1 Department of Medicine and Surgery, University of Milano-Bicocca, 20126 Milano, Italy
2 Department of Clinical Sciences and Community Health, University of Milano, 20122 Milano, Italy
3 Department of Cardio-Thoracic-Vascular Diseases, Foundation IRCCS Ca' Granda Ospedale Maggiore Policlinico, 20122 Milano, Italy
4 Department of Cardiology, University Hospital “Dr. Dragisa Misovic-Dedinje'', 11000 Belgrade, Serbia
Abstract
The hypertensive response to exercise testing, defined as exaggerated blood pressure response (EBPR), has been documented to be independently associated with unhealthy conditions, carrying an increased risk of future hypertension, cardiovascular (CV) morbidity and mortality. In treated hypertensives, EBPR is a marker of uncontrolled hypertension, a condition previously undetected by office blood pressure (BP) measurements at rest; EBPR may also detect masked hypertension, a phenotype characterized by normal BP values in the medical environment but elevated home or ambulatory BP monitoring (ABPM). The aim of the present review is to provide a comprehensive and up-dated information on the clinical importance of EBPR targeting the following issues: (I) definition and prevalence; (II) underlying mechanisms; (III) clinical correlates and association with subclinical organ damage; (IV) predictive value; (V) clinical decision making.
Keywords
- exaggerated blood pressure response to exercise
- hypertension
- target organ damage
- cardiovascular disease
In both normotensive and hypertensive individuals, physical exercise, either dynamic or isometric, carried out in the clinical setting for cardiovascular (CV) diagnostics, is associated to significant blood pressure (BP) variations, in particular sharp increments in systolic BP (SBP) and variable changes in diastolic BP (DBP), that may either decrease, increase or remain unchanged [1, 2]. In physiological conditions, BP changes during exercise are the result of the rise in cardiac output in response to the increased oxygen demand from working muscles via activation of the adrenergic tone [3, 4]. The electrocardiography (ECG)-monitored stress test for assessing cardiopulmonary fitness is routinely performed by means of dynamic exercise with a progressive workload. In response to the rapid increase in physical activity, stroke volume and heart rate are boosted by increased sympathetic activity and a vasodilation occurs at the level of arterioles supplying the exercising muscles, thus leading to a decrease in systemic vascular resistances [5].
Stress ECG testing with measurement of BP at incremental stages of exercise intensity is a validated non-invasive diagnostic tool carried out worldwide in cardiology practice [6, 7]. Measuring BP response to physical exercise during stress ECG testing represents a key procedure, as abnormal responses in terms of BP increases or decreases provide relevant information in addition to conventional ECG and clinical diagnostic criteria [8, 9]. In addition to dynamic exercise, other stimuli such as mental stress, cold stress and handgrip represent an alternative method for evaluating exaggerated pressure reactivity [10]. Exaggerated BP response (EBPR) to these stimuli has been also associated with poor prognosis; our review, however, will focus on the abnormal pressure reactivity to exercise ECG-testing [11].
Consistent evidence indicates that hypertensive response to exercise testing is independently associated with several CV risk factors and, more importantly, with an increased likelihood of CV morbidity and mortality [12, 13, 14, 15]. The strength of this association is more evident when EBPR occurs in the early stages of test or during submaximal exercise [16, 17]. Of note, EBPR in treated hypertensives may be seen as a marker of uncontrolled hypertension previously undetected by office BP measurements at rest [18]. On the other hand, EBPR in normotensive individuals may be a marker of masked hypertension and predictor of future hypertension [19, 20]. This review aimed to summarise available evidence about the clinical relevance of EBPR will be focused on the following issues: (I) definition and prevalence; (II) mechanisms; (III) clinical correlates and association with subclinical organ damage; (IV) prognostic value; (V) clinical management.
Several definitions of EBPR during treadmill and bicycle exercise testing have
been reported in the literature; this may be relate to the lack of consensus
about the ‘threshold’ value of exercise BP defining this phenotype. This
uncertainty is also reflected by the different recommendations issued by the
Cardiology and Sports Medicine guidelines. The American Heart Association (AHA)
guidelines define EBPR as systolic peak BP
| Guidelines | Blood pressure cut-offs | |
|---|---|---|
| Men | Women | |
| American Heart Association | 210/90 mmHg | 190/90 mmHg |
| European Society of Cardiology | 220/85 mmHg | 200/80 mmHg |
| American College Sports Medicine | 225/90 mmHg | |
A meta-analysis of 12 studies revealed that EBPR may be defined over a wide
interval of SBP values ranging from 180 to 275 mmHg [25]. A further source of
heterogeneity of EBPR diagnostic criteria is the intensity of physical exercise.
Differences in protocol, type of exercise (i.e., bicycle, treadmill), age,
gender, degree of physical activity of the individuals examined (i.e., sedentary,
trained, athletes) and comorbidities are major determinants of variability among
studies. A consequence of the lack of consensus about EBPR definition is the
difficulty in comparing and interpreting data provided by the studies. It is
worth noting that EBPR may occur in both normotensive individuals with no known
history of hypertension and in treated hypertensive patients. Thus, the variable
prevalence of EBPR reported in current literature may be related to numerous
factors including threshold values used to define this phenotype, demographic
(i.e., age, sex, ethnicity) and clinical characteristics of the populations
studied. In the Southall and Brent Revisited (SABRE) study, including 659 older
adults, EBPR (i.e., SBP
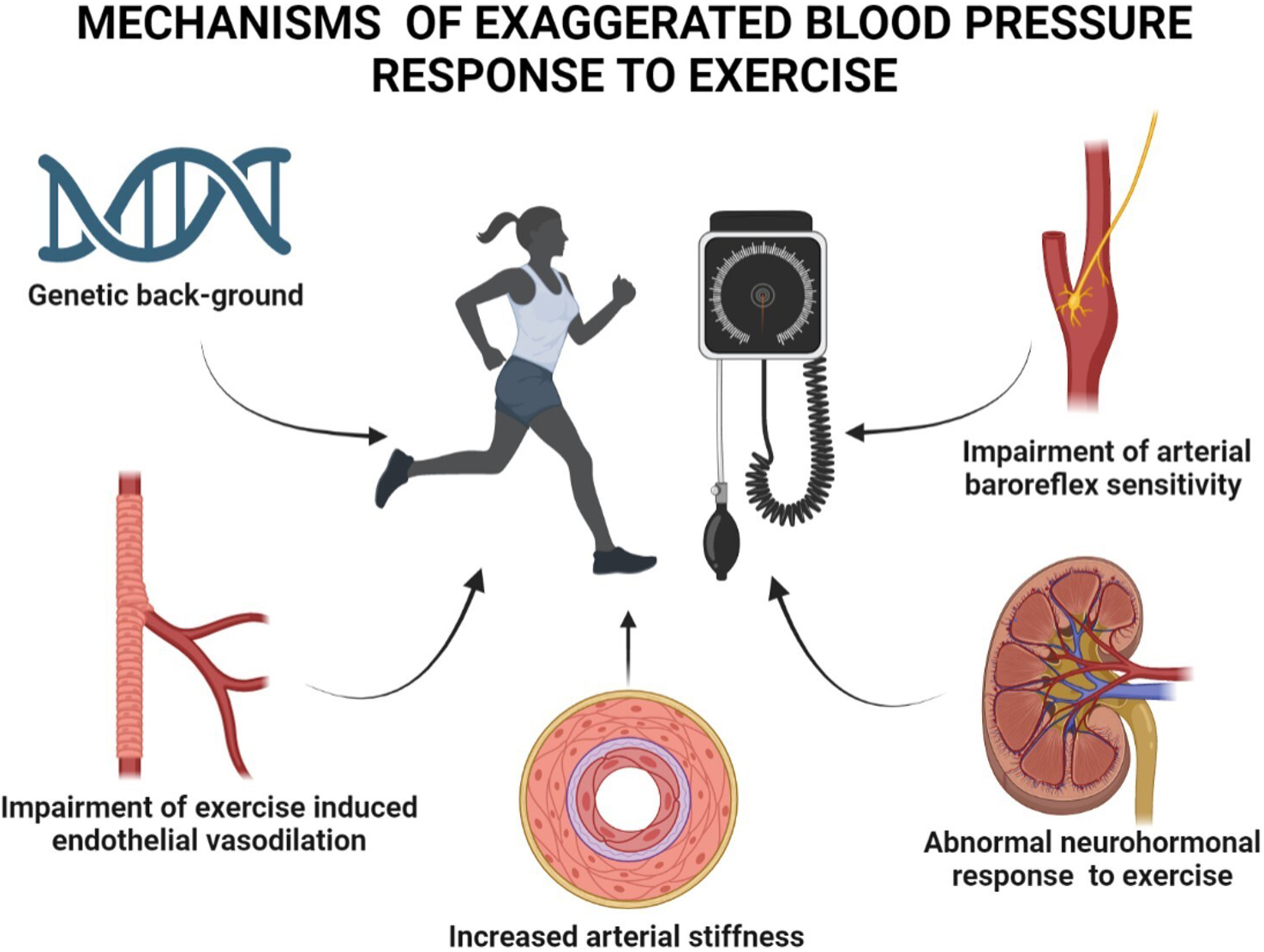
Although the complex pathophysiological mechanisms of EBPR remain poorly defined, growing evidence on this issue has been collected in individuals with and without CV diseases. The multiple factors implicated in EBPR development include genetic background, endothelial function, large artery stiffness, arterial baroreflex sensitivity and neurohormonal response to exercise (Fig. 1). Emerging findings suggest that exaggerated BP and sympathetic responses to physical stimuli may reflect a deranged neural-cardiovascular control in young adults with a genetic predisposition to hypertension [28]. A greater increase in BP and muscle sympathetic nerve activity in response to static exercise has been described in young otherwise healthy adults with a family history of hypertension as compared to controls matched for age, BP and heart rate [29]. Endothelium-dependent vasodilation in large arteries represents a key adaptive response to systolic wall shear stress occurring during physical exercise [30]. Thus, endothelial dysfunction by impairing the physiological vasodilation that counterbalances the increased shear stress, may trigger EBPR. Numerous studies during the last two decades have documented an association of EBPR with endothelial dysfunction, aortic stiffness and enhanced angiotensin II rise at exercise peak [31, 32]. Stewart et al. [33] measured endothelial vasodilator function, assessed as brachial artery flow-mediated vasodilation (FMD), in untreated individuals with high normal BP or mild hypertension. The authors documented that FMD was the only independent correlate of the difference between resting and maximal pulse pressure in both sexes.
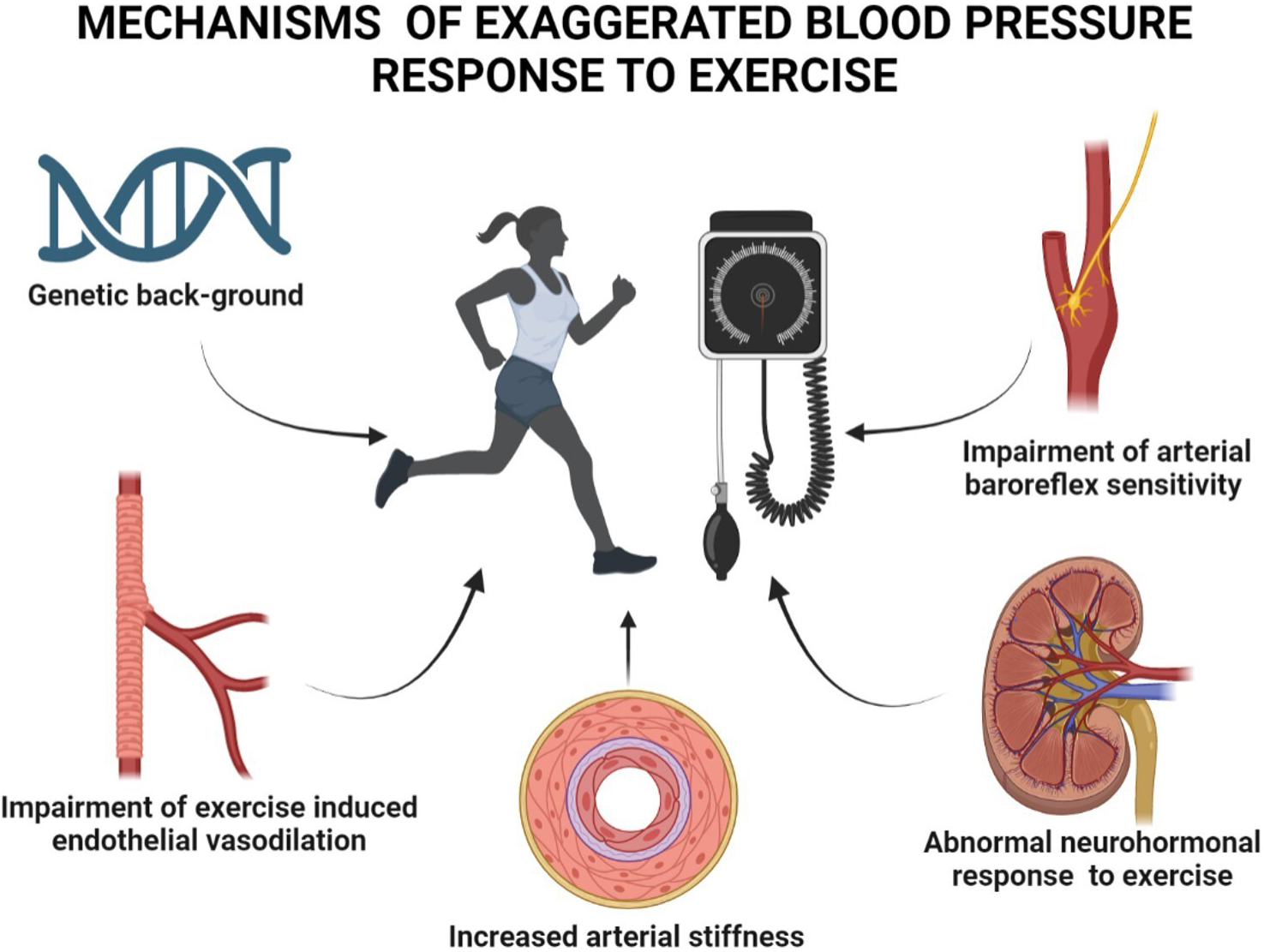 Fig. 1.
Fig. 1.Mechanisms and factors implicated in the development of exaggerated blood pressure response to exercise.
Increased arterial stiffness resulting in a reduced buffer function of large
arteries may, in turn, enhance excessive BP increments during exercise. A recent
study carried out in 92 untreated normotensive men without a history of CV
disease revealed that, compared with individuals with a normal response, those
with an EBPR exhibited a significantly higher brachial-ankle pulse wave velocity
(1412
Main research lines addressing the factors associated with EBPR have
investigated the relationship between masked hypertension and BP changes during
exercise. Masked hypertension is a condition characterized by normal BP values
measured in the medical environment but elevated home or ambulatory BP monitoring
(ABPM) values [42]. This term was coined in the early 2000’s by Pickering
et al. [43] in order to define a hypertensive status not identified by
routine office BP measurements. Since then, a large body of evidence has
accumulated on the adverse clinical and prognostic significance of masked
hypertension. Numerous cross-sectional and longitudinal investigations
demonstrated that, compared to normotensive individuals, those with masked
hypertension have an increased risk of hypertension-mediated organ damage, CV
morbidity and mortality substantially overlapping that of sustained hypertensive
patients [44, 45, 46]. The pathophysiological mechanisms underlying these observations
are related to the fact that out-of-office BP, either monitored at home or in
dynamic conditions over 24 h, has a closer relationship with CV events and a
greater predictive value for adverse outcomes compared to office BP [47]. One of
the first studies to investigate the relationship between masked hypertension and
EBPR was published a decade ago by Sharman et al. [48]. Among 72
non-diabetic individuals free of CV disease with EBPR to maximal treadmill
exercise (i.e., SBP
The presence of subclinical target organ damage in individuals with normal
office BP at rest has been proven to be associated with EBPR; in particular, left ventricular hypertrophy (LVH)
or concentric remodelling have been related to this abnormal BP phenotype. In a
pioneering study performed in 1978 including participants to Framingham Heart
Study free of CV disease and not taking any antihypertensive or CV medication,
individuals with an EBPR to exercise exhibited a 10% higher LV mass than those
with normal SBP responses to exercise [51]. In the previously mentioned study by
Sharman et al. [48] patients with EBPR had significantly higher values
of LV mass index (41.5
Two lines of research, based on prospective studies, investigated the predictive significance of EPBR targeting its association with: (I) new-onset hypertension and (II) fatal and non-fatal CV events.
The Coronary Artery Risk Development in Young Adults (CARDIA) study was the first investigation to address the association between
EBPR and incident hypertension in 3741 normotensive individuals undergoing
treadmill testing [52]. EBPR was found in 687 participants (18%) who exhibited 5
mmHg higher SBP and 1 mmHg higher DBP; after a 5-year follow-up period
(p
The value of EBPR in predicting future hypertension has also been demonstrated in the setting of athletes which, by definition, includes healthy subjects trained to perform intense physical activity. One hundred and forty-one normotensive athletes with EBPR to exercise were compared to 141 normotensive athletes with normal BP response matched for gender, age, body size, and type of sport [55]. A total of 19 athletes belonging to EBPR group developed hypertension (13.5%) compared with 5 of the normal BP response group (6.5%) during a mean follow-up period of 6.5 years.
Finally, moving from single studies to meta-analysis, the findings of a systematic review by Keller et al. [56] based on 18 prospective and retrospective studies including 35,151 healthy normotensive participants undergoing cardiopulmonary testing revealed a significant association between EBPR to exercise (systolic, diastolic or both) and new onset hypertension over a follow-up period lasting between 2 and 14 years regardless of the wide heterogeneity of the criteria used to define the EBPR.
The notion that EBPR in otherwise normotensive individuals may represent a risk factor for cardiovascular disease has been known for over thirty years. The Paris Prospective Study was among the first to investigate this topic by analysing the data collected in 4907 normotensive healthy middle-aged men over a mean followed-up period of 17 years [15]. The magnitude of exercise-induced SBP elevation was significantly associated with incident CV events and all-cause death, regardless of several major confounders including LVH. The value of SBP recorded during the maximal stress test for prediction of all-causes mortality, CV disease, and coronary heart disease was assessed in a large population-based sample of 20,387 men and 6234 women living in Dallas during a 8-year follow-up period [57]. In men, the adjusted risks of all-cause mortality for quartiles 3 and 4 of maximal SBP, compared to the lowest quartile, were: 1.36 (1.01–1.85), and 1.37 (0.98–1.92), respectively. This was also the case for the risk of CV disease and coronary morbidity and mortality. Similar findings for all-cause and CV mortality were observed in women across maximal SBP quartiles. Recently, the Oslo Ischemia Study provided further evidence of a positive association between the magnitude of BP responses to moderate exercise and the risk of CV disease and mortality [58].
Unlike the above mentioned studies, a recent report by Zafrir et al. [59], based on a retrospective analysis of 14,792 individuals followed for over 6 years, showed that the excessive increase in SBP during exercise did not predict CV disease and mortality after adjustment for confounding factors. Of note, when the SBP quartiles measured during the stress test were taken into account, the analysis documented that individuals belonging to the highest quartile were at higher risk than those in the lowest quartile.
A couple of meta-analyses have confirmed the unfavourable prognostic
significance of EBPR. Pooled data from 12 studies including 46,314 individuals
without overt cardiac disease suggested that the hypertensive response to an
exercise of moderate intensity carried a 36% higher rate of CV events and
mortality (95% CI 1.02–1.83, p = 0.04) compared to normal BP response
[17]. The meta-analysis by Perçuku et al. [25], carried out in
47,188 normotensive individuals from 8 studies, showed that individuals with EBPR
to exercise had a greater risk of CV death and coronary disease (HR: 1.36,
p
EBPR documented during exercise in normotensive subjects without history of hypertension and in treated hypertensives may require further diagnostic investigations, bearing in mind, however, that this BP phenotype is not a fully reproducible clinical trait. Grossman et al. [63], evaluating the data of exercise tests performed during annual health examinations for five consecutive years in 69 normotensive patients with high normal BP levels, found that only 11 patients (21.5%) out of the whole baseline EBPR group exhibited the same abnormal BP response during subsequent tests. Although limited data on EBPR reproducibility over time do not allow to correctly estimate the clinical relevance of this BP phenotype, the significance of EBPR in single patients should be evaluated in order to exclude both masked hypertension or uncontrolled masked hypertension. A comprehensive diagnostic approach in these patients should include BP measurements out-side the office environment (preferentially in dynamic conditions with the ABPM) and the search for subclinical hypertension mediated organ damage [64]. Available evidence of an association between EBPR, elevated ABPM values and/or target organ damage such as concentric remodelling, LVH, increased arterial stiffness, and carotid atherosclerosis should direct the clinician to plan a therapeutic intervention based on lifestyle modifications and drug treatment [65, 66, 67]. A large body of evidence supports the view that regular physical activity may favourably affect endothelial function, sympathetic activity, arterial stiffness and consequently BP levels both in normotensive individuals and hypertensive patients. Lifestyle modification programs consisting of aerobic exercise and diet counselling have been shown to reduce exercise-induced SBP elevation, improve arterial stiffness and nitric oxide bioavailability even in short-term studies (i.e., 12 weeks) [68]. Of note, the reversibility of EBPR to exercise was found to be less evident in elderly patients [69]. Nonetheless, non-pharmacological treatment of hemodynamic changes associated with EBPR represents the first mandatory step in sedentary overweight/obese individuals without history of hypertension. As for antihypertensive treatment, this option should be considered when EBPR is associated with masked hypertension, according to guideline recommendations. In treated patients with normal office BP at rest, but elevated BP during exercise, optimal treatment strategies are still uncertain [70]. As RAAS activation and increased adrenergic tone may affect vascular and myocardial responses to exercise, angiotensin converting enzyme inhibitors, angiotensin receptor antagonists and beta-blockers are the drugs of choice for hypertensive patients with EBPR [71]. Furthermore, it is useful to underline that in diabetic patients the improvement of metabolic profile may reduce and even normalize EBPR [72]. In addition, the insulin sensitizer rosiglitazone has been reported to play a beneficial effect on resting BP as well as on BP response to exercise in men with type 2 diabetes mellitus and coronary artery disease, especially in those with EBPR [73]. Finally, weight loss after bariatric surgery, a treatment increasingly used in morbid obesity, has been shown to effectively reduce the high prevalence of EBPR in these patients [74]. It is worth mentioning, however, that evidence on the effects of non-pharmacological and pharmacological interventions on CV outcomes in patients with EBPR is still lacking.
EBPR detected during ECG stress test is a common condition both in normotensive individuals without a known history of hypertension and in treated hypertensives with normal resting BP. Growing evidence suggests that EBPR is related to several CV risk factors such as endothelial and large artery dysfunction, increased sympathetic tone, metabolic alterations and obesity [75]. In clinical perspectives, EBPR may be regarded as a marker of multiple conditions, including masked hypertension, poor BP control, subclinical target organ damage and adverse CV outcomes [76, 77]. When this condition is ignored, CV risk is underestimated in a large proportion of the population undergoing stress testing, thus contributing to the increased burden of CV diseases with their public health consequences. It should be underlined, however, that some methodological and clinical aspects of this condition remain poorly defined, in particular clinical indications of stress test, diagnostic criteria of EBPR and screening criteria of individuals to unmask this condition. Furthermore, the role of EBPR treatment as an effective therapeutic target for reducing CV risk remains to be clarified. Thus, further studies are needed to investigate this important issue in order to prevent CV complications associated with EBPR.
CC and MT conceived and designed the review; CC drafted the manuscript; AF and EG contributed to the collection and analysis of literature, prepared figures and tables; CS and GG provided substantial revision of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Guido Grassi is serving as one of the Editorial Board members of this journal. We declare that Guido Grassi had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Carl J. Lavie.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.