1 Department of Gastroenterology, The First Affiliated Hospital of Shantou University Medical College, 515041 Shantou, Guangdong, China
2 Department of Endoscopy Center, Cancer Hospital of Shantou University Medical College, 515041 Shantou, Guangdong, China
Abstract
Non-alcoholic fatty liver disease (NAFLD), one of the most common chronic liver diseases with a prevalence of 23%–25% globally, is an independent risk factor for cardiovascular diseases (CVDs). Growing evidence indicates that the development of NAFLD, ranging from non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), advanced fibrosis to cirrhosis, and even hepatocellular carcinoma, is at substantial risk for CVDs, which clinically contribute to increased cardiovascular morbidity and mortality. Non-invasive serum markers assessing liver fibrosis, such as fibrosis-4 (FIB-4) score, aspartate transaminase-to-platelet ratio index (APRI), and NAFLD fibrosis score (NFS), are expected to be useful tools for clinical management of patients with CVDs. This review aims to provide an overview of the evidence for the relationship between the progression of NAFLD and CVDs and the clinical application of non-invasive markers of liver fibrosis in managing patients with CVDs.
Keywords
- biomarkers
- cardiovascular disease
- fibrosis
- non-alcoholic fatty liver disease
- non-alcoholic steatohepatitis
Non-alcoholic fatty liver disease (NAFLD) is replacing viral hepatitis as the most common chronic liver disease, with a global prevalence of 23–25% among adults. The prevalence of NAFLD varies from region to region, with the highest in the Middle East (32%) and the lowest in Africa (13%) [1]. Despite its rising burden on global public health and the economy, minimal attention has been focused on NAFLD. The prevalence of young NAFLD was augmented from 19.34 million in 1990 to 29.49 million in 2017 [2]. It was estimated that more than USD 100 billion in annual direct medical costs in the U.S. [3]. Another assessment model for NAFLD disease progression in 8 countries suggested that China had the greatest overall and relative growth in NAFLD prevalence, with up to 314 million NAFLD cases predicted by 2030 [4]. NAFLD is now considered a multisystem disease rather than a liver disease, which encompasses a spectrum of histological conditions ranging from liver steatosis non-alcoholic steatohepatitis (NASH) to liver fibrosis, increasing the prevalence of liver-related and extrahepatic complications. Moreover, a large amount of evidence has shown that NAFLD may be closely related to cardiovascular diseases (CVDs), such as atrial fibrillation [5], heart valve calcification [6], coronary artery disease [7], and heart failure [8, 9], independently of other well-known cardiovascular risk factors. Previous research has highlighted that people with NASH tend to be at a greater risk of CVDs than those with non-alcoholic fatty liver (NAFL) [10], which means the risk of CVDs might parallel the severity of NAFLD. Thus, early monitoring and identification of liver fibrosis in NAFLD patients may reduce the incidence of major adverse cardiovascular events (MACEs). Hence, non-invasive serum markers, such as fibrosis-4 index (FIB-4), aspartate transaminase-to-platelet ratio index (APRI), and NAFLD fibrosis score (NFS), which are now advocated in current guidelines to detect fibrosis, might be potential tools for CVD management. This review mainly focuses on the evidence for a relationship between the progression of NAFLD and CVDs and the clinical application of non-invasive markers of liver fibrosis in the management of patients with CVDs.
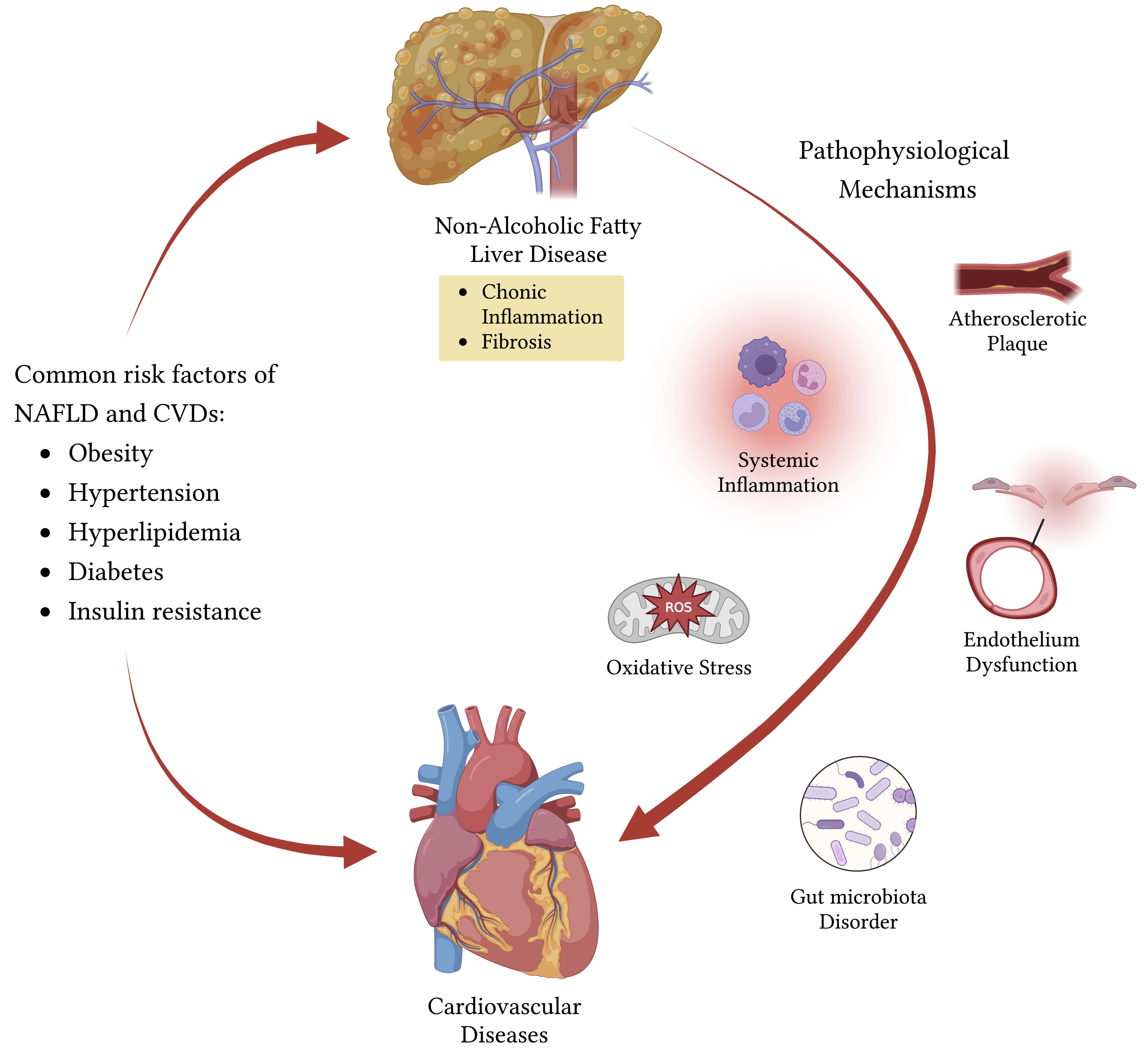
NAFLD and CVDs are both manifestations of metabolic syndrome, sharing common risk factors such as obesity, hypertension, hyperlipidemia, diabetes, and insulin resistance. The underlying mechanisms linking NAFLD to CVDs are still being researched, yet involve several complex pathways, such as insulin resistance, oxidative stress, low-grade systemic inflammation, endothelial dysfunction, and gut dysbacteriosis, which may be influenced by genetic and epigenetic variations [11] (Fig. 1). Low-grade inflammation is a key feature in the underlying mechanism between NAFLD and CVDs. Systemic inflammation promotes the occurrence of CVDs via endothelial dysfunction, enhanced plaque formation, and coagulation [12]. The vascular endothelium is involved in regulating various physiological and pathophysiological processes, such as platelet function, vascular tone, and inflammation. Recent research has indicated that impaired endothelial function plays a significant role in the interplay between NAFLD and CVDs [13, 14]. Insulin resistance would promote lipolysis in adipose tissue and increase the delivery of free fatty acids (FFAs) to the liver. FFAs induce inflammation and the production of very low-density lipoprotein (VLDL), which increases the concentration of VLDL in the circulation and leads to atherosclerosis [15, 16]. Early animal experiments demonstrated that the gut microbiota controls metabolic functions and is crucial for developing NAFLD [17]. Intestinal dysbiosis can also be involved in the development of CVDs. The gut microbiome secretes several molecules into the bloodstream; for example, many studies have suggested that trimethylamine-N-Oxide (TMAO) was associated with CVDs and considered a pro-atherogenic compound [18, 19, 20]. Emerging evidence suggested that NAFLD has been linked to CVDs, raising concerns about the early intervention of liver fibrosis in NAFLD patients with cardiovascular disease. A growing number of studies have discussed the relationship between NAFLD progression and CVDs, raising public attention to early intervention of liver fibrosis in patients with CVDs. A meta-analysis demonstrated that the fibrosis stage, determined by biopsy, was related to all-cause mortality and morbidity in patients with NAFLD, with and without adjustments for potential confounding factors [21], also providing a similar conclusion to another meta-analysis from Dulai et al. [22].
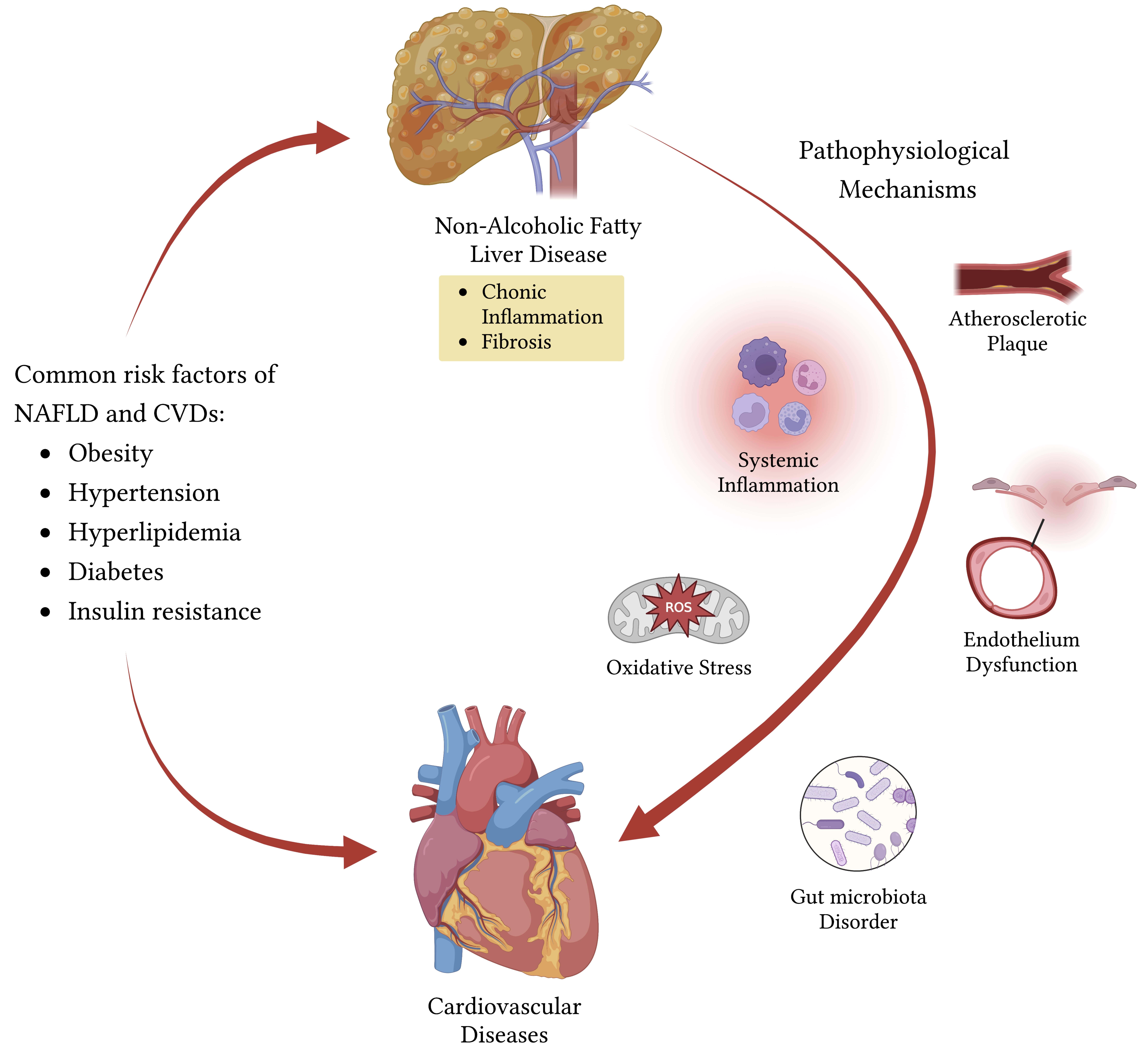 Fig. 1.
Fig. 1.
Potential pathophysiological mechanism linking NAFLD to CVDs. NAFLD and CVDs share common risk factors: obesity, hypertension, hyperlipidemia, diabetes, and insulin resistance. NAFLD drives multiple mechanisms that ultimately lead to CVDs, such as insulin resistance, oxidative stress, low-grade systemic inflammation, endothelial dysfunction, and gut dysbacteriosis. NAFLD, non-alcoholic fatty liver disease; CVDs, cardiovascular diseases; ROS, reactive oxygen species.
NAFL is characterized as triglyceride accumulation
in more than 5% of hepatocytes without evidence of hepatocellular injury or
fibrosis [23]. Fat accumulation in liver cells is a key point in the development
of NAFLD, further progressing to irreversible damage such as NASH and even
cirrhosis under the persistence of risk factors. Hepatic triglycerides are not
directly hepatotoxic, and it is estimated that hepatocyte injury is caused by
toxic triglyceride precursors or triglyceride metabolites [24]. Owing to its
significant relationship with metabolic syndrome, hepatic steatosis is more
common in patients with obesity and hyperlipidemia, which also serve as
independent risk factors for CVDs. However, individuals of normal weight (
Characterized by not only
| Authors | Region | Total cases | Diagnosed method | Outcomes | OR (95% CI) | HR (95% CI) | RR | |
| NAFLD/MAFLD | CVDs | |||||||
| Agaç MT et al. [36] | USA | 3976 | CT | CT | CAC | 1.37 (1.11–1.68) | - | - |
| Fudim M et al. [37] | USA | 870,535 | Database record | ICD-9/10 | HF | - | 1.23 (1.19–1.29) | 1.30 |
| Gummesson A et al. [38] | Sweden | 1015 | CT | Ultrasound | CAC | 1.77 (1.07–2.94) | - | - |
| Guo Y et al. [39] | China | 11,444 | Multiple criteria | ICD-10 | CVDs | - | 1.37 (1.20–1.56) | 2.04 |
| Kang MK et al. [40] | Korea | 772 | Ultrasound | CT | CA | 1.48 (1.05–2.08) | - | 1.49 |
| Lee H et al. [41] | Korea | 8,962,813 | FLI | ICD-10 | CVDs | - | NAFLD: 1.09 (1.03–1.15) | 2.29 |
| MAFLD: 1.43 (1.41–1.45) | ||||||||
| Lee SB et al. [7] | Korea | 5121 | Ultrasound | CT | CA | 1.18 (1.03–1.35) | - | 1.32 |
| Roh JH et al. [42] | Korea | 308,578 | FLI | ICD-10 | HF | - | 2.71 (2.380–3.085) | 1.62 |
| Simon TG et al. [43] | Sweden | 56,939 | Biopsy | ICD-10 | MACEs | - | 1.63 (1.56–1.70) | - |
| VanWagner LB et al. [44] | USA | 2424 | CT | CT | CAC | 1.33 (1.001–1.82) | - | 1.46 |
| Wong VW et al. [45] | China | 612 | Ultrasound | CC | CAD | 2.31 (1.46–3.64) | - | 1.32 |
| Yu MM et al. [46] | China | 1683 | CT | CT | MACEs | - | 1.63 (1.28–2.06) | 1.99 |
| Chung GE et al. [47] | Korea | 3300 | Ultrasound | Echocardiography | LV diastolic dysfunction | 1.29 (1.07–1.60) | - | - |
NAFLD, non-alcoholic fatty liver disease; MAFLD, metabolic associated fatty liver disease; CVDs, cardiovascular diseases; HF, heart failure; MACEs, major adverse cardiovascular events; FLI, fatty liver index; CT, computed tomography; CA, coronary atherosclerotic; CAC, coronary artery calcium; CAD, coronary artery disease; ICD, the International Statistical Classification of Diseases and Related Health Problems; CC, cardiac catheterization; LV, left ventricular; OR, odds ratio; HR, hazard ratio; CI, confidence interval; RR, relative risk (RR was calculated by the extracted data from the research).
A nationwide cohort of Swedish adults with biopsy-confirmed NAFLD performed by Simon et al. [43] demonstrated that rates of fatal and non-fatal MACEs outcomes, including ischemic heart disease (IHD), congestive heart failure (CHF) and cardiovascular mortality, were significantly higher in NAFLD patients than those without. Further, a significant risk was found across all stages of NAFLD and increased with the progression of NAFLD. Compared with patients with simple steatosis, those with non-cirrhotic fibrosis and cirrhosis had significantly elevated rates of MACE outcomes (4.1/1000 and 20.2/1000 person-years, respectively). Despite this, data remain limited regarding the relationship between CVDs and different stages of NAFLD, probably because of the difficulty in specifically identifying NAFL, NASH, and stage fibrosis in the absence of liver biopsy. Hence, non-invasive assessments for advanced fibrosis, such as non-invasive serum markers, FibroScan, and magnetic resonance elastography (MRE), have gradually been used in clinical practices. An increasing number of studies investigated the relationship between CVDs and NAFLD fibrosis assessed using non-invasive serum tests, most of which concluded that the liver fibrosis stage was associated with a high risk of cardiovascular events [48, 49, 50].
The assessment of the NAFLD fibrosis stage plays an essential role in evaluating the prognosis, establishing therapies, and evaluating the response to treatments. Liver biopsy, the golden standard for identifying fibrosis, provides direct measurement and exact stages of hepatic fibrosis. However, due to its invasiveness, it also has well-known limitations, such as poor acceptability, sample error, and potential complications, including pain, infection, and bleeding. Thus, it seems impractical and challenging to conduct liver biopsy in large-scale clinical screening for NAFLD. Therefore, several accurate, repeatable, dynamic, and non-invasive methods have been developed in clinical practice, including imaging techniques and serum markers.
Ultrasonography is the most common imaging method for diagnosing liver steatosis
owing to its low cost and easy operation, which is widely used in screening and
health check-ups. However, ultrasonography can only detect moderate-to-severe
hepatic steatosis (
MRE is a MRI-based method for quantitatively imaging tissue stiffness, which appears more accurate than sonographic elastography and is not significantly impacted by obesity with a lower risk of failure [59]. A meta-analysis evaluating the diagnostic accuracy of elastography and magnetic resonance imaging for liver fibrosis and NASH demonstrated that areas under the receiver operating characteristic curve (AUROC) of MRE for diagnosis of significant fibrosis, advanced fibrosis, and cirrhosis were all above 0.90 [60]. Proton density fat fraction (PDFF) is the ratio of proton density in free triglyceride to the total proton density in free triglyceride and water, while MRI-PDFF uses different resonance frequencies of water and fat protons to determine the proportion of total hepatic protons bound to fat. The AUROCs for identifying steatosis grades 1, 2, and 3 were 0.99, 0.90, and 0.92, respectively, for MRI-PDFF, which was superior to CAP for quantifying liver steatosis [61]. Furthermore, MRI-PDFF can detect small changes exactly for liver steatosis over time [62]. However, these MRI-based techniques have several limitations, such as an impossibility in the case of coronary artery metal stents, high cost, time-consuming, and limited availability, which are more suitable for research purposes than for clinical practice. Studies were too few to estimate the relationship between the NAFLD severity measured by MRE or MRI-PDFF and CVDs. Because of these above limitations, predicting cardiovascular risk in NAFLD patients measured by MRE or MRI-PDFF is difficult and impractical.
Non-invasive methods include the above imaging measurement and the quantification of biomarkers in serum samples. Current serum biomarkers include models for diagnosing hepatic steatosis (e.g., fatty liver index), grading fibrosis (e.g., NFS), and direct measurements for fibrosis, such as procollagen-Ⅲ N-terminal peptide (PⅢNP). Among these, some are specific for NAFLD (BARD score (body mass index, aspartate aminotransferase-to-alanine aminotransferase ratio, diabetes score) and NFS), whereas some are now suitable for NAFLD patients, such as APRI and FIB-4, initially designed for hepatitis C. These non-invasive scoring systems perform with high negative predictive values (NPVs) but poor positive predictive values (PPVs), suggesting that they might be applied to exclude advanced fibrosis [63, 64, 65]. Non-invasive serum markers are suitable as first-line tools in primary healthcare settings to exclude advanced fibrosis, whereas MRE and FibroScan are more suitable for selecting patients who require liver biopsy in specialized hospitals. Unlike imaging methods and liver biopsy, non-invasive serum markers fulfill the requirements of an optimal method that is low-cost, available, repeatable, and dynamic, which is why non-invasive biomarkers are becoming the ideal surrogate markers for identifying advanced fibrosis.
As a common pathogenesis mechanism of NAFLD and coronary artery disease (CAD), oxidative stress has
gradually been promoted to a position that cannot be ignored. Several reports
have shown that enhanced oxidative stress correlates with coronary artery
disease. Previous study has shown that 8-iso-prostaglandin (PG) F2alpha, a
specific class of isoprostanes produced from arachidonic acid, might be the most
valid marker to assess endogenous oxidative stress [66]. Recent research
predicted the incidence and progression of cardiovascular disease by measuring
urine or serum oxidative metabolite. For example, Schwedhelm et al. [67]
introduced urinary 8-iso-PG F2alpha as a novel marker in addition to known risk
factors of coronary heart disease. It was identified as an independent and
cumulative risk marker of coronary heart disease, together with diabetes,
hypertension, hypercholesterolemia, and elevated C-reactive protein (CRP). Beyond that, plasma levels
of 8-iso-prostaglandinF2
EASL-EASDEASO Clinical Practice Guidelines recommend APRI, NFS, and FIB-4 as part of the diagnostic regimen for ruling out advanced fibrosis and further recommend these serum biomarkers to stratify the risk of liver-related outcomes in NAFLD [71]. Recent studies have further observed that the severity of liver fibrosis assessed by non-invasive scoring systems is associated with the increased risk of liver mortality and cardiac-related outcomes (Table 2, Ref. [7, 8, 48, 49, 72, 73, 74, 75, 76, 77, 78, 79, 80]).
| Authors | Region | Total cases | Non-invasive methods | Outcomes | OR (95% CI) | HR (95% CI) |
| Chen Q et al. [49] | China | 3265 | NFS, FIB-4, APRI, GPR, Forns score | Cardiovascular mortality | - | NFS 3.02 (2.05–4.45) |
| FIB-4 3.34 (2.29–4.86) | ||||||
| APRI 1.99 (1.40–2.83) | ||||||
| GPR 1.80 (1.36–2.39) | ||||||
| Forns score 2.43 (1.28–4.61) | ||||||
| Lee SB et al. [7] | Korea | 5121 | NFS, FLI | Coronary atherosclerotic plaques | NFS 1.20 (1.08–1.42) | - |
| FLI 1.37 (1.14–1.65) | ||||||
| Niederseer D et al. [72] | Switzerland | 1956 | NFS | Framingham risk score | 1.30 (1.09–1.54) | - |
| Ishiba H et al. [73] | Japan | 366 | FIB-4 | CAC score | 3.34 (1.16–9.85) | - |
| Lee J et al. [48] | Korea | 1173 | FIB-4, NFS | CAC score | FIB-4 1.70 (1.12–2.58) | - |
| NFS 1.57 (1.02–2.44) | ||||||
| Song DS et al. [74] | Korea | 665 | FIB-4, NFS, APRI, Forns score | CAC score | FIB-4 2.573 (1.147–5.769) | - |
| NFS 3.91 (1.339–11.416) | ||||||
| APRI 2.151 (1.093–4.231) | ||||||
| Forns score 1.536 (0.698–3.383) | ||||||
| Kim D et al. [75] | USA | 11,154 | FIB-4, NFS, APRI | CVD | - | High APRI 2.53 (1.33–4.83) |
| High NFS 3.46 (1.91–6.25) | ||||||
| High FIB-4 2.68 (1.44–4.99) | ||||||
| Kim JH et al. [76] | Korea | 3,011,588 | Fatty liver index | MI | - | 2.16 (2.01–2.31) |
| Baratta F et al. [77] | Italy | 898 | NFS, FIB-4 | CVD | - | NFS 2.29 (1.17–4.47) |
| FIB-4 4.57 (1.61–12.98) | ||||||
| Lee CH et al. [78] | Korea | 3,003,068 | FLI | MI | - | 1 FLI points 1.21 (1.14,1.29) |
| 2 FLI points 1.26 (1.17,1.35) | ||||||
| 3 FLI points 1.22 (1.13,1.32) | ||||||
| 4 FLI points 1.30 (1.21,1.40) | ||||||
| Chung GE et al. [79] | Korea | 5,324,410 | FLI | MI | - | FLI 30–59 vs. FLI |
| FLI | ||||||
| Park J et al. [8] | Korea | 786,184 | BARD score | HF | - | Incident HF 1.12 (1.04–1.20) |
| Hospitalized HF 1.20 (1.07–1.35) | ||||||
| Han B et al. [80] | Korea | 7,958,538 | FLI | HF | - | FLI 30–60 vs. FLI |
| FLI |
NFS, non-alcoholic fatty liver score; FIB-4, fibrosis-4 score; ARPI, aspartate transaminase-to-platelet ratio index; GPR, gamma-glutamyltransferase-to-platelet ratio; FLI, fatty liver index; CAC, coronary artery calcium; MI, myocardial infarction; HF, heart failure; OR, odds ratio; HR, hazard ratio; CI, confidence interval; NAFLD, non-alcoholic fatty liver disease; CVDs, cardiovascular diseases; BARD score, body mass index, aspartate aminotransferase-to-alanine aminotransferase ratio, diabetes score.
Advanced liver fibrosis stage, assessed by NFS and FIB-4, was associated with a
high risk of coronary artery calcification (CAC) progression in NAFLD patients
[48]. A prospective observational study over a median follow-up time of 41.4
months demonstrated that NAFLD patients with liver fibrosis identified by FIB-4
and NFS had a 4-fold increase in cardiovascular risk [77]. Another observational
study involving 12,380 NAFLD patients concluded that patients with a FIB-4 score
Unfortunately, the optimal cut-offs for non-invasive serum biomarkers in ruling
out (e.g., FIB-4
According to the current research, NAFLD patients with high fibrosis scores should be considered at high risk of developing CVDs. Early intervention, such as lifestyle modifications and drug therapy, can reverse the pathological state of NAFLD and reduce cardiovascular risk. Non-invasive serum biomarkers seem to be the optimal method for detecting cardiovascular risks, stratification, and evaluating therapeutic response after interventions. However, lacking routine screening for cardiovascular risks among NAFLD patients with advanced fibrosis might be the barrier. Integrating hepatic fibrosis screening for CVD risk stratification with the application of non-invasive biomarkers seems feasible but warrants further research and analysis. The next important step is to figure out highly sensitive and specific biomarkers that identify patients at high risk of cardiovascular events, monitor disease progression, and evaluate therapeutic response after intervention. A large number of research studies ought to investigate further whether different thresholds of fibrosis markers should be implemented in different cardiovascular diseases. The aim of this review was not to prove that imaging methods can be replaced by non-invasive biomarkers to stratify NAFLD progression but rather to highlight that non-invasive tests, as easily accessible, inexpensive, and repeatable clinical assessments, can be beneficial for physicians to identify NAFLD patients with high cardiovascular risks. Once these fibrosis scores increase, further cardiovascular risk stratification can be conducted to determine whether appropriate treatment is needed. Overall, emerging evidence emphasizes the added prognostic value of non-invasive serum markers regarding CVDs in patients with NAFLD, which have clinical implications regarding the need for CVD screening, risk stratification, and intervention in NAFLD patients with increased serum biomarkers. Further cohort studies should focus on whether improving advanced fibrosis assessed by serum biomarkers can reduce cardiovascular risks.
Among the several fibrosis biomarkers, the specific marker that is more effective in assessing NAFLD fibrosis has yet to be determined. Sun et al. [89] demonstrated that the FIB-4 index with a 1.30 cut-off has better diagnostic accuracy than the FIB-4 index with a 3.25 cut-off, NFS, and BARD score. However, a retrospective, multicenter cohort study of 320 patients suggested that the NAFLD fibrosis that score appears to be the best indicator of patients at cardiovascular risk [90]. Moreover, some researchers recommend diagnostic accuracy can be improved by combining serum biomarkers or even constructing a combined model of serum markers and imaging, which has a higher predictive efficiency [91].
Regrettably, the relationship between sarcopenia, NAFLD, and CVD is not mentioned in the review, firstly because of the lack of a generally accepted definition and the difficulty in adopting common diagnostic criteria. Secondly, such an analysis is beyond the scope of this review, which focuses on discussing the role of non-invasive fibrosis markers in detecting CVD risks in NAFLD patients. Hence, further research is needed to confirm the correlation between these diseases.
Cardiovascular events are now considered the primary cause of death in NAFLD patients, which are significantly associated with NAFLD independent of recognized risk factors. It seems that the increase in cardiovascular risks parallels the progression of NAFLD. In addition to serum fibrosis biomarkers, many studies have explored the role of serum/urine oxidative stress markers in predicting cardiovascular risks. Unlike liver biopsy and imaging methods, non-invasive biomarkers have certain advantages in detecting cardiovascular risks due to the characteristics of being easily available, cheap, repeatable, and dynamic. Non-invasive serum tests are expected to be the first-line tools in CVD screening and risk stratification for NAFLD patients. Future research is needed to establish the corresponding cut-off values for specific CVDs and determine whether improved advanced fibrosis evaluated by fibrosis serum markers reduces cardiovascular risks.
LZC performed the review of the literature and wrote the original draft. XBC and XBJ had the idea for the paper, reviewed and edited it critically for important intellectual content, YC, XC and YCX were responsible for performing visualization, the literature search and curating data, and critically revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors have read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
The figure in this article was created in https://www.biorender.com/.
This research was supported by the Elite Scientists Sponsorship Program by the First Affiliated Hospital of Shantou University Medical College (NO.YCTJ-2023-7).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.