1 Department of Cardiology, Washington DC Veterans Affairs Medical Center, Washington, DC 20422, USA
2 School of Medicine and Health Sciences, George Washington University, Washington, DC 20052, USA
3 Department of Kinesiology and Health, School of Arts and Sciences, Rutgers University, New Brunswick, NJ 08901, USA
Abstract
Chronic and intense exercise programs lead to cardiac adaptations, followed by
increased left ventricular wall thickness and cavity diameter, at times meeting
the criteria for left ventricular hypertrophy (LVH), commonly referred to as
“athlete’s heart”. Recent studies have also reported that extremely
vigorous exercise practices have been associated with heightened left ventricular
trabeculation extent, fulfilling noncompaction cardiomyopathy criteria, as part
of exercise-induced structural adaptation. These changes are specific to the
exercise type, intensity, duration, and volume and workload demands imposed on
the myocardium. They are considered physiologic adaptations not associated with a
negative prognosis. Conversely, hypertrophic cardiac adaptations resulting from
chronic elevations in blood pressure (BP) or chronic volume overload due to
valvular regurgitation, lead to compromised cardiac function, increased
cardiovascular events, and even death. In younger athletes, hypertrophic
cardiomyopathy (HCM) is the usual cause of non-traumatic, exercise-triggered
sudden cardiac death. Thus, an extended cardiac examination should be performed,
to differentiate between HCM and non-pathological exercise-related LVH or
athlete’s heart. The exercise-related cardiac structural and functional
adaptations are normal physiologic responses designed to accommodate the
increased workload imposed by exercise. Thus, we propose that such adaptations
are defined as “eutrophic” hypertrophy and that LVH is reserved for pathologic
cardiac adaptations. Systolic BP during daily activities may be the strongest
predictor of cardiac adaptations. The metabolic demand of most daily activities
is approximately 3–5 metabolic equivalents (METs) (1 MET = 3.5 mL of O
Keywords
- exercise
- physical activity
- left ventricular mass
- cardiac structure
- cardiac function
- arrhythmias
- athletes
Chronic exaggerated increases in the hemodynamic load, lead to balancing
responses and changes in cardiac myocytes, typically leading to an increase in
left ventricular mass (LVM) and finally in established left ventricular
hypertrophy (LVH) [1]. Specifically, chronically elevated blood pressure (BP) is
likely to result in increased ventricular wall thickness and left ventricular
mass index [(LVMI), (
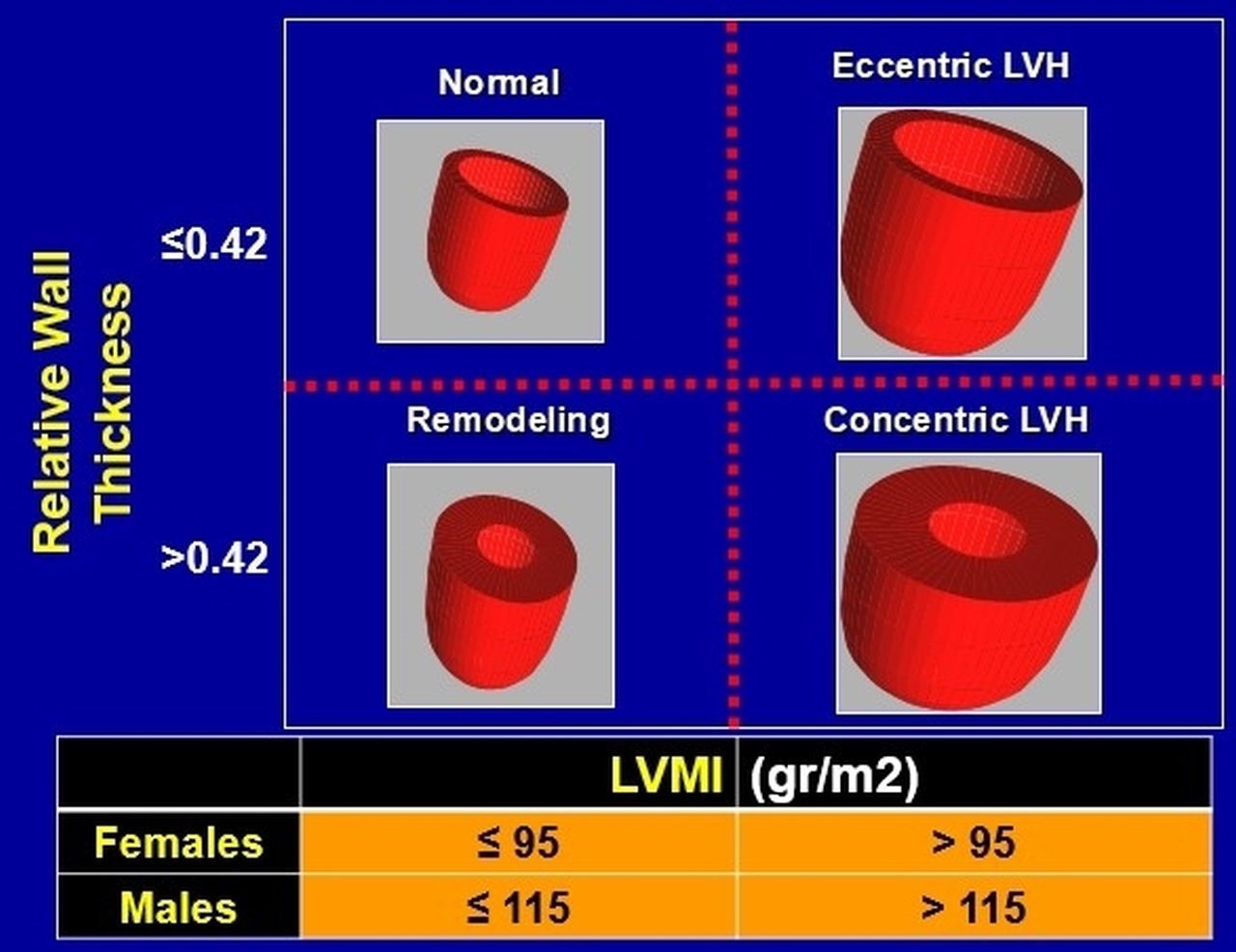
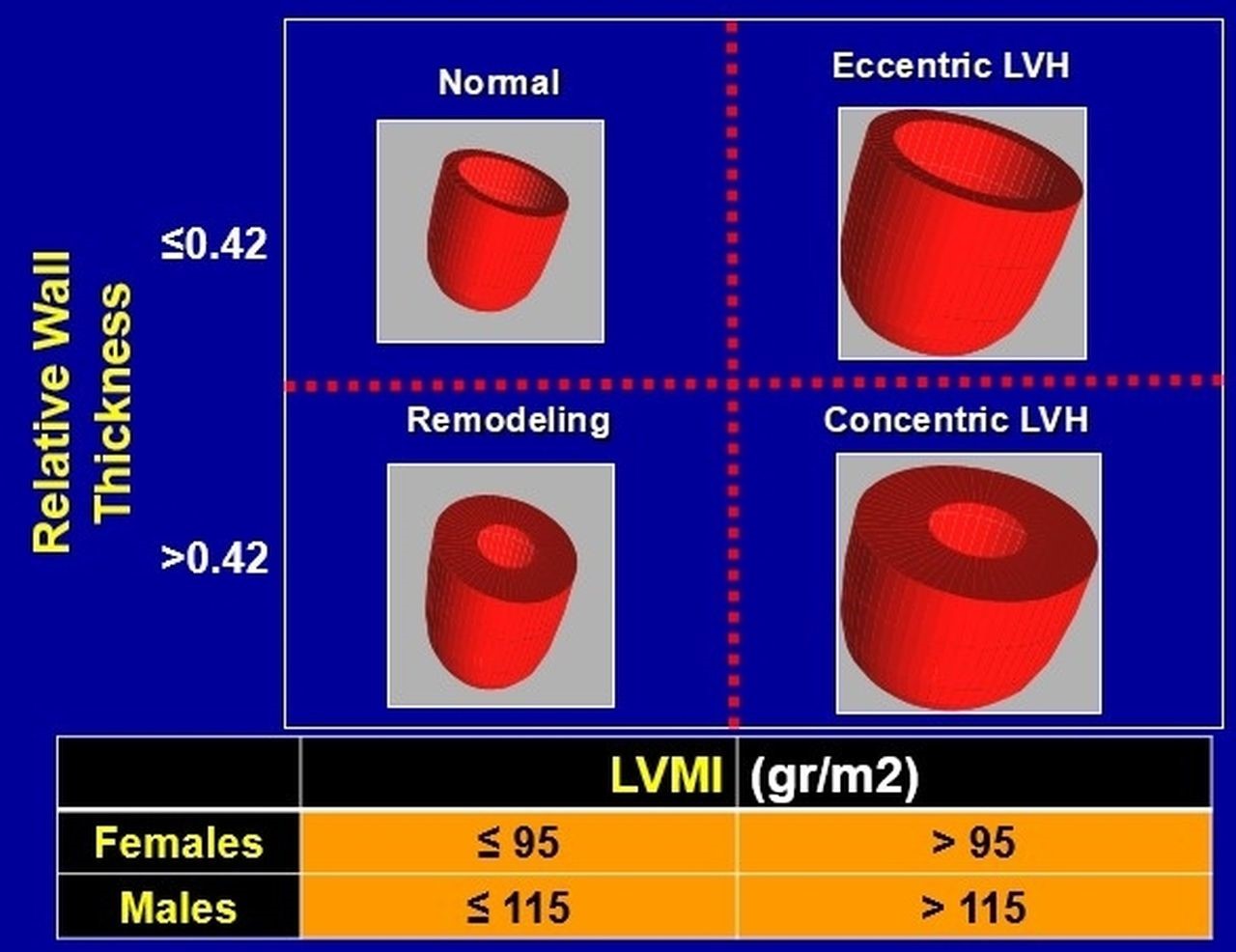 Fig. 1.
Fig. 1.Classification of all left ventricular hypertrophy patterns, based on calculated relative wall thickness and left ventricular mass index (LVMI). LVH, left ventricular hypertrophy; LVMI, left ventricular mass index.
The presence of LVH, especially the concentric geometry pattern is a strong and independent predictor of cardiovascular events and all-cause mortality. The risk of cardiac events, as well as sudden cardiac death, increases three-fold in this population [4, 11, 12]. Conversely, LVH regression, the outcome of resting BP reduction and hypertension control achieved by most antihypertensive medications, is associated with a significant reduction in cardiovascular events and death [13, 14, 15, 16]. The degree of LVH regression is strongly related to the degree of BP reduction, supporting partly the pathophysiologic mechanism of the stimulus role of pressure overload in the development of concentric LVH [17, 18, 19, 20].
Although the exercise-related favorable health outcomes have been described extensively [21] the exercise-related BP management in hypertensive patients with Stage II hypertension is less known. In addition, the impact of exercise training on BP response during physical work or exercise and its association with cardiac structure and function is also poorly understood [22]. In our previous work we have provided evidence indicating that exercise BP response during relatively low workload of 3–5 metabolic equivalents (METs; 1 MET = 3.5 mLof oxygen consumed per kg of body weight per minute) may be a strong indicator of LVH. Furthermore, achieving lower exercise BP at an absolute and relative workload may lead to LVH regression [23, 24]. In this review, we summarize the findings of select studies on the association between exercise and cardiac structural changes and their clinical significance. We also present evidence on the exercise-BP-LVH interaction, and LVH regression associated with proper exercise training.
Temporary sudden or long-standing increases in physical workload or exercise also pose an increased hemodynamic demand on the cardiovascular system. Subsequently, appropriate, acute, and chronic cardiac adaptations occur to accommodate this increased workload.
In 1975, Morganroth and colleagues [25] described 2 distinctly different cardiac
morphological adaptations in athletes, as the outcome of the specific hemodynamic
load imposed on the ventricles during repeated exercise bouts of different
exercise modes. In general, they reported that left ventricular end-diastolic and
cardiac mass were increased in athletes engaging in repetitive isotonic
contractions such as running, and normal wall thickness (
Although this hypothesis has been challenged recently, the general concept that cardiac adaptations reflect the demand imposed the exercise type remains. However, it should be emphasized that most physical activities combine a static and an isotonic component. Thus, cardiac adaptations are more diverse and can consist of concentric and eccentric morphology which can coexist to accommodate the imposed exercise demands on the cardiovascular system [26, 27].
Exercise-related cardiac alterations, including hypertrophy, dilatation,
bradycardia, and arrhythmias coming from chronic physical activity experienced by
athletes have been reported more than two centuries ago, and continue to be of
interest to physicians and scientists. Early cases of an enlarged heart (end of
19th century) were reported in Harvard University rowers [28], elite Nordic
skiers [29], and Boston Marathon runners [30, 31], mostly viewed as useful
modifications in response to exercise [30, 31]. The evolution of the
electrocardiogram (ECG) revealed cardiac hypertrophy abnormalities in the
electrical activity of the heart [32, 33, 34, 35, 36], while advances in echocardiography and
magnetic resonance imaging have led to a better understanding of the athlete’s
heart. An important study on the long-term consequences of endurance
training in Olympic athletes reported no adverse cardiac events during 8.6
As mentioned, exercise-induced anatomical and functional cardiac changes are specific to the type and intensity of the activity. Accordingly, acute hemodynamic cardiovascular responses to these two types of exercise differ markedly. Therefore, long-term exposure to either of them is likely to lead to specific chronic cardiovascular adaptations to accommodate the specific demands imposed on the cardiovascular system by the exercise type or physical work.
Some studies reported that the upper limits of wall thickness resulting from
engaging in aerobic exercise regularly to be
| Left ventricular wall thickness | ||
|---|---|---|
| Gender | Family history | Family history |
| Race | Gender | Gender |
| Exercise type | Exercise type | Exercise type |
| Cardiac function | Symptoms | Symptoms |
| Cardiac function | Cardiac function | |
| Asymmetrical LVH | Asymmetrical LVH | |
| Specific echocardiographic findings | Specific echocardiographic findings | |
| Specific ECG findings | Specific ECG findings | |
| Cardiac Echocardiographic findings | Cardiac Magnetic Resonance Imaging | |
| Detraining LVH regression | Detraining LVH regression | |
| No further evaluation in most cases | Cardiac evaluation in many | Cardiac evaluation in all |
| Left ventricular wall thickness thresholds and need for further cardiac evaluation, based on demographic, clinical and cardiac imaging criteria. LVH, left ventricular hypertrophy; ECG, electrocardiographic. | ||
Female athletes usually have smaller frame, lower lean body mass, different
hormonal profile, and lower peak exercise systolic BP, stroke volume and
VO
In conclusion, vigorous exercise-related chronic cardiac adaptations are considered “physiologic” responses to the specific hemodynamic load imposed to of the particular sport, exercise, or physical activity. These adaptations are not associated with diastolic dysfunction, atrial or ventricular complex arrhythmias, or worse prognosis, conditions observed in hypertension-related LVH [7, 8]. Additionally, there is evidence that exercise-induced LVH regression is observed after 3 or more months of exercise training discontinuation [46, 50], further supporting the concept that the cardiac adaptations are in response to an increased workload and not pathological.
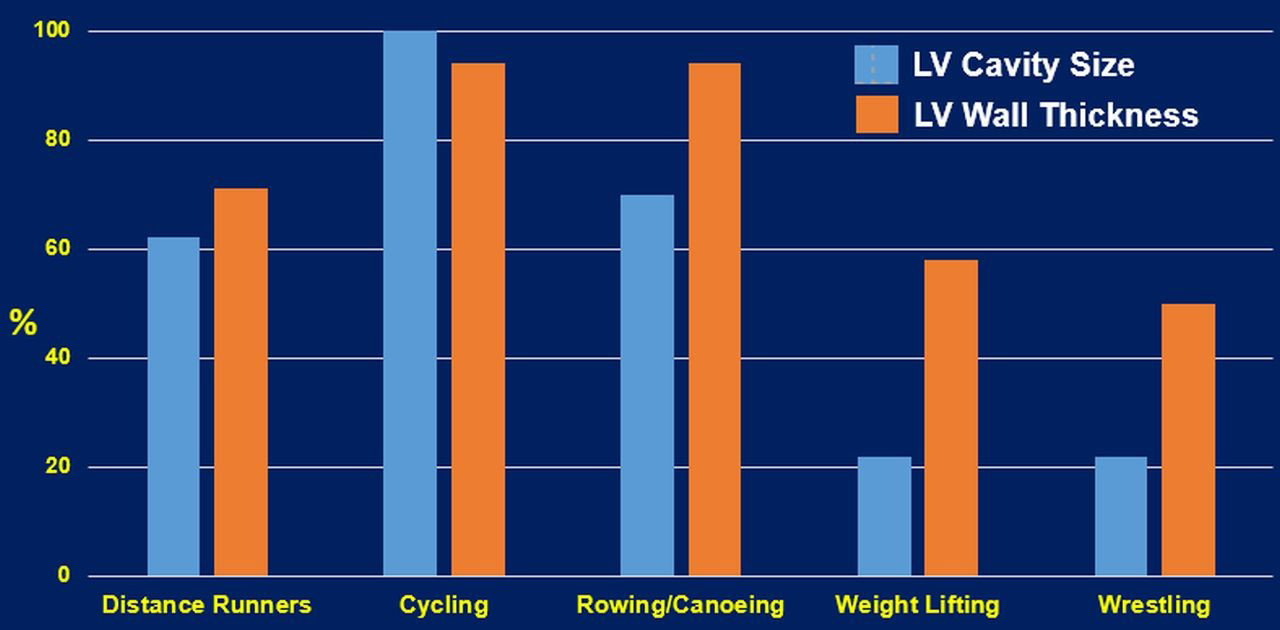
Resistance exercises are typically accompanied by increased ventricular wall thicknesses, asymmetrically to chamber inner dimensions. Whether the concentric LVH is induced by resistance training alone is debatable [32]. Most sports or daily activities contain both aerobic and anaerobic types of exercises. Therefore, cardiovascular adaptations are likely to reflect the combined types of the workload of the sport or activity, leading to mixed cardiac remodeling patterns. This is supported by the finding of elite heavy-trained athletes engaging in sports such as cycling, rowing, and swimming, are a typical example of combined both aerobic and resistance exercises. These athletes (Fig. 2) often present the most excessive increase in all left ventricular geometry parameters (wall thickness and cavity dimension) [36]. Finally, it is important to emphasize that an increase in wall thickness or LV diastolic dimension alone should not be considered a favorable physiological adaptation. LV dilatation without concomitant wall hypertrophy will lead to an undesirable increase in wall tension that is detrimental to the heart [50]. Such conditions are usually observed in patients with chronic heart failure.
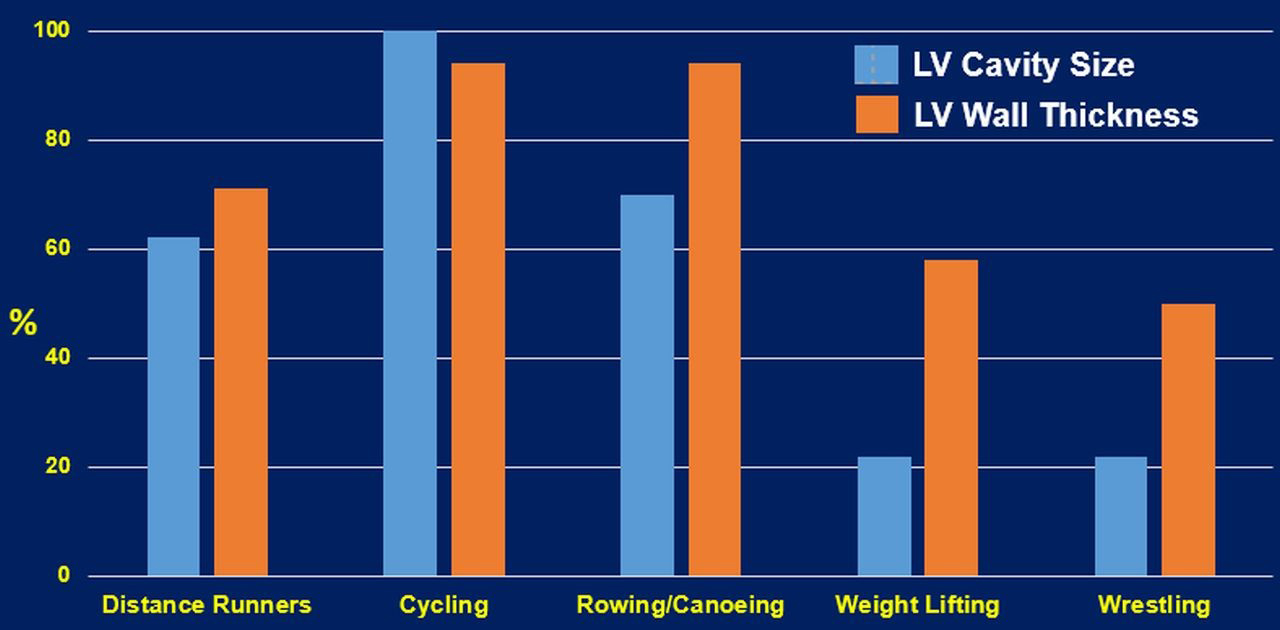 Fig. 2.
Fig. 2.Cardiac structural changes of select sports that represent aerobic, resistance and the combination of the two exercise types [27]. LV, left ventricular.
There is no consensus as to whether adaptations observed in the cardiac architecture and function in athletes are considered favorable or at least benign to the cardiovascular system or pathological changes favoring increased risk of arrhythmias. Provocation of exercise-related complex arrhythmias in certain situations [50] and a higher incidence of atrial fibrillation (AF) in middle-aged and older heavy-trained athletes engaged in chronic high-intensity exercises, as compared to non-athletes have been reported [57, 58, 59, 60, 61, 62, 63]. This relationship does not appear to be directly related to the amount of exercise-related physiologic LVH [49, 64]. It is more directly related to intensity as well as the duration of strenuous exercises [57, 58, 61, 63].
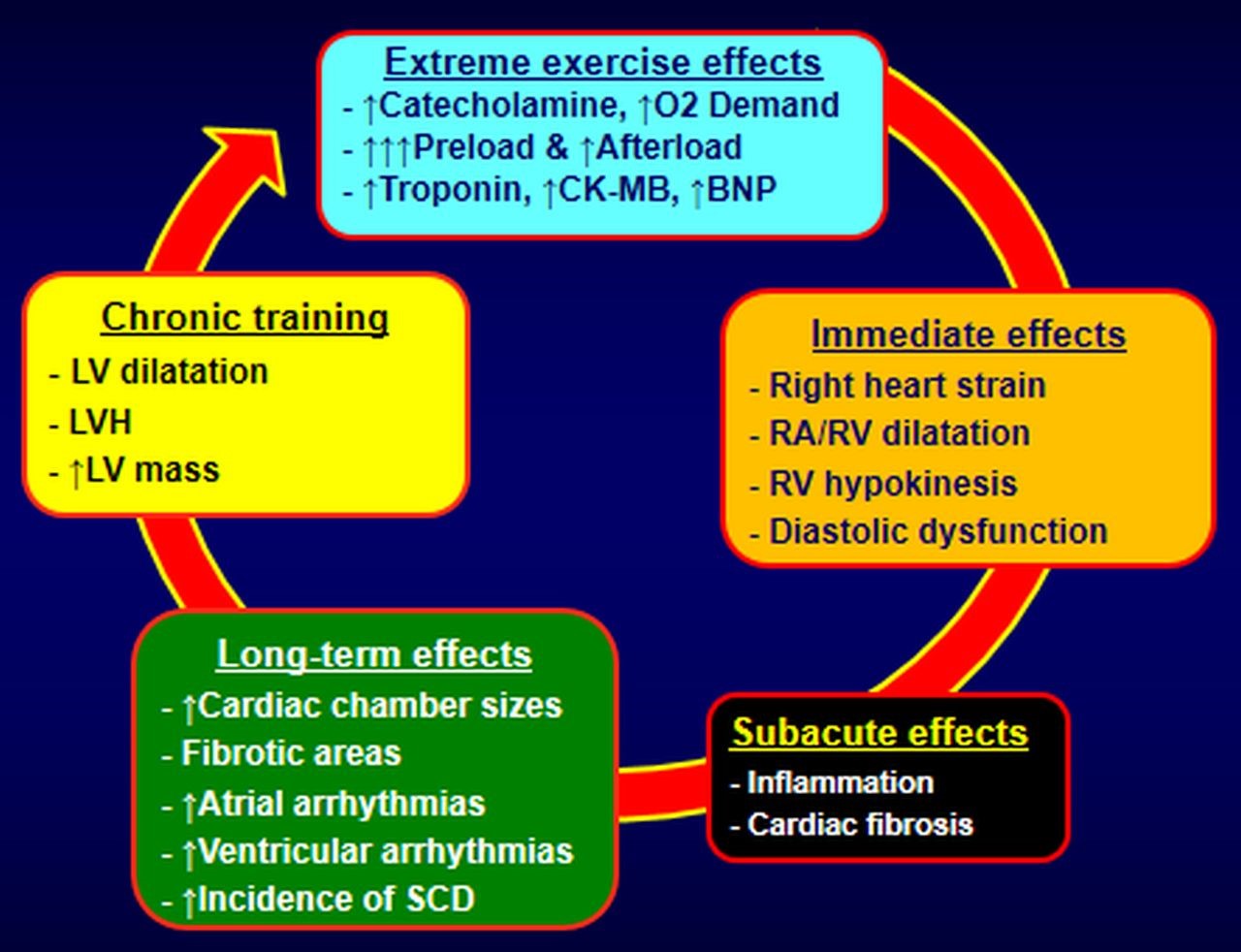
Whether exercise-related atrial arrhythmogenicity and chaotic ectopy from the pulmonary veins, are the main cause of atrial arrhythmias, or whether other pathophysiologic mechanisms are responsible has not been determined. A survey of elite cyclists did not document increased atrial ectopy [65]. Additionally, increased vagal tone is observed in many endurance athletes [60], leading to bradycardia and reduced atrial refractory period, major modulators of heart rhythm, triggering re-entry arrhythmias. It is well-accepted that the pressure in the pulmonary arteries increases during exercise [66]. During intense exercise, higher pressures typically notable in athletes, are measured in the right cardiac chambers, with a continuing decline in right ventricular ejection fraction as the duration of vigorous exercise increases [67]. In case of long-term intense exercise stress and without reasonable recovery time, dilatation of the less-muscular chambers (atria and right ventricle) is observed, leading to some degree of inflammation, minor injury, and fibrotic lesions. These events are the usual pathophysiologic suspects for electrical instability and cardiac complex arrhythmias [68]. The presence of an exercise-related arrhythmogenic right ventricular cardiomyopathy has been the subject of considerable debate in some studies [66, 69]. Finally, another study in elite athletes [70] observed no relationship between ventricular ectopy and the magnitude of exercise induced LVH, indicating the benign nature of ventricular ectopy and the expression of athlete’s heart. Although the proposed pathogenic pathways for the development of exercise-related cardiomyopathy, summarized in Fig. 3 are noteworthy, no established pathophysiologic mechanism exists to explain any relationship between high exercise intensity and risk of AF.
 Fig. 3.
Fig. 3.Proposed pathophysiologic mechanisms of cardiomyopathy and arrhythmias in endurance athletes. LVH, left ventricular hypertrophy; LV, left ventricular; CK-MB, creatine kinase-myocardial band; BNP, B-Type natriuretic peptide; RA/RV, right atrium/right ventricle; SCD, sudden cardiac death.
Nevertheless, such arrhythmias rarely have been associated with adverse cardiac events and are usually disappeared or to a large degree reduced after reasonably short periods of deconditioning [49].
We examined the role of cardiorespiratory fitness (CRF) on new-onset AF in 5962 middle-aged and older Veterans. Our findings showed an inverse and graded relationship between CRF and AF risk. The AF incidence was 21% lower for each 1-MET increase in exercise capacity. Compared with the least fit individuals, the AF-risk was 20%, 45%, and 63% lower for moderately fit, fit, and highly fit individuals, respectively [71]. Recently we reported comparable findings (ACC 2022-Highlights) in a large cohort of 459,592 hypertensive veterans, showing an independent, inverse, and graded association between CRF and AF-risk [72]. Compared with the least fit (4.5 METs) hypertensives (reference group), the AF-risk was 39% lower for moderately fit (8.3 METs), 48% for fit (10.3 METs), and 55% for highly fit (13.1 METs). Similar findings were observed in both, younger and older than 65 years, suggesting that CRF achieved by moderate or even high-intensity exercise programs, protects from the risk of new onset AF, regardless of age.
Relatively small reductions in BP achieved by antihypertensive therapy (even 5 mmHg), lead to impressive beneficial effect, including mortality and morbidity risk reduction [73, 74]. LVH regression comparable to the degree of BP reduction [17, 18, 19, 20] is responsible in part for the health benefits [13, 14, 15, 16]. Several well-controlled studies [22, 75, 76, 77, 78] have shown significant exercise-related BP reduction in both systolic and diastolic BP (4–10 mmHg and 3–8 mmHg respectively), independent of age, gender, and weight loss. Therefore, it is reasonable to assume that similar exercise-related reductions in BP should yield similar health outcomes, including the effects of exercise-induced LVH regression. This assumption is supported by the findings of large and well-designed epidemiologic studies that have shown a significant, inverse, and graded relationship between CRF (expressed by exercise capacity), and mortality risk in hypertensive and pre-hypertensive individuals [21, 79, 80, 81, 82, 83].
LVH regression resulting from exercise-related BP reduction has not been evaluated extensively. However, most exercise studies in patients with LVH confirm the beneficial effect of lowering BP by exercise, in this phenotype of target organ damage [84, 85, 75, 76, 77, 78].
We studied 46 men with resistant hypertension under multi-drug therapy (57
Contrary to these findings, in a study including 23 obese individuals with
high-normal BP, no LVM regression was reported, regardless of the notable
reduction in BP [89]. There were also no beneficial effects on cardiac geometry
or LV diastolic function, in 51 overweight and obese individuals (BMI: 29.5
It is reasonable to assume that exercise training or other lifestyle changes (as complementary “interventional therapy”), will not “repair” what is not damaged. The exercise groups were engaged in a combination of both muscle strength and aerobic training, with different long-term cardiac reactions and structural alterations, leading often to undesirable impaired cardiac geometry and progression to LVH [38]. Overall, despite the small body of knowledge and poor quality of data on exercise-related LV structural adaptations, LVH regression is achieved (mostly in individuals with LVH), by engaging in an appropriate type of aerobic exercise training.
The current evidence supports a strong, and direct association between the grade
of LVH regression and the level of BP reduction with antihypertensive drugs
[17, 18, 19, 20]. A meta-analysis of 4 echocardiographic studies, following 1064
hypertensive individuals for 3–10 years (45–51 years of age, 59% men), noted
only 8% LVH regression with parallel cardiovascular risk reduction [19]. Another
meta-analysis including 80 trials, with 3767 patients in the treatment group and
346 patients in the placebo group, showed a wide spectrum of LVM reduction among
antihypertensive agents (6%–13%). Specifically, the greater LVM reduction was
induced by renin-angiotensin-aldosterone-system (RAAS) inhibitors and calcium
channel blockers (10%–13%), while a smaller reduction was seen by beta-blockers
(6%) and diuretics (8%) [92]. The degree of exercise-related LVH regression is
analogous to what has been reported by most antihypertensive drugs [93].
Additionally, the observed exercise-related reduction in BP was significantly
lower (average 7/5 mmHg in our studies and similar in others) [22, 75, 76, 77, 78],
compared with the overall drug-induced BP reduction (26.6/16.6 mmHg) [89].
Despite the significant BP difference, a 12.3% reduction in LVMI has been
reported by exercise studies, which is comparable to the reduction obtained by
RAAS blockers (13% with angiotensin receptor blockers (ARBs) and 10% with angiotensin-converting enzyme (ACE)-inhibitors), and clearly greater
than that realized by beta-blockers (6%) [92]. The BP reduction in the
drug-related LVH regression studies, was roughly 13%, representing 1% LVMI
regression for every 1% reduction in BP. In our study [22], supervised aerobic
exercise training by stationary bicycle, reduced systolic BP by 5% and LVMI by
12.3%, a 2.5% LVMI reduction per 1% reduction in BP. In subsequent studies,
our findings suggest that the degree of LVH regression may not be the sole
outcome of the exercise-related reduction in resting BP but also the lower
exercise systolic BP response at absolute workloads. Specifically, we noted that
the systolic exercise BP following 16 weeks of aerobic training was 14% systolic
BP reduction at 3 METs, 15% at 5 METs and 9% at peak exercise [22, 94, 95].
Interestingly, the metabolic demand of most daily activities, is equivalent to
3–5 METs [27, 28]. These findings suggest that the hemodynamic load was reduced
during daily activities. Additionally, the daily hemodynamic load may be an
important predictor of LVH regression, stronger than the resting BP. This
hypothesis is supported further by our findings, in 790 prehypertensive men and
women (BP
| Sensitivity | Specificity | |
| Exercise SBP |
88% | 74% |
| Daytime ABP |
85% | 73% |
| ECG | 6%–53% | 89%–100% |
| Exercise SBP and Daytime ABP at 5 METs provide a better sensitivity, and comparable specificity for prediction of LVH development. SBP, systolic blood pressure; ABP, ambulatory blood pressure; METs, metabolic equivalents; LVH, left ventricular hypertrophy; ECG, electrocardiogram. | ||
Collectively, these findings indicate that aerobic exercise training lowers the exercise systolic BP response during submaximal and peak workloads. The clinical significance of this is that lower BP during daily activities, leads to lower hemodynamic loads daily, subsequently reducing the impetus for LVH development or LVH progression.
The chronic adaptations of vigorous exercise in competitive (basketball, soccer, football, etc.) and non-competitive athletics (marathon, cycling, weightlifting, etc.) on cardiac structure and function in hypertensives with LVH, are of limited knowledge. Possibly high-intensity exercises impose an exaggerated demand on the cardiovascular system, prolong further abnormal changes, and therefore, are not recommended. Alternatively, all scientific societies recommend a “prescription” of low-to-moderate intensity aerobic exercise training (brisk military walk) of approximately 30–45 minutes per day, 5 days per week, as part of global management [65, 96, 97, 98]. Such an exercise program is safe and feasible for a wide range of ages and hypertensives with co-morbidities [87] and has been shown to have a protective effect on the major cardiovascular risk factors [75], including LVH regression [22].
Long-term exposure to exercise programs of proper intensity, duration, and volume increases the hemodynamic and cardiac workload. To accommodate this increased demand on the myocardium, cardiovascular adaptations that include increased LV cavity size and LV wall thickness or both ensue. These adaptations are specific to the cardiac demand imposed by the exercise type, intensity, and volume. LV wall thickness observed with prolonged exercises usually does not exceed 13 mm. However, left ventricular wall thickness as high as 16 mm has been reported in some highly trained athletes engaging in extreme exercise practices such as ultramarathon running or a combination of vigorous aerobic and isometric/resistance exercise programs. These adaptations are relatively benign in the absence of hypertrophic cardiomyopathy (HCM) or other cardiac malformations. In these athletes, the clinical distinction between exercise-related “benign” LVH and the presence of HCM, the main reason for sudden cardiac death in apparently healthy athletes, may not be easily discernable. Thus, we strongly recommend that the individuals are examined by a cardiologist with experience in this sports cardiology.
Evidence supports that exercise systolic BP response
Exercise-related cardiac structural changes that lead to improved cardiac function are physiological and necessary to meet the increased demand posed by exercise. Thus, the exercise-related LVH may be considered as “eutrophic” LVH and reserve “hypertrophic” cardiac adaptations solely those imposed by pathophysiologic mechanisms (hypertension, cardiac injury-fibrosis, HCM) and intrude upon cardiac function, usually leading to “malignant” LVH, cardiac dysfunction, complex arrhythmias and even death.
AP—Conceptualized the content of the manuscript and took the lead role in the writing of the manuscript. CF and MD—Assisted in the writing of the manuscript, made substantial contributions to conception and design, and additionally to analysis and interpretation of data. CG—Assisted in the writing and editing of the manuscript, made substantial contributions to conception and design, and additionally to analysis and interpretation of data. PK—Conceptualized the content of the manuscript and directed the writing and editing of the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Peter Kokkinos is serving as one of the Editorial Board members and Guest Editors of this journal. We declare that Peter Kokkinos had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Hirofumi Tanaka.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.