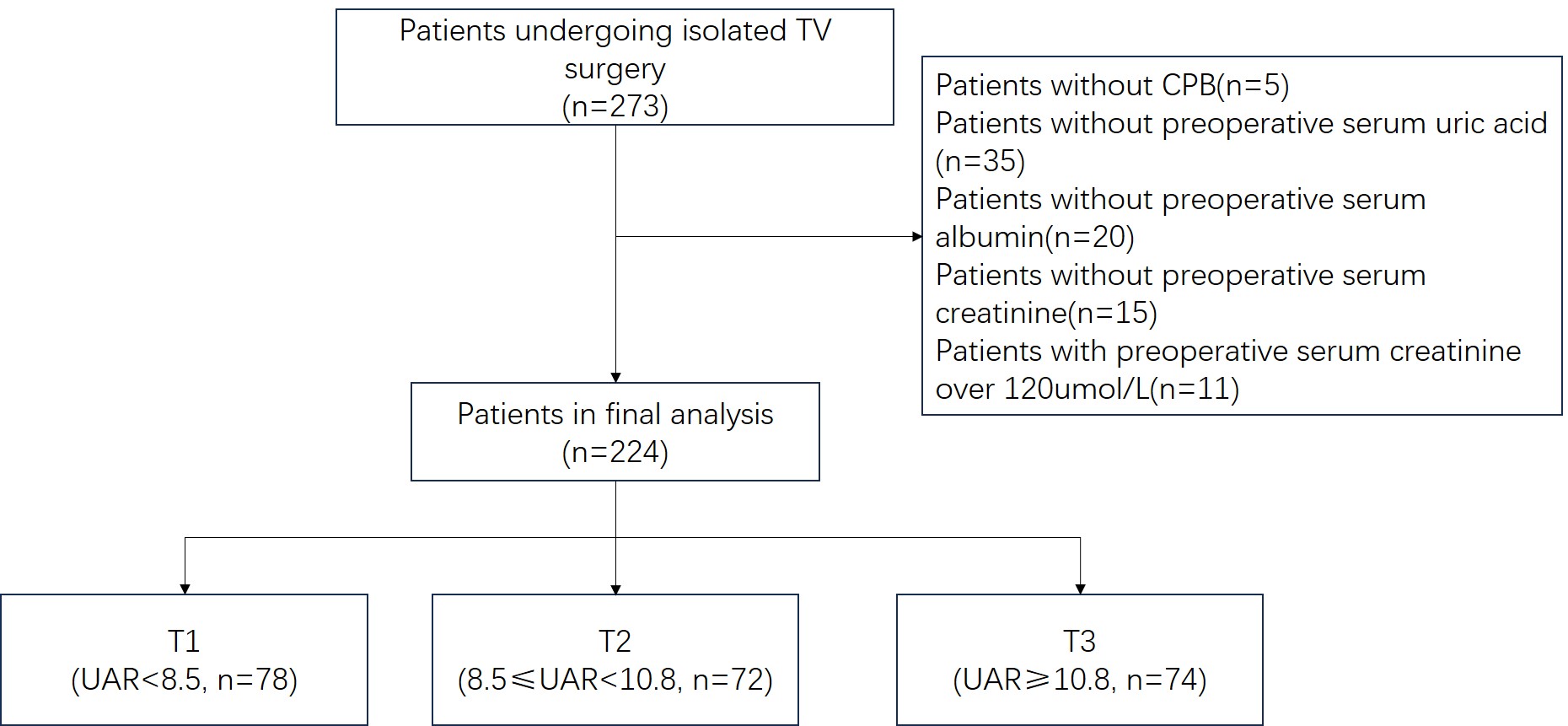 Fig. 1.
Fig. 1. Schematic chart of enrolled patients. TV, tricuspid valve; CPB, cardiopulmonary bypass; UAR, uricacid/albumin ratio.
Baseline demographic and clinical data, including age, sex, height, weight, and co-morbidities, were extracted from the hospital’s electronic medical records system. Preoperative cardiac function, specifically left ventricular ejection fraction (LVEF) and pulmonary artery pressure, was assessed using the latest echocardiography. Additionally, preoperative laboratory parameters such as creatinine, serum uric acid, albumin, hemoglobin, blood glucose levels, and platelet counts were documented before surgery. Intraoperative data were also recorded, including cardiopulmonary bypass (CPB) circulation time and aortic cross-clamp time. Postoperative outcomes were monitored, specifically the incidence of AKI, mechanical ventilation duration, and ICU stay length. The UAR was calculated as the ratio of serum uric acid to serum albumin. AKI was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria [17] and was assessed by a trained physician in the ICU.
Continuous variables are described as the mean
The clinical characteristics of patients are presented in Table 1. A total of 224 patients participated in the analysis; 223 patients underwent tricuspid valve repair, and one patient underwent tricuspid valve replacement. Patients were further divided into three tertiles, and the median (IQR) of the UAR levels were 7.4 (6.5–8.0) in T1 (n = 78), 9.5 (9.0–10.1) in T2 (n = 72), and 12.7 (11.7–14.4) in T3 (n = 74). Sex, age, weight, UAR, uric acid, albumin, creatinine, CPB duration, and blood transfusion significantly differed among the three groups. Patients in the T3 group were predominantly males with higher age, weight, uric acid, creatinine, and lower albumin. As the UAR tertile increased, patients experienced longer durations of mechanical ventilation and ICU stay, yet there were no differences in the in-hospital mortality rates among the groups. A total of 41 out of 224 (18.3%) patients developed AKI. The incidence of AKI increased significantly across the increasing UAR tertiles, with rates of 3.8%, 22.2%, and 29.7% in each successive tertile, respectively (p
| Variables | T1 (n = 78) | T2 (n = 72) | T3 (n = 74) | p-value | |
| UAR | 8.5 | UAR | |||
| Male, n (%) | 0.006 | ||||
| No | 62 (79.5) | 50 (69.4) | 41 (55.4) | ||
| Yes | 16 (20.5) | 22 (30.6) | 33 (44.6) | ||
| Age, years | 40.0 [28.0; 51.0] | 40.0 [30.5; 54.0] | 48.5 [36.0; 58.0] | 0.01 | |
| Height, cm | 159.2 | 159.4 | 161.3 | 0.2 | |
| Weight, kg | 52.5 [47.0; 60.0] | 54.9 [50.0; 62.0] | 58.2 [51.5; 66.0] | 0.016 | |
| Hypertension, n (%) | 0.626 | ||||
| No | 73 (93.6) | 68 (94.4) | 67 (90.5) | ||
| Yes | 5 (6.4) | 4 (5.6) | 7 (9.5) | ||
| Diabetes, n (%) | 0.155 | ||||
| No | 77 (98.7) | 67 (93.1) | 72 (97.3) | ||
| Yes | 1 (1.3) | 5 (6.9) | 2 (2.7) | ||
| Atrial fibrillation , n (%) | 0.078 | ||||
| No | 67 (85.9) | 59 (81.9) | 53 (71.6) | ||
| Yes | 11 (14.1) | 13 (18.1) | 21 (28.4) | ||
| LVEF, % | 64.0 [61.0; 68.0] | 63.0 [60.0; 67.0] | 64.0 [60.0; 69.0] | 0.254 | |
| Pulmonary artery pressure, mmHg | 45.5 [35.0; 62.0] | 40.5 [32.0; 54.0] | 45.0 [35.0; 56.0] | 0.203 | |
| Uric acid/albumin ratio | 7.4 [6.5; 8.0] | 9.5 [9.0; 10.1] | 12.7 [11.7; 14.4] | ||
| Uric acid, umol/L | 297.9 [264.2; 332.0] | 384.5 [362.0; 419.3] | 494.0 [434.0; 554.0] | ||
| Albumin, g/L | 40.8 [38.8; 42.5] | 40.4 [38.3; 42.4] | 38.0 [35.8; 40.3] | ||
| Hemoglobin, g/L | 131.0 [117.0; 140.0] | 132.5 [122.0; 147.0] | 136.5 [121.0; 147.0] | 0.132 | |
| Platelets, 109/L | 225.5 [186.0; 278.0] | 223.0 [182.0; 254.0] | 202.5 [161.0; 251.0] | 0.069 | |
| Blood glucose, mmol/L | 4.6 [4.2; 5.0] | 4.4 [4.2; 4.9] | 4.5 [4.2; 4.9] | 0.923 | |
| Creatinine, mg/dL | 59.5 [53.0; 67.9] | 66.3 [57.0; 74.4] | 73.5 [61.9; 84.6] | ||
| CPB duration, minutes | 101.0 [81.0; 128.0] | 102.0 [80.0; 138.0] | 124.0 [92.0; 148.0] | 0.022 | |
| Cross-clamp time, minutes | 48.0 [35.0; 70.0] | 40.5 [26.5; 64.0] | 41.0 [0.0; 75.0] | 0.235 | |
| Blood transfusion, n (%) | 0.048 | ||||
| No | 68 (87.2) | 51 (70.8) | 58 (78.4) | ||
| Yes | 10 (12.8) | 21 (29.2) | 16 (21.6) | ||
| Acute kidney injury, n (%) | |||||
| No | 75 (96.2) | 56 (77.8) | 52 (70.3) | ||
| Yes | 3 ( 3.8) | 16 (22.2) | 22 (29.7) | ||
| Duration of mechanical ventilation, hours | 9.0 [5.0; 17.0] | 9.5 [5.0; 19.5] | 16.0 [6.0; 23.0] | 0.035 | |
| Length of stay in ICU, hours | 39.0 [20.0; 46.0] | 41.5 [22.0; 67.0] | 47.0 [39.0; 86.0] | ||
| In-hospital death | 0.119 | ||||
| No | 78 (100.0) | 70 (97.2) | 74 (100.0) | ||
| Yes | 0 (0.0) | 2 (2.8) | 0 (0.0) | ||
Abbreviations: LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; ICU, intensive care unit; UAR, uric acid/albumin ratio.
Potential factors associated with AKI development are shown in Table 2. Sex, height, age, hypertension, atrial fibrillation, creatinine, platelet count, blood transfusion, and UAR were significantly associated with AKI development. Compared to the lowest tertile (T1), the middle (T2) and the highest (T3) tertiles of the UAR were associated with a seven-fold (odds ratio (OR): 7.14, 95% confidence interval (CI): 1.99–25.7) and ten-fold (OR: 10.58, 95% CI: 3.01–37.17) increased risk of AKI following TV surgery, respectively.
| Variables | Acute kidney injury | |||
| OR | 95% CI | p-value | ||
| UAR tertile | ||||
| T1 | reference | |||
| T2 | 7.14 | 1.99–25.70 | 0.003 | |
| T3 | 10.58 | 3.01–37.17 | ||
| Male, % | 3.59 | 1.79–7.23 | ||
| Height, cm | 1.05 | 1.00–1.09 | 0.037 | |
| Weight, kg | 1.01 | 0.98–1.05 | 0.41 | |
| Age, years | 1.04 | 1.01–1.07 | 0.002 | |
| Hypertension, % | 2.97 | 1.01–8.69 | 0.048 | |
| Diabetes, % | 2.81 | 0.64–12.27 | 7.169 | |
| Atrial fibrillation, % | 3.4 | 1.62–7.14 | 0.001 | |
| Hemoglobin, g/L | 1 | 0.98–1.01 | 0.57 | |
| Platelets, 109/L | 0.99 | 0.98–0.99 | ||
| Blood glucose, mmol/L | 0.93 | 0.62–1.40 | 0.735 | |
| Creatinine, mg/dL | 1.03 | 1.00–1.05 | 0.018 | |
| LVEF | 0.99 | 0.97–1.02 | 0.636 | |
| Pulmonary artery pressure, mmHg | 0.98 | 0.97–1.00 | 0.102 | |
| Blood transfusion, % | 2.72 | 1.30–5.71 | 0.008 | |
| CPB duration time, minutes | 1.01 | 1.00–1.01 | 0.052 | |
| Cross-clamp time, minutes | 0.99 | 0.98–1.00 | 0.146 | |
Abbreviations: UAR, uric acid/albumin ratio; OR, odds ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; AKI, acute kidney injury.
Table 3 presents the results of multivariate logistic regression analyses assessing the association between UAR and the development of AKI in both the unadjusted and adjusted models. When the UAR was used as a continuous variable, the unadjusted model and Models I and II adjusted for confounding variables demonstrated an accelerated risk of AKI by 1.69, 1.56, and 1.7 times, respectively, for each standard deviation increase in the UAR level. The UAR was categorized into three levels, with the lowest tertile used as the reference group. In the unadjusted model, both the middle and the highest tertiles demonstrated a statistically significant increase in the risk of developing AKI, with ORs of 7.14 (95% CI: 1.99–25.7; p = 0.003) and 10.58 (95% CI: 3.01–37.17; p
| Crude model | Model I | Model II | ||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| UAR, per 1SD increased | 1.69 | 1.22–2.34 | 0.001 | 1.56 | 1.11–2.20 | 0.011 | 1.7 | 1.06–2.74 | 0.027 | |
| UAR tertile | ||||||||||
| T1 | 1 (Ref) | 1 (Ref) | 1 (Ref) | |||||||
| T2 | 7.14 | 1.99–25.70 | 0.003 | 8.75 | 2.18–35.13 | 0.002 | 7.7 | 1.61–37.14 | 0.011 | |
| T3 | 10.58 | 3.01–37.17 | 0.001 | 9.28 | 2.43–35.41 | 0.001 | 9.3 | 1.96–44.60 | 0.005 | |
| p-value for the trend | 0.002 | 0.013 | ||||||||
Crude model: unadjusted; Model I: adjusted for male, height, weight, age, hypertension, diabetes, and atrial fibrillation; Model II: adjusted for Model I and hemoglobin, platelets, blood glucose, creatinine, LVEF, pulmonary artery pressure, blood transfusion, CPB duration time, and cross-clamp time. UAR, uric acid/albumin ratio; OR, odds ratio; CI, confidence interval; SD, standard deviation; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; AKI, acute kidney injury.
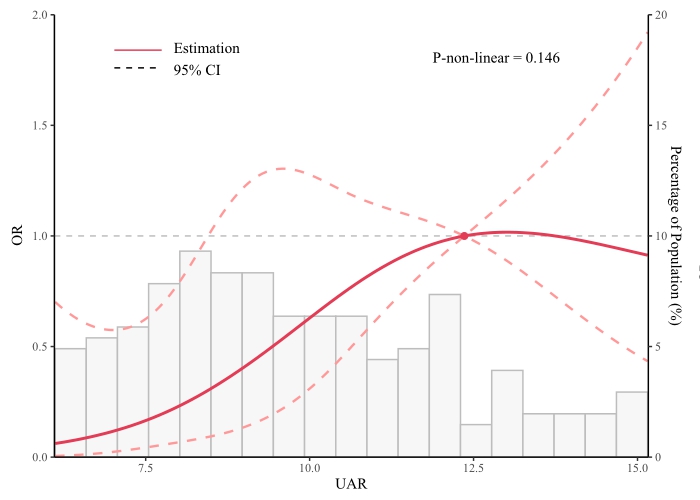 Fig. 2.
Fig. 2. Restricted cubic spline curves for AKI by TV surgery. OR, odds ratio; CI, confidence interval; UAR, uric acid/albumin ratio; AKI, acute kidney injury; TV, tricuspid valve.
ROC analysis presented in Fig. 3, the area under the curve (AUC) of the UAR was 0.713 (95% CI: 0.633–0.793; p
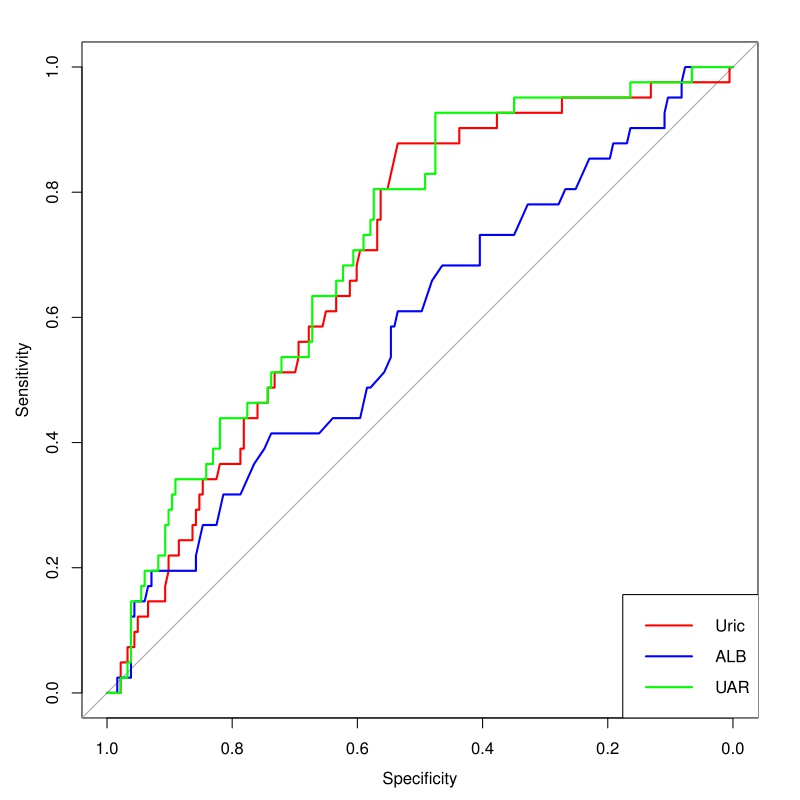 Fig. 3.
Fig. 3. Receiver operating characteristic curve analysis of the UAR, uric acid, and albumin levels to predict AKI. Uric, uric acid; ALB, albumin; UAR, uric acid/albumin ratio; AKI, acute kidney injury.
Our study confirms that the UAR can be used as an independent predictor of AKI in patients undergoing isolated tricuspid valve repair or replacement surgery. A linear relationship between the UAR levels and AKI incidence suggests that an elevated UAR is linked to an increased risk of AKI, even after accounting for confounding factors. The UAR has a moderate predictive value for AKI, with its predictive efficacy equivalent to uric acid.
Our study found that individuals in the higher UAR tertile were older, predominantly male, and had elevated levels of creatinine and uric acid and lower levels of albumin. These findings are consistent with previous studies [15, 18]. For example, the study by Li et al. [18] observed that patients with unstable angina pectoris who underwent percutaneous coronary intervention and had a high UAR of
Acute kidney injury is characterized by a sudden decline in renal function within hours to days, indicated by a rapid rise in serum creatinine levels, a decrease in urine output, or both [7]. The incidence of AKI related to cardiac surgery is estimated to be between 20% and 30% [2, 7, 20, 21]. In this study, 18.3% of patients developed AKI following surgery. Several risk factors, including age, male gender, hypertension, cardiac arrhythmia, transfusion, and preoperative serum creatinine levels, have been associated with the development of AKI following cardiac surgery [21, 22, 23]. Our study corroborates these associations, demonstrating that older male patients with higher creatinine levels, lower platelet counts, history of transfusion, hypertension, and atrial fibrillation are at an increased risk of AKI development. Our study determined that the UAR is a risk factor for AKI, with a linear relationship indicating that higher UAR levels are associated with an increased risk of AKI. Furthermore, elevated uric acid and decreased albumin levels may contribute to this condition. Serum uric acid has been widely evaluated as a predictive biomarker for AKI after cardiac surgery. A study of 190 patients undergoing coronary artery bypass surgery revealed that for each 1-unit increase in serum uric acid concentration, the risk of AKI rose by 1.18 times [24]. Another study involving 247 cardiac surgery patients showed that, after adjusting for confounders with multivariate logistic regression, a preoperative uric acid level of
Studies by Şaylık et al. [29] and Yeter et al. [14] have demonstrated the predictive value of the UAR for kidney impairment post-percutaneous coronary intervention. Şaylık et al. [29] identified a UAR cutoff of
The metabolic disorder of uric acid and decreased albumin synthesis before cardiac surgery may underlie the mechanism through which the UAR predicts AKI. Firstly, the kidney plays a significant role in uric acid excretion and absorption. Therefore, before undergoing cardiac surgery, most patients grapple with diminished cardiac function, potentially triggering a cascade of events known as cardiorenal syndrome, which in turn can impair kidney function [31]. Consequently, this leads to reduced excretion of uric acid in the urine and increased reabsorption, resulting in elevated uric acid levels in the bloodstream [32]. Secondly, sustained elevation in uric acid levels may promote pathogenic inflammation, cellular proliferation, and maladaptive changes. These pathological changes can result in glomerulosclerosis and fibrosis in the tubulointerstitium, resulting in renal damage even after cardiac surgery [33]. Chronic kidney disease is characterized by persistent chronic inflammation, which may decrease albumin concentration due to a reduced synthesis rate [31, 34]. As a result, low serum albumin levels indicate the severity of the inflammation [35] and reflect the extent of kidney function impairment.
The UAR has a wide range of applications. Moreover, it is effective in predicting AKI and has demonstrated significant prognostic value in forecasting outcomes for patients with ST-elevation myocardial infarction (STEMI) [36] and complications following various procedures, including cryoballoon catheter ablation [37], coronary artery bypass grafting (CABG) [38], and PCI. Compared with single markers, the UAR provides stronger results and a multidimensional impact, making it particularly advantageous in clinical practice.
The current study has several inherent limitations. Firstly, the retrospective design of the study prevents us from establishing a causality relationship between the UAR and AKI. Secondly, the study focuses solely on patients who underwent isolated TV surgery, which may limit the generalizability of our findings to other cardiac surgery populations. Additionally, the absence of long-term follow-up data limits our understanding of AKI incidence over an extended period. Finally, despite applying a multivariate analysis, residual confounders, such as the duration of tricuspid valve disease, right heart failure, and diuretic therapy, could not be fully addressed. For these reasons, future research should be prospective and include diverse patient populations to validate our findings. We strongly recommend incorporating long-term follow-up data to gain a deeper understanding of the incidence and progression of AKI.
Our analysis establishes a clear linear correlation between preoperative UAR levels and the incidence of AKI in patients undergoing isolated tricuspid valve repair or replacement. An elevated UAR is linked to an increased risk of AKI. The UAR demonstrates a moderate level of predictive accuracy for AKI; while it is comparable to uric acid alone, it has wider clinical application value. As a cost-effective biomarker, UAR could be instrumental in identifying patients at risk of AKI who may require closer monitoring and management.
All data relevant to the study are included in the article. Data can also be requested from the corresponding author.
CJZ: Conceptualization, Formal analysis, and Writing—review & editing; YJL: Conceptualization and Writing—original draft; LYL: Conceptualization, Writing—review & editing and Project administration; JL and FFZ: Investigation, Data curation, and Writing—review & editing. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects.
This study was performed in accordance with the principle of the Declaration of Helsinki, and the research protocol was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (KY2024-605–02), and all of the participants provided signed informed consent.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



