1 Department of Cardiology, Hebei Yanda Hospital, 065201 Langfang, Hebei, China
2 Department of Hematology, Hebei Yanda Lu Daopei Hospital, 065201 Langfang, Hebei, China
3 Heart Center & Beijing Key Laboratory of Hypertension, Beijing Chaoyang Hospital, Capital Medical University, 100016 Beijing, China
Abstract
Cardiovascular disease (CVD) remains one of the primary causes of morbidity and mortality worldwide. Classic cardiovascular risk factors, such as hypertension, diabetes mellitus (DM), hyperlipidemia, and smoking, have been well identified and given increased attention in clinical practice. However, the incidence and prevalence of CVD remains high, especially in developing countries. Therefore, there has been more attention to non-traditional CVD risk factors such as gut microbiota, sleep disorders, dietary structure, and psychosocial factors in their important roles in the development of CVD. In this review we summarize the association of non-traditional risk factors with CVD with the aim of further reducing the risk of CVD.
Keywords
- cardiovascular disease
- cardiovascular risk
- gut microbiota
- sleep disorder
- psychosocial factors
- Vitamin D deficiency
- environmental exposure
- hyperhomocysteinemia
- hyperuricemia
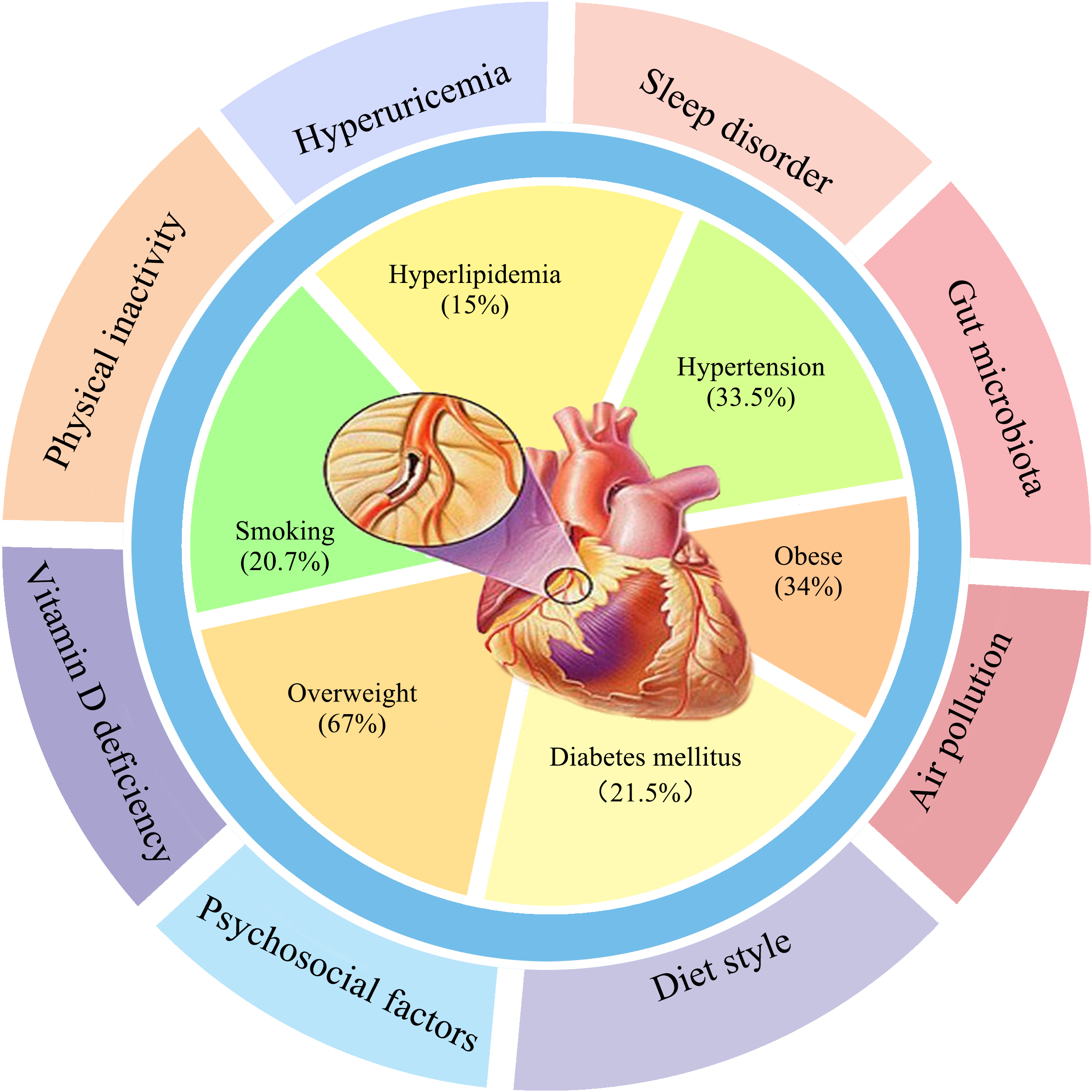
Cardiovascular disease (CVD) remains a major public health issue and one of the primary causes of morbidity and mortality worldwide [1]. The prevalence of well-known modifiable cardiovascular risk factors include hypertension (33.5%), smoking (20.7%), hyperlipidemia (15%), diabetes mellitus (21.5%), overweight (67%) and obesity (34%) [2, 3] (Fig. 1). Age and male sex are traditional non-modifiable risk factors. The multicenter case-control INTERHEART study showed that common risk factors accounted for 90% of the population attributable risks (PAR) in men and 94% in women with myocardial infarction, and included smoking (PAR 35.7%), increased apolipoprotein B/apolipoprotein A1 (ApoB/ApoA1) ratio (PAR 49.2%), hypertension (PAR 17.9%), diabetes (PAR 9.9%), and abdominal obesity (PAR 20.1%) [4]. However, these traditional risk factors by themselves cannot explain the increased incidence of CVD. It is known that 25% of patients with premature CVD and 12% of patients with an acute myocardial infarction, do not have any established risk factors [5, 6].
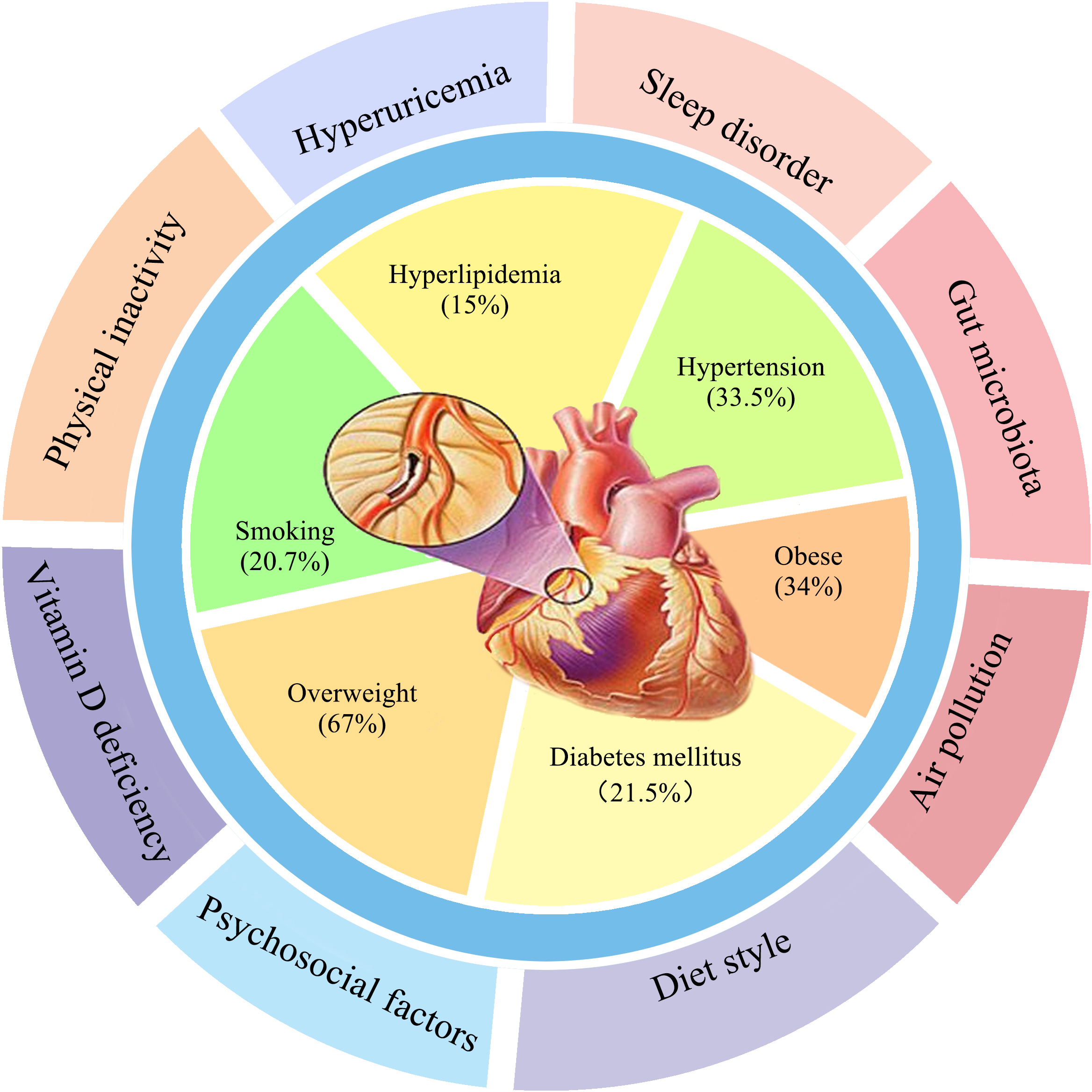 Fig. 1.
Fig. 1.The first circle is traditional CVD factors, and the second circle is new non-traditional CVD risk factors.
Despite great efforts to control these conventional risk factors, there are
still residual cardiovascular risks. These may be largely due to
“non-traditional” cardiovascular disease factors, which have been identified
based on studies of the pathogenesis of atherosclerosis and atherothrombotic
cardiovascular events since 1999 [7]. These risk factors include chronic
inflammation and its markers, such as C-reactive protein, oxidative stress and
endothelial dysfunction; lipoprotein (a) [Lp(a)]; psychosocial factors, such as
environmental stress and responsiveness to stress; plasma insulin levels and
markers of insulin resistance; and activation of the renin-angiotensin system
[7]. However, the strength of the associations of these risk factors with CVD
events and therapy still need to be defined [7]. The 2018 US Preventive Services
Task Force studies of asymptomatic adults with no known cardiovascular disease,
found that non-traditional CVD risk factors such as ankle brachial index (ABI),
high-sensitivity C-reactive protein (hsCRP) levels, and coronary artery calcium
(CAC) scores, were not independent factors for CVD [8]. Recently, Whayne
et al. [9] summarized the non-Traditional CVD risk markers as
ApoA, ApoB, hsCRP, homocysteine, interleukin 1 (IL1), Lp(a), the density of
low-density lipoprotein (LDL) particles, the LDL particle number, tissue/tumor
necrosis factor-
The traditional risk factors refer to the risk factors that are well proven and
have a clear relationship with CVD, such as hypertension, diabetes, dyslipidemia,
obesity, and smoking. The non-traditional risks discussed in this article include
relatively common “new” risk factors, such as gut microbiota, vitamin D
deficiency, and lack of exercise (Fig. 1). Non-traditional risk factors
with unclear relationships, such as COVID-19, and rare non-traditional risk
factors such as poly genomic scores, pregnancy related complications, IL1, and
TNF-
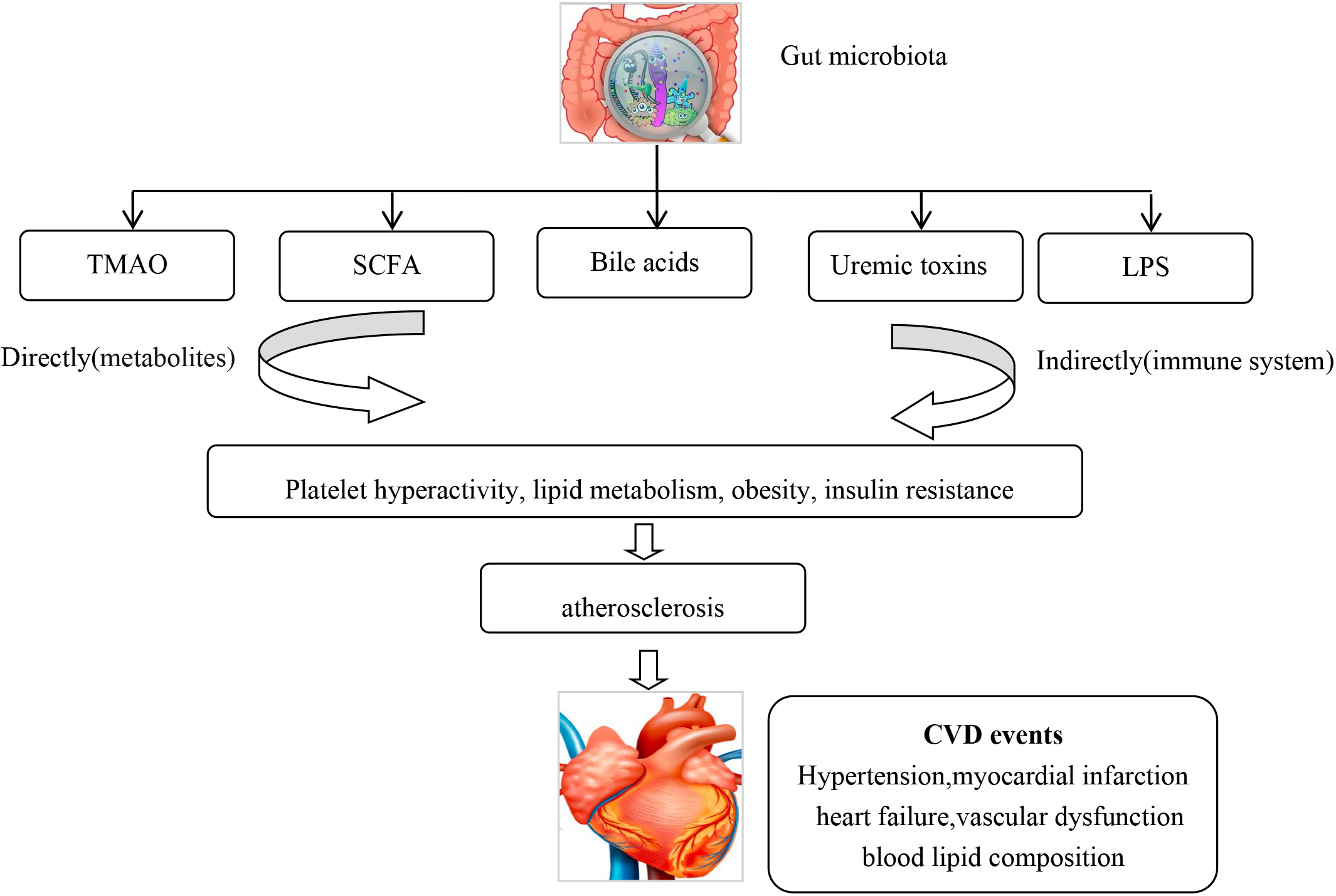
The human gut is inhabited with approximately 100 trillion bacteria, which can modulate both physiology and body metabolism [10]. Emerging evidence has showed an association of gut microbiota and their metabolites with CVD. Asymptomatic and symptomatic atherosclerotic plaques contain different pathogenic microbiomes [11], such as Pseudomonas, Streptococcus and Chlamydia pneumoniae [12, 13, 14]. Studies have found that specific gut microbial species are associated with inflammation and atherosclerosis [15], hypertension and vascular dysfunction [16, 17], symptomatic stroke and transient ischemic attack [18], heart failure [19] and blood lipid composition [20]. Pathogenic bacteria can make vessel walls more vulnerable to atherosclerotic plaque formation, either by infection or indirectly by an auto-immune inflammatory reaction [21, 22]. Gut microbiome was associated with an increased incidence of acute myocardial infarction (AMI) in animal models [23]. Gut microbiota can generate trimethylamine N-oxide (TMAO) [24], short chain fatty acids (SCFA) [25], uremic toxins [26], bile acids [27], and lipopolysaccharides (LPS) [28], which can alter the hosts metabolism. TMAO increases CVD risk by affecting platelet hyperactivity, lipid metabolism, obesity, and insulin resistance [29, 30]. TMAO and phenylacetylglutamine (PAGln) have been shown to induce platelet hyper-reactivity and increase thrombotic formation [29]. TMAO has been associated with an increased incidence of CVD and could predict the risk for myocardial infarction (MI), stroke or death even after adjustment for traditional CVD risk factors [30, 31]. TMAO has also been associated with an increased risk of atrial fibrillation [32]. SCFAs regulate blood pressure by the angiotensin-renin system pathway [33, 34]. Lipopolysaccharide-binding protein (LBP) levels have been found to be significantly higher in coronary artery disease (CAD) patients and was shown to be an independently biomarker for total and CVD related mortality [35]. A cohort study on stools from 218 patients with atherosclerotic cardiovascular disease (ACSVD) and 187 healthy controls demonstrated that gut microbiome in ASCVD patients had an increased abundance of Enterobacteriaceae and Streptococcus spp. [36]. CVD and diabetic medication may also effect gut microbiota, which may alter the absorption of these drugs, especially lipid lowering and diabetic medications [37, 38, 39] (Fig. 2).
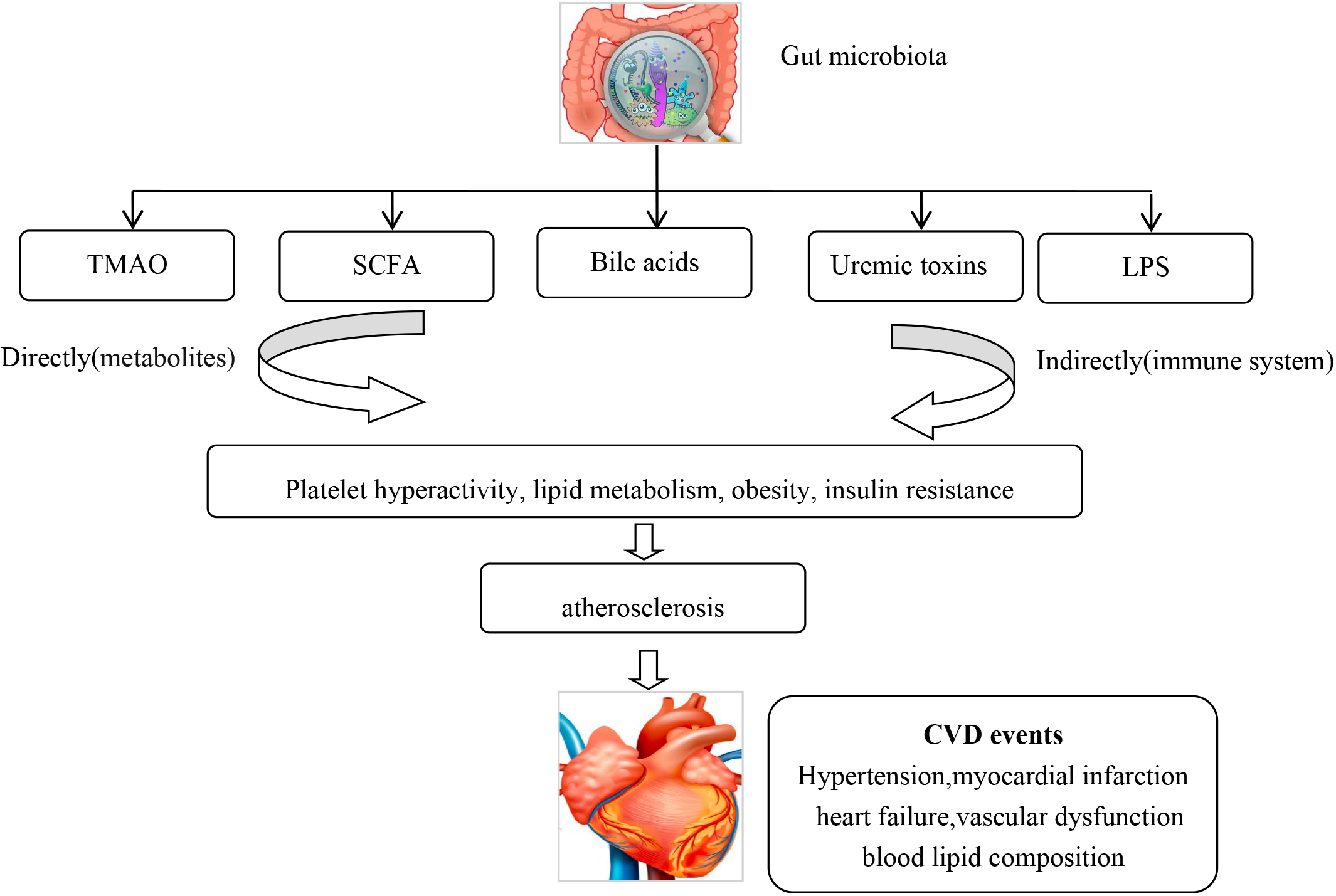 Fig. 2.
Fig. 2.The gut microbiota can via metabolites and the immune system lead to CVD. CVD, Cardiovascular disease.
Age, sex, ethnicity, diet, and lipid levels may also affect gut microbiota and add to the difficulty in determining the role of gut microbiota in CVD [40]. Although accumulated evidence shows gut microbiota and their metabolites play a vital a role in inflammation and CVD [41], a recent meta-analysis found that antibiotic treatment had no beneficial effect on the risk of CVD [42]. Further work is required to establish the role of gut microbiota in preventative and therapeutic regimes to reduce the risk of CVD.
Physical inactivity is an import risk factor for numerous metabolic diseases. About 1.9 million deaths annually are estimated to be due to physical inactivity [43] and $117 billion healthcare dollars annually are spent on diseases attributed to inadequate physical activity [44]. The risk of CVD increases in individuals with physical inactivity and a sedentary lifestyle [45]. It has been estimated that physical inactivity is responsible for 6% of CVD [46]. There is evidence for a relationship between increased physical activity and a lower incidence of CVD and mortality, conversely greater amounts of sedentary behavior are linked to an increased risk of CVD and mortality [44]. Sedentary behavior in CVD patients increases the risk of depression and leads to insufficient physical activity [47]. A meta-analysis showed that reducing sedentary behaviors result in an increase in life expectancy [48]. A study examining sitting time and mortality in 17,013 Canadians showed a dose-response relationship between sitting time and all-cause mortality and CVD. There was a 54% increased risk of CVD mortality among adults with sitting “almost all of the time” compared to those with sitting “almost none of the time” [49]. Conversely, a meta-analysis including 160 randomized controlled trials with 7487 participants revealed that exercise could lower fasting insulin, triglycerides, Glycosylated Hemoglobin-type A1C (HbA1c), leptin, fibrinogen, and angiotensin II, and raised high-density lipoprotein cholesterol, apolipoprotein A1, and interleukin-18 [50]. In addition, 17 meta-analyses and one systematic review with 594,129 adults confirmed that physical activity reduces blood pressure on a dose-response relationship among adults with normal range blood pressure, prehypertension, and hypertension [51].
The strong evidence from these studies supports the premise that adults should reduce their sedentary time to less than 9 hours per day, or even less than 6–8 hours per day [52, 53]. Physical activity is one of the most important modifiable risk factors, which can decelerate the atherosclerotic process, control CVD risk factors, such as hypertension, diabetes, and obesity [54]. More than 7000 steps/day or even 10,000 steps/day are recommended for the secondary prevention of CVD to achieve a decrease in blood pressure and BMI [55, 56, 57]. A meta-analysis which included six studies with 693 patients suggested self-monitoring of physical activity by patients with CVD had a significant effect on decreasing the risk of CVD [58]. The 2018 Physical Activity guidelines recommended at least 150–300 minutes per week of moderate intensity aerobic exercise, or 75–150 minutes of vigorous exercise for adults and those exceeded this level by at least 3–5 times continued to show a 40% reduction in CVD mortality [44].
There is a strong correlation between diet and incidence of CVD. Diets rich in saturated fat and cholesterol are the major cause of CVD and atherosclerosis, and have been termed “the diet–heart hypothesis” [59]. This has led to numerous diets to alter the risk of CVD. The Mediterranean diet has been found to lower blood pressure, fasting glucose, and blood lipids [60]. The Lyon Diet Heart Study, enrolling 505 patients with a myocardial infarction, showed that patients assigned to the Mediterranean diet had a lower incidence of cardiac deaths, overall mortality, and nonfatal myocardial infarctions [61]. A study enrolling patients with acute coronary syndromes showed that a Mediterranean diet was associated with preserved left ventricular systolic function, more favorable myocardium remodeling, and a decreased incidence of recurrent CVD events [62]. A beneficial effect of a Mediterranean diet on diastolic and systolic function was also demonstrated in patients with chronic heart failure (CHF) [63]. A large prospective, multicenter cohort study of Spanish university graduates with an average follow-up of 11.5 years, demonstrated that compliance with the Mediterranean diet and lifestyle significantly lowered the risk of CVD [64]. Alternative diet styles have also been studied. A 10 year cohort study of Greek adults found no correlation between a dietary approaches to stop hypertension (DASH)-style diet and the risk of CVD on long-term follow-up [65]. In contrast, a meta-analysis of prospective cohort trials found that compliance with the DASH diet showed a 20% decrease in the incidence and mortality in CVD [66].
A ‘High-salt’ diet has been shown to increase the risk of CVD [67]. A cross-sectional study including 2632 coal miners showed that ‘High-salt’ and ‘Refined grains’ diets significantly increased 10-year atherosclerotic CVD risk scores and 10-year ischemic CVD risk scores [68]. Refined foods can be digested rapidly with none of the fiber-rich structure of high-starch vegetables. However, it has been reported that a refined food diet is associated with an increased risk of CVD [69].
Dietary habits are an important adjustable factor in the prevention of CVD [70]. Compliance with the Mediterranean dietary pattern can significantly decrease the incidence of CVD and its adverse events [64].
Sleep disorders are an important health issue. Approximately 30% of the general
population has a sleep related condition [71]. A series of meta-analyses have
confirmed both too little (
The mechanisms responsible for the association of sleep disorders with CVD may be attributed to increased appetite and food intake, and altered glucose intolerance, and metabolic changes [85]. In addition, longer sleep duration is related to poor lifestyle behaviors, such as unhealthy diets, decreased exercise, increased psychologic disorders, and impaired metabolic syndromes, all of which increase the incidence of heart failure and stroke [86]. Sleep disorders have emerged as a target for interventions to reduce the incidence and risk of CVD.
Psychosocial stress includes stress symptoms and stressors, such as loneliness and critical life events. The WHO estimated the prevalence of depression among CVD patients at 3%–9% worldwide [87]. It was especially higher in China and Iran, with a prevalence of 35% to 47% [88, 89]. Seldenrijk et al. [90] found that depression had a 3-times higher risk for progression to CVD during a six-year follow-up. The INTERHEART study showed self-reported psychosocial factors were independently associated with the risk for an acute MI [91]. Depression has been found to be another non-traditional risk factor for cardiovascular morbidity and mortality in patients with CHD [92]. Depression can result in deterioration of heart function [93]. A meta-analysis including 10,785 AMI patients demonstrated that approximately 20% suffered from severe depression, and one of three patients had mild to moderate depression [94]. Another meta-analysis of 30 prospective cohort trials found that depression was independently associated with an increased risk of CAD and AMI [95]. On the other hand, CVD is also associated with an increased risk of depression [96]. Up to 40% of patients who had a major cardiovascular event also had major depression [97]. Fotopoulos et al. [98] found that over half of patients with myocardial disease (53.8%) had depression, anxiety, or both.
Studies have also demonstrated a dose-dependent relationship between the severity of depression and anxiety and CVD [99]. Both anxiety and depression have been shown to be an associated with AMI [100, 101]. Acikel et al. [102] reported that excellent management of psychological status during and post cardiac surgery could improve quality of life and cardiovascular prognosis. Depression and anxiety are also often observed in CHF patients [103]. Approximately 60% of heart failure (HF) patients have anxiety and 30% suffer from depression [104, 105, 106]. Depression or anxiety were associated with a lower exercise capacity in HF patients [107, 108]. Anxiety was also associated with rehospitalization in CHF patients [109]. Stress can also influence cardiovascular health. Studies have shown that men are more likely to be faced with job-related stress; while women more frequently face chronic stress, both of which are related to CVD risk [110, 111, 112]. In addition, women had higher anxiety levels compared with men [113]. Serpytis et al. [114] demonstrated that women were at an increased risk of anxiety and/or depression in comparison to men in patients with a myocardial infarction. Several studies have demonstrated that women were more likely to suffer from psychological stress during and after the recent pandemic. This may be attribute to preexisting depressive and anxiety disorders, chronic environmental strain, and domestic violence, which leads to sedentary behavior and deteriorating lifestyle habits. In addition social, economic, and cultural factors may also contribute to the effect of gender on CVD [115, 116, 117].
The exactly mechanism of mental disorders increasing the risk for CVD remains uncertain. Currently it is known that psychosocial stress has direct biological effects and is indirectly correlated with socioeconomic and behavioral risk factors [118]. This directly effects the amygdala-based fear-defense system and other pathophysiological pathways [119]. Psychosocial risk factors can also activate the sympathetic system and hypothalamic-pituitary-adrenal axis resulting in endothelial dysfunction, and generation of proinflammatory cytokines, platelet activation, and activation of cardiogenic sympathetic nerves, which leads to an increased heart rate, myocardial infarction, arrhythmias, and even sudden death [113].
Almost 23% of the Australia population, 24% of USA, 37% of Canada, 6%–76%
of Europe, and 6–70% of South East Asia suffer from vitamin D deficiency
(25(OH)D level
Vitamin D deficiency is another CVD risk factor, which could cause ventricular
hypertrophy, arterial stenosis, heart failure and arrhythmias [124]. The
relationship between vitamin D levels and MI, HF, coronary artery disease
(CAD) and stroke is significant (p
However, not all the data is consistent with a beneficial effect of Vitamin D on the reduction of CVD. Several RCTs and meta-analyses failed to confirm the cardiovascular benefit with vitamin D supplements. Mendelian randomization studies did not imply a causality between genetically predicted 25(OH)D concentrations and CVD outcomes [132]. A systematic review conducted in 2010 did not confirm the association between vitamin D and cardiometabolic outcomes [133]. In addition, several well-designed trials also did not confirm cardiovascular benefits from vitamin D supplements. One randomized controlled trial (RCT) of 5108 community dwelling residents aged 50 to 84 who were randomized to vitamin D3 versus placebo, found that during a median of 3.3 years, 25(OH)D concentrations increased by more than 20 ng/mL compared with placebo, but this did not result in a reduction in the primary endpoint of the incidence of CVD and death (HR: 1.02, 95% CI: 0.87–1.20) [134]. Another large randomized double-blind, placebo-controlled study, Vitamin D Assessment (VIDA), found that vitamin D supplements did not have beneficial effects on the cumulative incidence of CVD [135]. Meta analyses of RCTs also have not showed a reduction in BP with vitamin D supplements [136]. This trial enrolled 25,871 participants without CVD at baseline, randomized to a 2000 IU/daily dose of vitamin D3 versus placebo. During a mean follow-up period of 5.3 years, there was no significant reduction in CVD events (HR: 0.97, 95% CI: 0.85–1.12) for vitamin D compared with placebo [137].
Vitamin D signaling is thought to alter the pathophysiology of atherosclerosis
by reducing the expression of TNF-
| Author | Publication year | Study type | No. Patients or studies | Central message |
|---|---|---|---|---|
| N I Zhernakova [124] | 2021 | Case control study | 187 | The deficit level of D-(25(OH)D) should be considered a laboratory predictor of MI in the elderly. |
| Jeffrey L Anderson [125] | 2010 | Observational study | 41,504 | Vitamin D might play a primary role in CV risk factors and disease. |
| Hamidreza Norouzi [126] | 2019 | Randomized prospective, case-control study | 732 | Lower levels of vitamin D is associated with increased risk and extent of coronary artery involvement as well as some of the risk factors of CAD, including male gender, hypertension and positive family history for CVD. |
| Cassianne Robinson-Cohen [127] | 2013 | Prospective cohort study | 6436 | Lower serum 25(OH)D concentration was associated with an increased risk of incident CVD events among participants who were white or Chinese but not black or Hispanic. |
| Richa Nepal [128] | 2021 | Descriptive cross-sectional study | 51 | Prevalence of vitamin D deficiency among patients of ACS was comparable to various other homologous international studies. |
| Ang Zhou [129] | 2022 | Non-linear Mendelian randomization | 295,788 | Vitamin D deficiency can increase the risk of CVD. Burden of CVD could be reduced by population-wide correction of low vitamin D status. |
| Emerging Risk Factors Collaboration/EPIC-CVD/Vitamin D Studies Collaboration [130] | 2021 | Observational analyses | 386,406 | Stratified Mendelian randomisation analyses suggest a causal relationship between 25(OH)D concentrations and mortality for individuals with low vitamin D status. |
| Setor Kwadzo Kunutsor [131] | 2013 | Meta-analysis | 283,537 | Participants with the highest 25(OH)D levels had a 30% lower risk of hypertension. |
| Tao Huang [132] | 2019 | Mendelian randomisation | 205,923 | No evidence to support that genetically increased 25(OH)D was associated with a lower risk of ischaemic stroke, intracerebral haemorrhage, subarachnoid haemorrhage, and lipid levels in both Chinese and European adults. |
| Anastassios G Pittas [133] | 2010 | Systematic review | 13 observational studies (14 cohorts) and 18 trials | The association between vitamin D status and cardiometabolic outcomes is uncertain. Trials showed no clinically significant effect of vitamin D supplementation at the dosages given. |
| Robert Scragg [134] | 2017 | A Randomized Clinical Trial | 5108 | Monthly high-dose vitamin D supplementation does not prevent CVD. |
| RKR Scragg [135] | 2019 | A randomised, double-blind, placebo-controlled trial | 5110 | Vitamin D supplements did not have beneficial effects on the cumulative incidence of CVD. |
| JoAnn E Manson [137] | 2019 | A nationwide, randomized, placebo-controlled trial | 25,871 | Supplementation with vitamin D did not result in a lower incidence of invasive cancer or CVD events than placebo. |
| Dongdong Zhang [136] | 2020 | Meta-analysis | 11 cohort studies and 27 RCTs, with 43,320 and 3810 participants | Supplementation with vitamin D does not lower blood pressure in the general population. |
| CVD, Cardiovascular diseases; ACS, acute coronary syndrome. | ||||
Hyperuricemia is defined as serum urate concentrations
Prospective cohort studies indicated that the levels of uric acid were independently and significantly related to cardiovascular mortality [144, 145]. High levels of uric acid (UA) are also related to an increased incidence of hypertension. A meta-analysis including 97,824 participants showed that hyperuricemia could increase the risk of hypertension [146]. In addition, a cross-sectional cohort of 285,882 individuals demonstrated that UA levels were significantly associated with atrial fibrillation (AF) [147]. A meta-analysis confirmed that high UA was associated with AF in both cross-sectional and cohort studies [148]. Hyperuricemia is also seen in patients with HF. A Japanese registry study showed that hyperuricemia frequently occurred in HF patients and that higher UA levels were associated with increased adverse long-term outcomes [149], suggesting the level of UA is a prognosis related marker in patients with HF.
Hyperuricemia also plays a significant role in other cardiovascular diseases. Abbott et al. [150] studied 5209 subjects who developed CHD in the Framingham Study and found that the two-year incidence of gout was 6 times greater in men in comparison to women during 32 years of follow-up. However, some analyses have reported contrary conclusions [151, 152]. In a study including 63,127 participants without a history of CVD, after a median follow-up of 7.04 years, there was no significant relationship between changes in SUA and CVD [153]. In contrast, a meta-analysis involving 341,389 adults showed that for every 1 mg/dL increase in UA, the incidence of CHD and all-cause mortality increased by 20% and 9%, respectively [154]. Another meta-analysis involving almost one million patients (n = 949,773) diagnosed with gout demonstrated the prevalence of MI was 2.8%, HF was 8.7% and hypertension was 63.9%; and that the risk of CVD was higher in subjects with gout compared to non-gout controls [155]. Of note, some studies have suggested a U-shaped relationship between UA levels and CVD [156]. Li et al. [157] in a cohort study of 82,219 participants demonstrated that the age at hyperuricemia onset was an important predictor of CVD and all-cause risk for mortality.
Studies have shown that hyperuricemia could induce inflammation, oxidative stress, and endothelial dysfunction, which directly or indirectly increases the risk for CVD [158].
Air pollution is a global health care challenge that has harmful effects on the cardiovascular system. Almost 20% of CVD deaths globally were attributed to air pollution [159]. Either short-term or long-term exposure to air pollutants can result in cardiac dysrhythmias, due to oxidative stress, autonomic dysfunction, coagulation dysfunction and inflammation [160].
Among diverse air pollutants, particulate matter (PM) plays a major role in CVD,
especially when its diameter is equal or less than 2.5
The possible mechanism for the harmful effects of environmental pollution results from the fact that even a short duration of exposure to PM2.5 could result in initiation of oxidative stress or inflammation, autonomic imbalance, and translocation of PM mixture components into the systemic circulation [170], which in turn increases heart rate, blood pressure, plaque vulnerability, and thrombosis formation [164], leading to plaque formation and ultimately rupture and acute coronary syndromes [171].
Non-traditional factors indirectly contribute to CVD. Control of these factors will reduce the numbers of traditional CVD risk factors and contribute to a lower prevalence of diabetes, lower blood pressure, and lower cholesterol levels, which would ultimately lead to better prevention of heart attacks, strokes, and mortality from CVD. This supports the current prevention and intervention guidelines that recommend lifestyle modification, diet and increased physical activities, in an effort to reduce the incidence and risk from CVD. Non-traditional factors have been associated with an increased risk of CVD (Table 2), such as sleep disorders and environmental and psychosocial factors such as depression. Therefore, reducing air pollution exposure, controlling negative psychosocial factors, ensuring proper sleep duration and optimal sleep quality, play an importance role in reducing the risk of CVD. Non-traditional factors can be a possible explanation for the residual risk of CVD in patients with coronary heart disease who do not have any traditional risk factors.
| Risk factors | Consistency of conclusions | Effectiveness after Intervention |
|---|---|---|
| Gut microbiota | Not confirm | Not sure |
| Physical inactivity | Consistency support | Yes |
| Diet style | support | Yes |
| Sleep disorders | support | Yes |
| Psychosocial factors | support | Yes |
| Vitamin D deficiency | Not confirm | Not confirm |
| Hyperuricemia | support | Yes |
| Environmental exposure | support | Yes |
| CVD, Cardiovascular diseases. | ||
Therefore, it may be appropriate to update current guidelines to include non-traditional risk factors as significant risk factors for CVD.
Several studies have established the relationship between non-traditional risk factors and CVD. These non-traditional risk factors include intestinal flora dysbiosis, sleep disorders, psychosocial stress, physical inactivity, vitamin D deficiency, hyperuricemia, air pollution, and poor diets. Furthermore, ethnicity [172], socioeconomics [173], chronic pain [174], obesity [175], and air pollution [176] have also been reported to be associated with CVD. Since most non-traditional risk factors are identified as important modifiable factors in CVD prevention, we should recognize all of these reversible risk factors for each patient and provide them with personalized preventive and therapeutic strategies to further reduce the risk for CVD and its adverse events.
LW: Writing the original draft, acquisition of the funding and visualization. JL: Supervision, visualization and was involved in drafting, revising it for important intellectual content. RW: Design and involved in drafting, revising it for important intellectual content. KL: Put forward the ideas, supervision, visualization, also contributions to the conception and design, and editing of the article. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research was funded by the Study on the cut-off value of inflammation-related indicators in the diagnosis of acute myocardial infarction combined with infection (grant number 20220967).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.