1 Department of Radiation Oncology, School of Medicine, Kocaeli University, 41001 Kocaeli, Turkey
Abstract
One of the most significant long-term toxicities of breast cancer radiotherapy is major adverse cardiac events (MACE). In current radiotherapy practice, the mean heart dose is the most commonly used parameter. The aim of our study was to reduce the doses of organs at risk (OAR) in the left anterior descending artery (LAD) and left ventricle (LV) by including the LAD and LV in planning radiotherapy while maintaining adequate dose coverage for patients with left-sided breast cancer.
We retrospectively analyzed left-sided breast cancer cases treated at the Kocaeli University Faculty of Medicine. Only patients with local and locally advanced breast cancer were included in the analysis. A total of 77 patients who were treated between 2020 and 2024 were included. The doses to the LAD and LV were added to the optimization algorithms. Two volumetric modulated arc therapy (VMAT) plans were created for each patient. A total of 154 plans were made, including standard and LAD and LV sparing plans.
There was no statistically significant difference in all VMAT plans regarding planning target volume (PTV) D2, D50, and D98 (dose receiving volume of PTV 2%, 50%, and 98%) (p > 0.05). However, a significant decrease was observed in heart V5 (the percentage of the heart receiving at least 5 gray (Gy)) and mean heart dose. A decrease in the mean heart dose was observed in the standard plan compared with the LAD and LV sparing plan (p < 0.001). Similarly, the heart V5 value decreased significantly (p < 0.001). Additionally, significant reductions were measured in all LAD and LV parameters after re-optimization.
We achieved significant reductions in all heart, LAD, and LV parameters without making any changes to the planned treatment volume coverage by adding LAD and LV OARs to the optimization algorithms. The potential risk of MACE can be significantly reduced by implementing this strategy.
Keywords
- left breast cancer
- left anterior descending artery
- left ventricle
- major adverse cardiac events
The World Health Organization reports that breast cancer is one of the most common cancers worldwide, with approximately 2.3 million new diagnoses annually [1]. The majority of recently diagnosed patients are treated with radiotherapy as adjuvant therapy to breast-conserving surgery [2]. Radiotherapy is associated with longer overall survival and longer local progression-free survival [3]. Radiotherapy is also used in palliative settings to enhance patients’ quality of life.
Due to anatomical proximity and the limitations of current techniques, the spread of radiation to normal tissues is inevitable. The most frequent acute toxicity in breast cancer radiotherapy is skin toxicity, whereas major adverse cardiac events (MACE) are late toxicities. The RTOG0617 study showed that in locally advanced non-small cell lung cancer (NSCLC) patients, overall survival is related to heart V5 and V30 volumes [4]. In current radiotherapy practice, the heart is generally contoured as a single organ and the mean heart dose (MHD) is the most commonly used organ at risk (OAR). According to a study by Darby et al. [5], an increase of 1 gray (Gy) in MHD was associated with a 7.4% increase in the risk for MACE. To mitigate MHD and the MACE risk, deep inspiration breath hold (DIBH) is used [6]. DIBH can also be used to reduce the doses to cardiac structures, especially left anterior descending artery (LAD) [7]. In 2011, Vikström et al. [8] showed that the MHD and LAD can be reduced by utilizing the DIBH method by as much as 54% and 65%, respectively. A study by Tang et al. [9] has shown significant correlations between MHD and doses received by the left ventricular (LV) and right ventricular (RV) anterior and apical walls. Tjong et al. [10] found that the key factors in predicting MACE risk after radiotherapy are pre-existing hypertension, coronary heart disease, and LAD V15 (LAD volume receiving 15 Gy in standard plan). The Cardiac disease, Hypertension, and Logarithmic Left anterior descending coronary artery radiation dose (CHyLL) score incorporates LAD V15 rather than MHD to calculate personalized LAD V15 constraints based on cardiac risk factors [10].
This study aimed to dosimetrically reduce LAD and LV doses as OARs by including them in planning optimization for patients with left-sided breast cancer.
We retrospectively analyzed cases of left breast cancer treated at the Kocaeli University Faculty of Medicine. In this analysis, only patients with local and locally advanced disease were included. These patients were expected to have a target volume near the heart. Patients who underwent free-breathing (FB) or DIBH computed tomography (CT) scans were included. Exclusion criteria was a predated second cancer diagnosis. All patients who met the inclusion criteria were enrolled in this study.
Institutional review board approval was obtained for this study. The Non-Interventional Clinical Research Ethics Committee of Kocaeli University approved the study, which convened on 14.02.2022 and assigned protocol number 2022/106.
In standard radiotherapy, the heart is contoured and evaluated as a single organ. In this study, the LAD and LV were contoured as substructures and incorporated into the optimization algorithms to minimize the doses while maintaining target volume coverage and adhering to dose constraints for other critical thoracic OARs. An overview of the optimization parameters for both the standard and sparing plans is shown in Table 1. The planning target volume (PTV) and OAR parameters in the standard plan were left unchanged, and only the LAD and LV parameters were added to the optimization algorithm.
| Standard plan | Sparing plan |
| PTV | PTV |
| Heart | Heart |
| Contralateral breast | Contralateral breast |
| Left lung | Left lung |
| PRV | PRV |
| Left anterior descending artery | |
| Left ventricle |
PTV, planned target volume; PRV, planned risk volume.
DIBH and FB CT images were contoured by a radiation oncology specialist according to the Radiation Therapy Oncology Group (RTOG) contouring atlases [11]. The contoured organs included the right and left breasts, lungs, heart, and intracardiac structures, specifically the left anterior descending artery and left ventricle. An experienced medical physicist designed new cardiac-optimized volumetric modulated arc therapy (VMAT) plans by integrating the previously mentioned structures into the Varian (Palo Alto, CA, USA) Eclipse V13.6 treatment planning system plan optimizer. Integration and planning were standardized, and treatment plans for all patients were calculated using the same optimization parameters. The goal was to maximize the protection of intracardiac substructures while ensuring PTV coverage and following dose constraints for OARs.
VMAT plans were developed for all patients. This involved two arcs. The first arc started at 293° with a collimator angle of 30° and was a clockwise arc of 240°. The second arc started at 173° with a collimator angle of 330° and was a 240° counterclockwise arc. Subsequently, the LAD and LV sparing plans (LADLVSP) aimed to spare the LAD and LV. The treatment plans were normalized to ensure that 95% of the PTV would receive a dose of 50 Gy.
Dosimetric data for the conventional and LADLVSP VMAT plans were collected using the Varian (Palo Alto, CA, USA) Eclipse V13.6 planning software. A comparison was made between the standard and LADLVSP plans. V15 is the percentage of volume receiving 15 Gy, and D2 is the maximum dose received to 2% of the PTV. D50 is the median dose received by 50% of the PTV. D98 is the minimum dose applied to 98% of the PTV [12]. Dosimetric data were obtained using the dose-volume histogram. Mean heart dose, heart V5, and heart V30 were used to compare differences in heart doses between plans. PTV D2, D98, and D50 were used to compare dose distribution. Mean LAD dose, LAD D2, and LAD V15 were used to compare LAD doses. Mean LV dose, LV D2, and LV V23 were used to compare LV doses.
Dosimetric data were analyzed for normal distribution using Kolmogrov-Smirnov Test. Non-parametric data were compared between the conventional and LADLVSP groups using a Wilcoxon‘s test with Bonferroni correction. The parametric data were compared using the paired t-test. The significance was assessed at the
77 patients treated from 2020 to 2024 met the inclusion criteria. Table 2 illustrates the demographic and clinical characteristics of the patients. 77 Standard and 77 LADLVSP plans were created for each patient. A total of 154 plans were analyzed. Re-optimized plans met the prescribed treatment dose, while critical organ doses remained within safety limits according to guidelines [13]. Table 3 summarizes the mean changes in the LAD, LV, PTV, and heart in both VMAT plans. No statistically significant differences were found in the VMAT plans for PTV D2, D98, and D50 (p
| Age group (0–39) | Age group (40–69) | Age group (70+) | Total | ||
| Age | 56 (31–80) | ||||
| Stage | |||||
| 1a | 1 | 36 | 10 | 47 | |
| 1b | 0 | 1 | 0 | 1 | |
| 2a | 2 | 15 | 1 | 18 | |
| 2b | 0 | 10 | 0 | 10 | |
| 3a | 1 | 0 | 0 | 1 | |
| Size | |||||
| 4 | 59 | 11 | 74 | ||
| 0 | 3 | 0 | 3 | ||
| ER status | |||||
| Negative | 2 | 11 | 2 | 15 | |
| Positive | 2 | 51 | 9 | 62 | |
| Her-2 status | |||||
| Negative | 4 | 62 | 11 | 77 | |
| Positive | 0 | 0 | 0 | 0 | |
| Tumor quadrant | |||||
| LI (Lower inner) | 0 | 19 | 4 | 23 | |
| LO (Lower outer) | 2 | 3 | 0 | 5 | |
| UO (Upper outer) | 2 | 26 | 3 | 31 | |
| UI (Upper inner) | 0 | 14 | 4 | 18 | |
| Grade | |||||
| 1 | 1 | 14 | 5 | 20 | |
| 2 | 2 | 31 | 5 | 38 | |
| 3 | 1 | 17 | 1 | 19 | |
According to AJCC Cancer Staging Manual, Eighth Edition, the letters a and b are used to further subdivide a stage based on tumor characteristics. For instance, stage 1a typically indicates a small tumor with no or minimal lymph node involvement, whereas stage 1b may denote similar tumor size but with microscopic nodal involvement. Similarly, the subdivisions in stage 2 (2a vs 2b) reflect differences in tumor size or extent of nodal involvement. Abbreviations: ER, estrogen receptor.
| Standard plan | LADLVSP | Difference | p value | |
| PTV D2 (Gy) | 55.69 | 55.68 | 0.01 | p = 0.773 |
| PTV D98 (Gy) | 48.83 | 48.82 | 0.01 | p = 0.464 |
| PTV D50 (Gy) | 53.21 | 53.21 | 0.00 | p = 0.797 |
| Heart mean (Gy) | 6.65 | 6.03 | 0.62 | p |
| Heart V5 (%) | 50.8 | 45.41 | 5.40 | p |
| Heart V30 (%) | 0.54 | 0.20 | 0.34 | p |
| Lad mean (Gy) | 17.32 | 12.41 | 4.91 | p |
| Lad D2 (Gy) | 29.52 | 24.26 | 5.26 | p |
| Lad V15 (%) | 51.89 | 29.59 | 22.30 | p |
| LV mean (Gy) | 7.46 | 5.59 | 1.87 | p |
| LV D2 (Gy) | 22.14 | 17.28 | 4.86 | p |
| LV V5 (%) | 58.59 | 37.17 | 21.42 | p |
| LV V23 (%) | 2.46 | 0.91 | 1.55 | p |
| RV V20 (%) | 1.28 | 0.82 | 0.46 | p = 0.056 |
| Right breast mean (cGy) | 381.25 | 395.62 | 14.37 | p |
| Left lung V20 (%) | 21.22 | 19.92 | 1.30 | p |
| Right lung V5 (%) | 36.29 | 37.19 | 0.90 | p = 0.316 |
Abbreviations: LADLVSP, left anterior descending coronary artery and left ventricle–sparing plan; PTV, planning target volume; Vn, percentage of the volume receiving n Gy; D2, D50, and D98, dose receiving volume of PTV 2%, 50%, and 98%, respectively; LAD, left anterior descending artery; LV, left ventricle; LAD V15, LAD volume receiving 15 Gy in standard plan; cGy, centigray; Gy, gray; VMAT, volumetric modulated arc therapy; RV, right ventricular.
Data are presented as mean
When evaluated individually, 26 patients with an initial LAD V15 dose greater than 50% experienced a reduction to 50% or below after VMAT plan re-optimization (Fig. 1). The number of patients with an LAD V15 dose greater than 50% in the standard plan was 40. Additionally, we successfully reduced the LAD mean dose from above 20 Gy to below 20 Gy in 13 patients. The number of patients with an LAD mean dose above 20 Gy in the standard plan was 16. Cross-sectional CT images of a patient whose mean LAD dose was reduced to below 20 Gy are shown in Fig. 2.
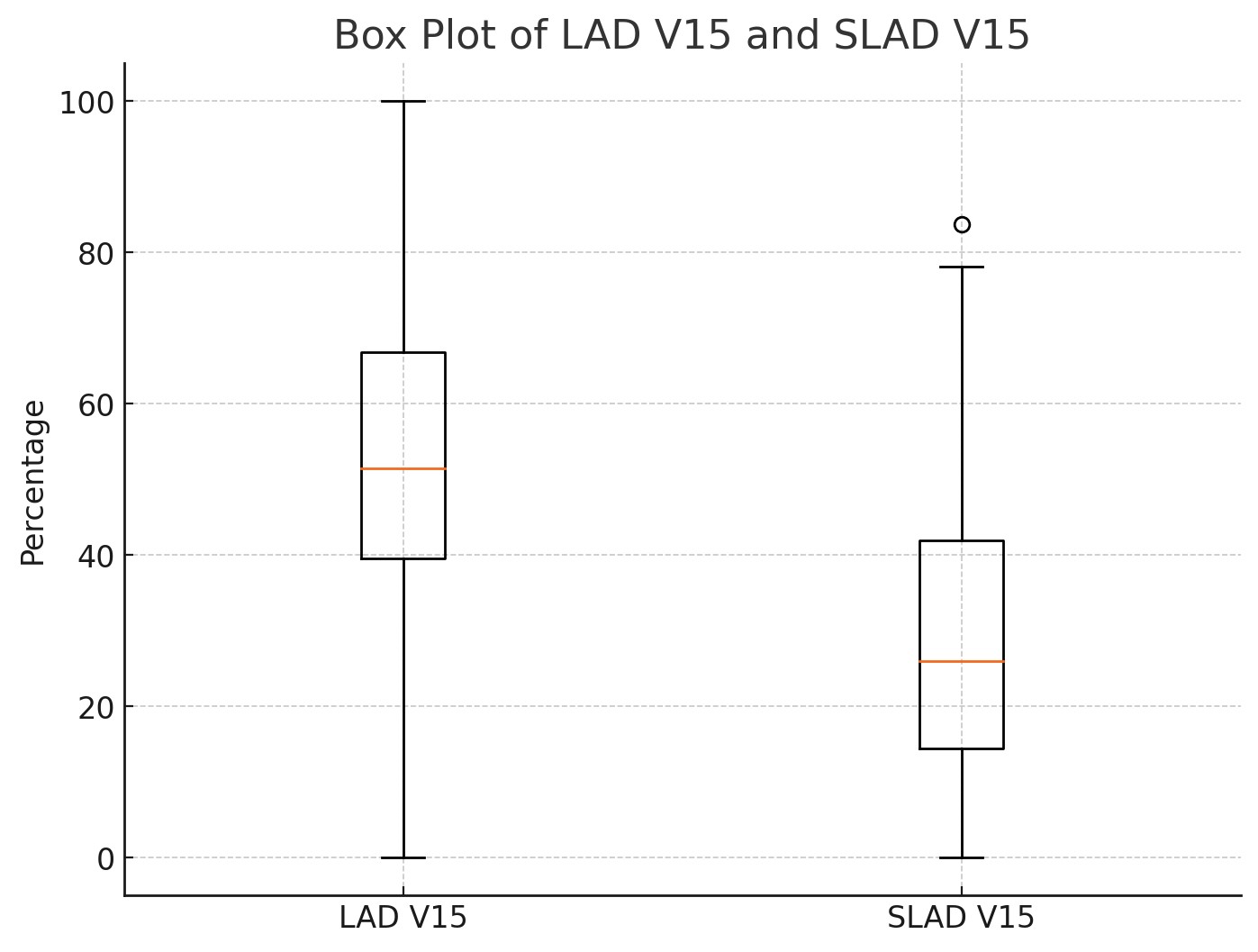 Fig. 1.
Fig. 1. Box plots of LAD V15 in standard and sparing planning techniques, shown side by side. LAD V15, LAD volume receiving 15 Gy in standard plan; SLAD V15, LAD volume receiving 15 Gy in sparing plan; LAD, left anterior descending artery.
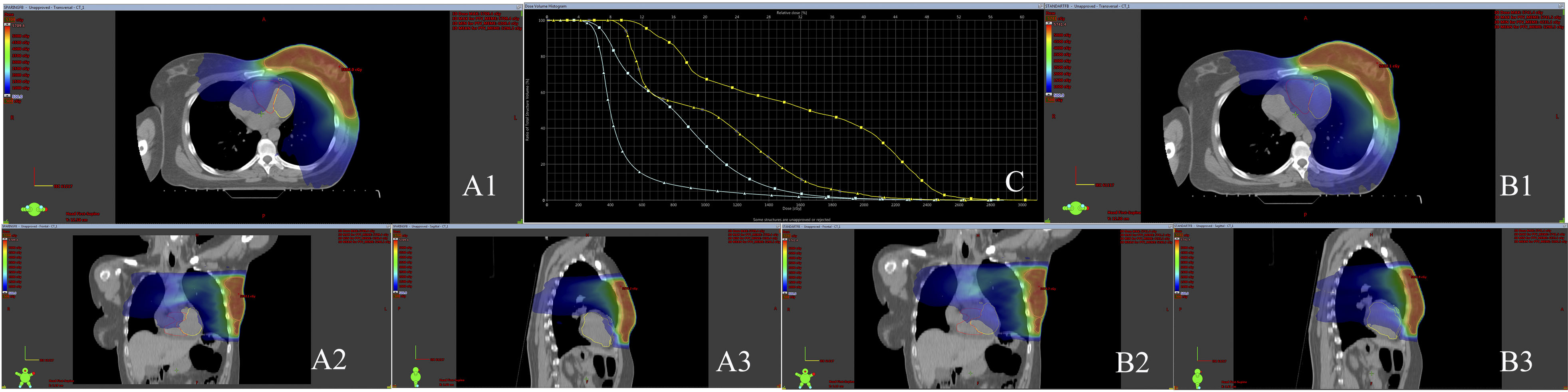 Fig. 2.
Fig. 2. Cross-sectional CT images of a patient with the LAD and LV delineated. (A1,A2,A3) LAD/LV sparing plan transverse, coronal and sagittal sections. (B1,B2,B3) Standard plan transverse, coronal and sagittal sections. (C) DVH image of LAD and LV cardiac substructures in sparing and standard plans. Isodose line represents 5 Gy. Additionally, the dose-volume histogram (DVH) displays the dose distributions for the LAD (yellow) and LV (green). LAD, left anterior descending artery; LV, left ventricle; 3D, three-dimensional; PTV, planning target volume; cGy, centigray; Gy, gray; CT, computed tomography;
In this study, we created new treatment plans by incorporating the LAD and LV organs into standard radiotherapy plans. We observed significant reductions in heart, LV, and LAD doses. For the other specified OARs, a significant increase in the mean dose to the right breast was observed. The clinical relevance of the mean contralateral breast dose is not well established in the current literature, especially compared to genetic and histological risk factors. Zurl et al. [14] stated that the increased mean contralateral breast dose associated with DIBH should not impact clinical decision-making regarding the excess risk of contralateral breast cancer (CBC). Current CBC risk assessment tools do not account for the dose received by the contralateral breast, and only ask whether radiotherapy was administered [15]. We also believe that a 3% increase in the contralateral breast dose during VMAT plan re-optimization should not affect clinical decision-making, especially considering the substantial 43% reduction in LAD V15.
Given the stronger association between the LAD and MACE compared to the MHD, the significant reduction in the LAD V15 dose suggests that protecting the LAD may be crucial in lowering the risk of MACE. Darby et al. [5] showed a significant elevation in the risk of ischemic heart disease after radiotherapy, and found a 7.4% increase in the risk of MACE per gray of MHD delivered to the patient. Our findings support the notion that this potential risk can be mitigated by protecting the LAD by including the OARs in treatment optimization. Similar dose reductions have been reported in the literature for patients using DIBH. Parlar et al. [16] observed a 50% reduction in MHD with DIBH compared with free-breathing techniques. Wolf et al. [17] also reported significant reductions in heart and LAD doses using the DIBH technique, highlighting its effectiveness in sparing cardiac structures. For patients unable to perform DIBH, the method developed in this study is a viable alternative to reduce the risk of MACE.
The use of intensity-modulated radiotherapy (IMRT) plans instead of three-dimensional conformal radiotherapy (3D-CRT) has been documented in the literature to reduce doses to the LAD and the heart. Garg and Kumar [18] found that IMRT plans significantly reduced the MHD and LAD by 30% and 25%, respectively, compared to 3D-CRT plans. The improved dose distribution offered by IMRT and VMAT techniques provide significant advantages for protecting critical organs. The literature reports that when the LAD dose exceeds 20 Gy, the risk of radiation-induced coronary stenosis increases by five-fold. Wennstig et al. [19] observed that patients receiving more than 20 Gy to the LAD had a significantly higher frequency of coronary artery interventions. In our study, we successfully reduced LAD doses to below 20 Gy in 13 out of 16 patients, thereby demonstrating the efficacy of our approach.
Van den Bogaard et al. [20] examined the impact of a LV V5 dose on MACE and found a significant risk reduction with lower LV V5 doses. Similar significant dose reductions were achieved in our patients. For instance, we observed a reduction in the LV V5 dose by 21% compared with conventional planning methods.
Arslan et al. [21] previously incorporated the LAD and LV into their optimization algorithm to minimize doses to these critical structures. Despite these efforts, the study’s limited sample size resulted in a statistically significant reduction in only D98, whereas the reduction in LV V5 was not statistically significant. Arslan et al. [21] reported a 12% reduction in the mean LAD dose and an 8% reduction in the mean LV dose in their cohort of 20 patients. Our study corroborates their findings. Using an improved study design with a larger sample size, we have established that this method can effectively lower doses to the LAD and LV. We achieved these results even when the differences between treatment plans were not statistically significant, highlighting the eficacy of our optimization algorithm.
Wang et al. [22] found that even low-dose radiation can cause perfusion damage in the irradiated areas. Therefore, adhering to the ALARA (As Low As Reasonably Achievable) principles, we aimed to minimize radiation doses to the lowest possible levels.
Radiotherapy-induced cardiotoxicity is a significant concern in the treatment of breast cancer. In a study by Díaz-Gavela et al. [23], the doses received by the heart during radiotherapy increased the risk of cardiac disease. The study revealed that the average heart and LAD doses were associated with increased cardiotoxicity. Specifically, higher doses to the LAD artery were associated with a greater risk of cardiac events. The maximum heart dose also plays a crucial role in determining this risk. These findings underscore the importance of minimizing doses using advanced radiotherapy techniques. Heart-sparing radiotherapy methods and multidisciplinary approaches are critical for reducing long-term cardiac complications and improving overall survival and quality of life for breast cancer patients [23].
Yeşildere and colleagues [24] conducted a dosimetric comparison between proton and photon therapies for the treatment of left-sided breast cancer. The study found that proton therapy significantly reduced doses to the heart and LAD. Specifically, proton therapy reduced the mean heart dose by 45% and the mean LAD dose by 50%. This dose reduction underscores the potential of proton therapy to minimize cardiac side effects. These findings suggest that proton therapy may be more advantageous for breast cancer treatment [24].
The limitations of this study include that only patients with left-sided breast cancer were included in this study. Therefore, the results are not applicable to right-sided breast cancer. Due to the low number of FB patients, comparative analysis of the sparing technique between DIBH and FB CT scans should be performed. This study was conducted retrospectively, which may introduce potential biases inherent to retrospective analyses, such as sampling bias and incomplete data collection.
Our method can be used with other advanced radiotherapy techniques to reduce the risk of MACE in patients with early-stage breast cancer. Future prospective studies should more comprehensively evaluate the effectiveness of LAD and LV protection strategies and their role in reducing MACE in clinical practice.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conceptualization, UD, AOK, IHS, NT, OA, HFC, DG; clinical reasoning, UD, MGA, EBS, AUK; data analysis, NT, IHS, AOK, OA, DG; writing—original draft preparation, UD, AOK, IHS, NT, OA; writing—review and editing, UD, HFC, MGA, EBS, AUK, DG; visualization, IHS, OA, HFC, AOK, NT; supervision, MGA, EBS, AUK; project administration, UD, HFC, EBS, MGA, AUK, DG. All authors have read and agreed to the final version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was conducted in accordance with the guidelines of the Declaration of Helsinki, the Ethics Committee of Kocaeli University (Ethic Approval Number: 2022/106), and all of the participants provided signed informed consent.
We gratefully acknowledge the assistance of Dr. Ozlem Ay and Dr. Deniz Celik from Kocaeli University in the acquisition and contouring of treatment CT images.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


