1 Department of Cardiology, The First Affiliated Hospital of Kunming Medical University, 650000 Kunming, Yunnan, China
Abstract
Myocardial fibrosis is a common pathological feature of various terminal cardiovascular diseases. Progressive fibrosis is the pathological basis for the development and progression of many cardiac arrhythmias and heart failure. There are no effective reversal drugs for myocardial fibrosis due to the lack of understanding of the molecular mechanisms. Noncoding RNAs, a class of RNAs that do not function in coding proteins, have been found to be intimately involved in the life cycle of cardiomyocyte differentiation, transcription and apoptosis and are important regulators of cardiovascular disease. An increasing number of studies have shown that noncoding RNAs regulate the proliferation and transformation of cardiac fibroblasts through related signaling pathways and can be used as potential biomarkers and novel therapeutic targets for cardiac fibrosis. This article reviews the relationship between noncoding RNAs and cardiac fibrosis.
Keywords
- non-coding RNAs
- myocardial fibrosis
- biomarker
- gene regulation
- molecular mechanism
Myocardial fibrosis is a common maladaptive pathological change in multiple advanced cardiovascular diseases in response to cardiac pressure or volume overload, which promotes the proliferation and activation of cardiac fibroblasts (CFs) and excessive extracellular matrix (ECM) production within the myocardium. Excessive synthies of multiple cytokines, growth factors and chemokines can alter CF biological activity, leading to cardiac remodeling that results in cardiac dysfunction, conduction abnormalities and reduced compliance, ultimately leading to arrhythmia and heart failure (HF).
Most sequences in the human genome do not encode proteins, and only 1.5–2% of the genome is capable of being transcribed into RNA that encodes proteins [1]. With 200 nucleotides as the maximum length, noncoding RNAs (ncRNAs) are divided into long-chain noncoding RNAs (lncRNAs) and short-chain noncoding RNAs, of which microRNAs (miRNAs) are the most characteristic ncRNAs. In addition, there is a nonlinear ncRNA called circular RNA (circRNA). Recent studies have confirmed that ncRNAs are important regulators of cardiovascular diseases and are involved in the life cycle of cardiomyocyte differentiation, transcription and apoptosis [2] (Fig. 1). CFs are important components of cardiomyocytes with diverse origins, and ncRNAs are differentially expressed in myocardial fibrotic tissues. Therefore, exploring the pathophysiological functions and mechanism of ncRNAs in cardiovascular disease-induced myocardial fibrosis can provide new strategies for the diagnosis, treatment and prognosis of cardiovascular diseases. This review discusses the relationship between noncoding RNAs and cardiac fibrosis.
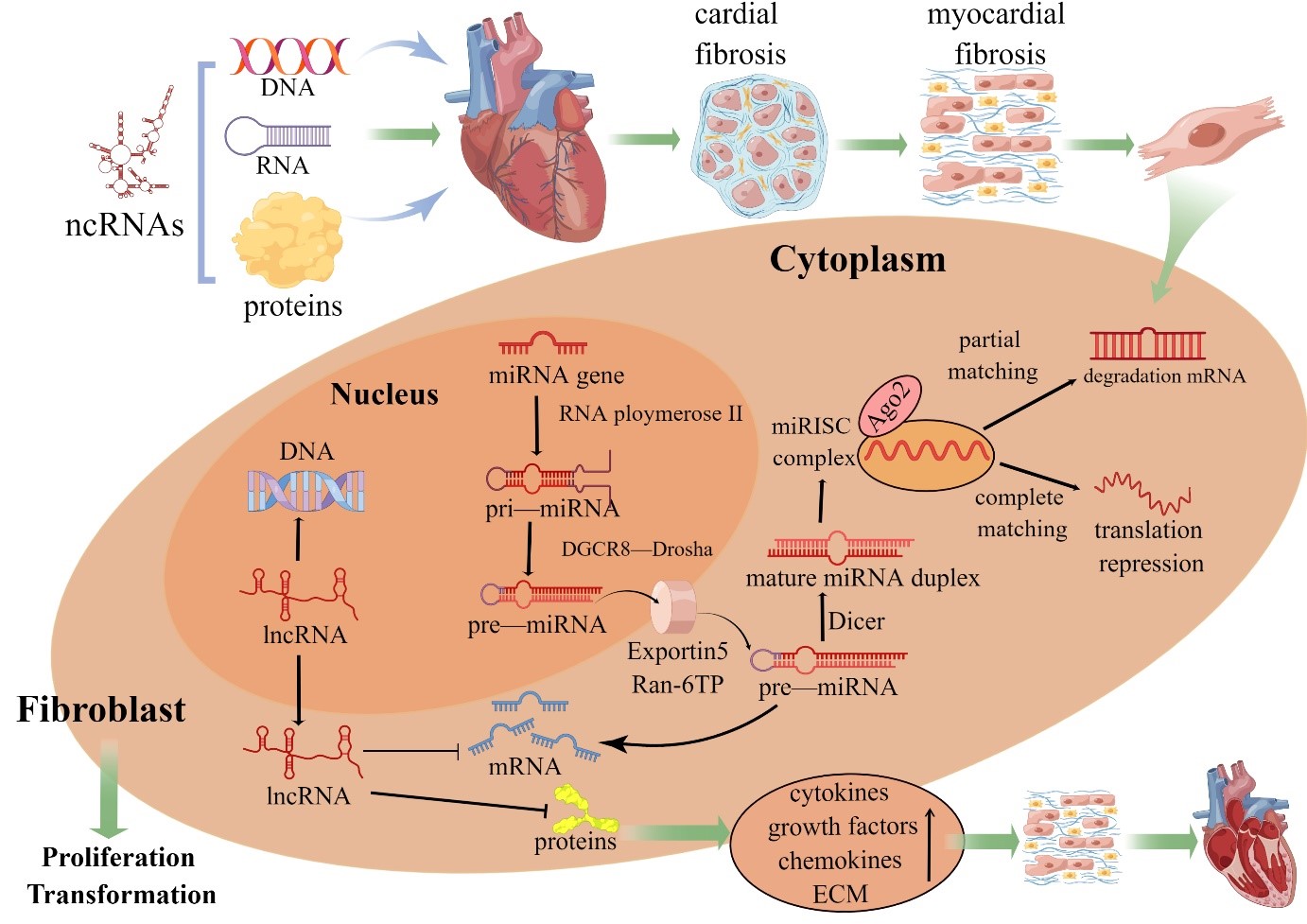
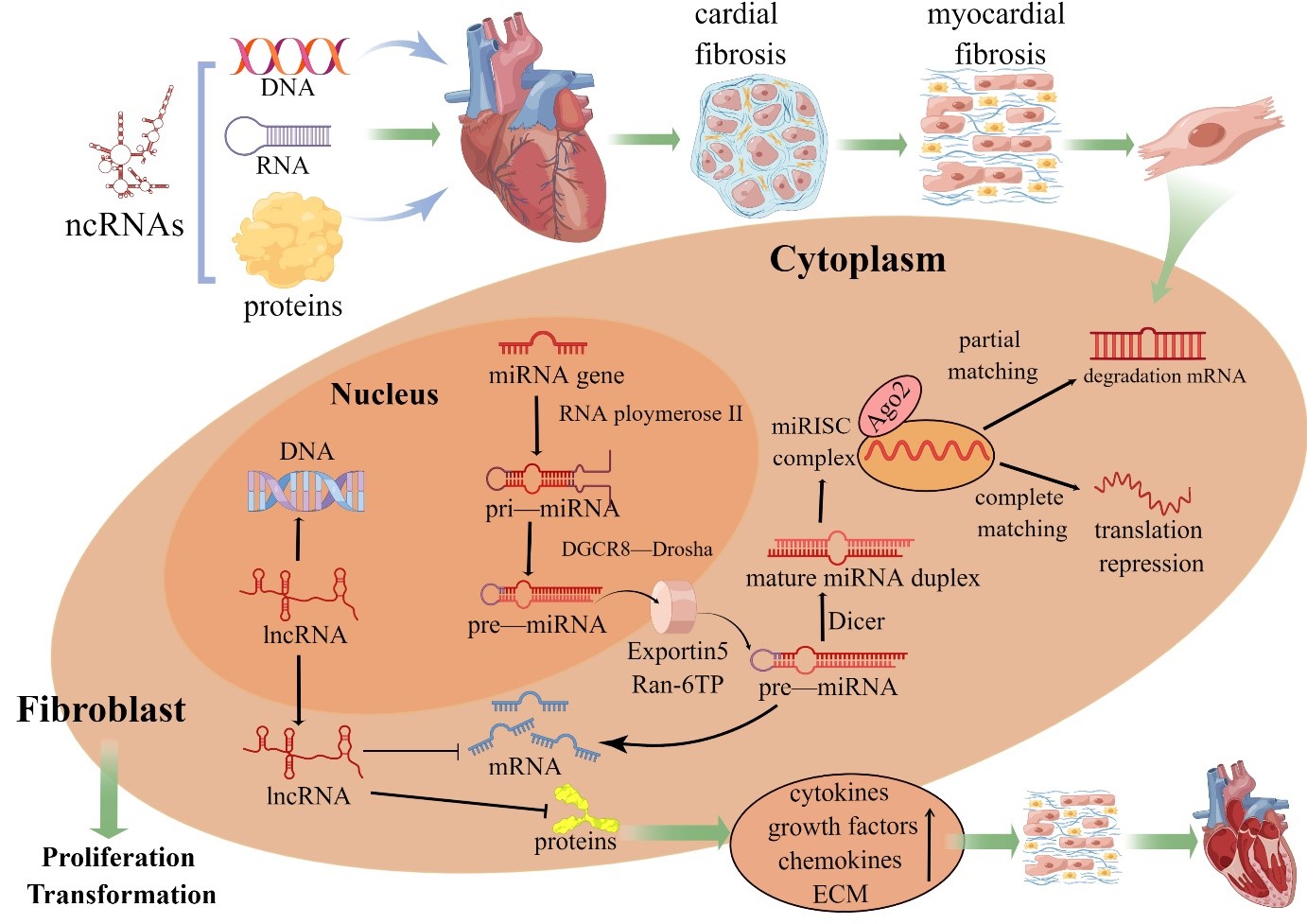 Fig. 1.
Fig. 1.ncRNAs in fibroblast biology. Pri-miRNAs are processed by Drosha into pre-miRNAs before the endonuclease Dicer generates a mature miRNA. Functional miRNAs are ultimately coupled to Argonaute 2 protein and then incorporated into the RNA-induced silencing complex. When a miRNA and target mRNA are completely complementary, the target mRNA is degraded; when two sequences are partially complementary, the specific gene is silenced through translational suppression of the target mRNA. The mechanism of mRNA regulation by miRNAs depends on the degree of sequence complementarity between the miRNA and 3´UTR motif in the target mRNA gene. One miRNA sequence can target different mRNAs, and the expression of one mRNA sequence can be downregulated by different miRNAs. In contrast, lncRNAs are involved in the proliferation and transformation of cardiac fibroblasts through complementary interactions with DNA, miRNA sponges and indirect or direct regulation of proteins. MiRNAs and lncRNAs are important regulators of intracellular gene expression, and excess synthesis of multiple cytokines, growth factors and chemokines alters the biological activity of CFs, resulting in increased extracellular matrix (ECM) synthesis that leads to cardiac remodeling (By Figdraw).
MiRNAs are single stranded RNAs molecules about 22 nucleotides in length that can regulate gene expression at the post-transcriptional level and play a critical role in cardiac fibrosis, mainly including left ventricular fibrosis, right ventricular fibrosis and atrial fibrosis (Table 1, Ref. [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37]).
| miRNA | Stimulation | Target gene | Function | Reference |
| miR-10a | AF | TGF- |
Anti | [27] |
| miR-20a-5p | DCM | ROCK2, JNK/NF-kB | Anti | [11] |
| miR-21 | HF, DCM, TAC, AF | WWP-1, TGF- |
Anti | [9, 13, 21] |
| miR-21-3p | DM | FGFR1,FGF21,PPAR |
Anti | [28] |
| miR-21-5p | VMC, ARVC | TNF |
Anti | [16, 31] |
| miR-27a-5p | TAC | Egr3 | Anti | [14] |
| miR-29 | MI | PI3K/mTOR/HIF-1 |
Anti | [6] |
| miR-29a-3p | PAH | THBS2 | Anti | [17] |
| miR-29b | TAC, AS, AF | TGF- |
Anti | [15, 25] |
| miR-30c | AF | TGF- |
Anti | [26] |
| miR-30d | HF | ITGA5 | Anti | [7] |
| miR-34a | Age, AMI, DOX | PNUTS, Bcl-2, SIRT1 | Anti | [20, 32] |
| miR-101 | MI | RUNX1/TGF- |
Anti | [5] |
| miR-129-5p | HF | Smurf1/PTEN | Anti | [8] |
| miR-132 | AF, Ang II | CTGF | Anti | [23] |
| miR-133b | DOX, HT | PTBP1, TAGLN2 | Anti | [33, 34] |
| miR-135b | ARVC | Vnt, Hippo | Anti | [16] |
| miR-146b-5p | AF, MI | TIMP/MMP9 | Anti | [30] |
| miR-155 | MI, VMC | Anti | [4, 35] | |
| miR-195 | HBP | TGF- |
Anti | [10] |
| miR-205 | AF | P4H |
Anti | [29] |
| miR-325-3p | PAH | HE4, PI3K/AKT | Anti | [18] |
| miR-495 | DOX | AKT | Anti | [36] |
| miR-4443 | AF | THBS1, TGF- |
Anti | [24] |
| miR-1 | MI | UPS | Pro | [3] |
| miR-23b-3p | AF | TGF- |
Pro | [22] |
| miR-27b-3p | AF | TGF- |
Pro | [22] |
| miR-148-3p | PAH | HIF-1 |
Pro | [19] |
| miR-340-5p | DCM | Mcl-1 | Pro | [12] |
| miR-1468-3p | Age | TGF- |
Pro | [37] |
| Bcl-2, B lymphocytoma-2; DNMT1, DNA methyltransferase 1; FGFR1,
fibroblast growth factor receptor 1; HBP, high blood pressure; HE4, human
epididymis protein 4; HIF-1 | ||||
An increasing number of studies have shown that miRNAs participate in the
development of myocardial fibrosis, but their functions and mechanisms remain to
be fully elucidated [38]. Myocardial infarction (MI) is the leading cause of death
from cardiovascular disease. The myocardium slowly enters a long-term progressive
fibrotic process after the acute phase of infarction, which is an adaptive
remodeling in the early stage, but the persistent fibrotic response accelerates
HF after MI. MiRNAs were significantly differentially expressed in MI, with miR-1
expression levels increasing after MI. Inhibiting miR-1 expression through the
use of a miR-1 antagomir significantly reduced left ventricular end-diastolic
internal diameter, collagen proliferation and TGF-
Post-MI myocardial interstitial ischemic edema can decrease myocardial
compliance and contractility, increase left ventricular end-diastolic pressure
and volume, and eventually progression to HF or even death. MiR-30d inhibited
fibroblast proliferation and activation by directly targeting integrin
In the early stage of cardiovascular diseases, such as chronic diseases,
degenerative valve diseases and cardiomyopathies, left ventricular wall
thickening and myocardial fibrosis progression result in reduced cardiac
compliance and function, which leads to left ventricular enlargement and the
development of left heart failure in the late stage. Studies have shown that
miRNAs are also involved in left ventricular fibrosis due to these diseases [39]. For
example, the miR-195 expression level was remarkedly reduced in hypertensive
rats, with disorganized myocardial cells, thickened myocardial fibers and
myocardial fibrosis in a hypertension group compared to controls. Overexpression
of miR-195 inhibited TGF-
The right ventricle is under volume or pressure overload due to left heart
failure, heart valve diseases, pulmonary artery hypertension (PAH) and other
diseases, which leads to the formation of right ventricular fibrosis, and scar
tissue causes right ventricular dysfunction. In PAH, sustained pressure overload
exerts mechanical stress on the right ventricular interstitium and CFs, which
increases collagen production by releasing TGF-
Hsu et al. [17] found that the levels of circulating miR-29a-3p and
thrombospondin-2 (THBS2) decreased and increased, respectively, in mice and
patients with PAH. MiR-29a-3p directly targets and regulates THBS2 expression to
inhibit the proliferation of CFs, which exerts a direct antifibrotic effect on
PAH-induced cardiac fibrosis. MiR-325-3p targets and regulates human epididymis
protein 4 to activate the PI3K/AKT signaling pathway, and the action of this
miRNA was found to inhibit CF transformation and attenuate right ventricular
fibrosis in PAH rats [18]. A mitochondrial metabolism study of PAH-induced right
ventricular fibrosis revealed that increased pyruvate dehydrogenase activity
inhibited mitochondrial superoxide dismutase 2 (SOD2) and H
Atrial fibrillation (AF) is the most common cardiac arrhythmia and a result of
atrial remodeling. Atrial remodeling, characterized by persistent biventricular
enlargement and fibrosis of myocardial tissue, is caused by atrial volume or
pressure overload and eventually progresses to diastolic insufficiency or even
sudden death by thromboembolism. MiRNAs are significantly differentially
expressed in AF. For example, miR-21, miR-23b-3p and miR-27b-3p expression has
been found to be significantly increased in atrial tissue of patients with AF
[21, 22, 43], but the levels of miR-132 and miR-443 were decreased [23, 24]. The main
target of miR-21 is TGF-
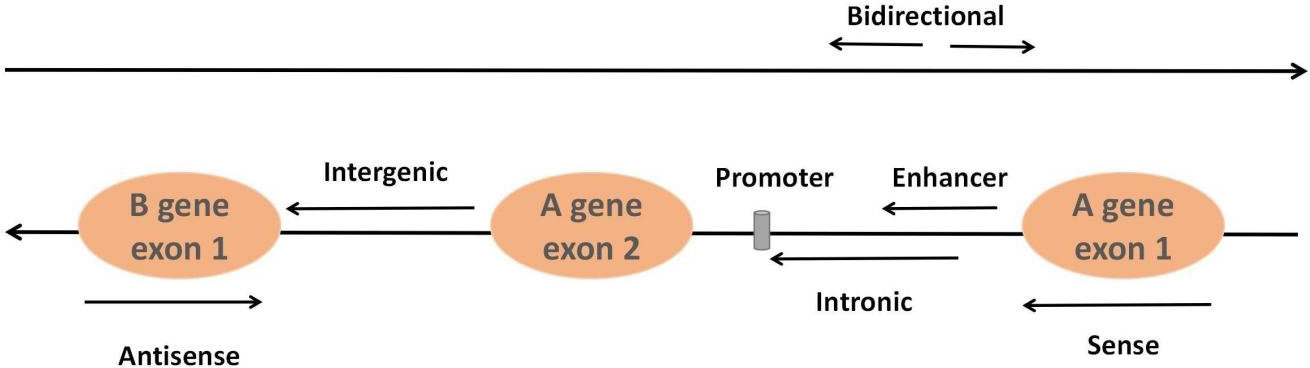
LncRNAs, accounting for approximately 80%–90% of all ncRNAs, constitute a class of ncRNAs more than 200 nucleotides in length. Most lncRNAs are transcribed by RNA polymerase II and modify both ends of the seven-methylguanosine triphosphate cap at the 5´ end and the polyadenylate tail at the 3´ end by utilizing the same splicing signal as the coding gene. They can be classified into six types based on their relative position in the genome of neighboring coding regions: sense lncRNAs, antisense lncRNAs, intergenic lncRNAs, intronic lncRNAs, enhancer lncRNAs and bidirectional lncRNAs [45] (Fig. 2). The classical mechanisms of lncRNAs can be divided into four categories: signal, decoy, guide and scaffold [46]. Signal: lncRNAs not only regulate neighboring genes in cis conformation but also play a trans-regulatory role in the expression of genes that are not closely related to their transcription sites. Decoy: the lncRNA-miRNA-mRNA axis, and lncRNAs adsorb and inhibit miRNAs to regulate mRNA molecular functions through molecular sponge action. Guidance: lncRNAs can recruit and bind related proteins through molecular interactions and guide complexes to specific targets. Scaffolding: lncRNAs interact with chromatin modification complex components or proteins such as transcription factors to bind to molecular scaffolds that influence gene expression. The advantages of lncRNAs that can be used as potential biomarkers include high stability and detectability, accurate quantification by highly sensitive methods such as real-time PCR and the fact that changes in the levels of lncRNAs may reflect the underlying mechanisms of disease.
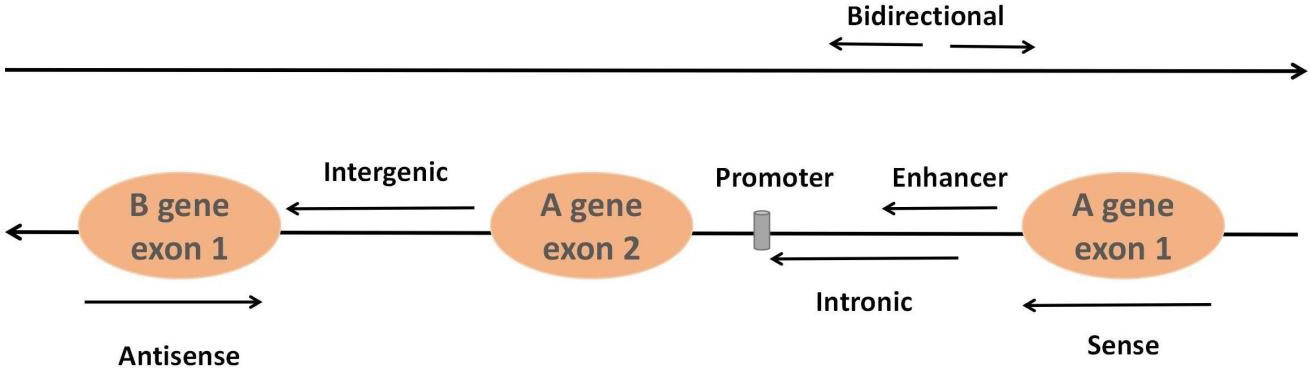 Fig. 2.
Fig. 2.Classification of lncRNAs. Sense lncRNAs are transcribed from the sense coding chain with exons, and they usually cover or overlap with protein-coding genes. In contrast, antisense lncRNAs are transcribed from genes encoding antisense proteins. Intergenic lncRNAs refer to lncRNAs in the genomic interval between two genes. Intronic lncRNAs are formed from the introns of second transcripts. Bidirectional lncRNAs are transcribed from the opposite direction and spaced approximately 1 kb apart. Enhancer lncRNAs originate from enhancer regions in protein-coding genes.
In recent years, significant progress has been made in the study of lncRNAs
regulating cardiac fibrosis (Table 2, Ref. [47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62]). Zhang et al. [47] demonstrated
that the lncRNA H19 level was significantly downregulated in mice with MI.
Functionally, enforced H19 expression dramatically reduced infarct size and
improved cardiac functions by mitigating myocardial apoptosis and decreasing
inflammation. Mechanistically, H19 regulated the expression of KDM3A to
ameliorate MI-induced myocardial injury in a miR-22-3p-dependent manner. The
expression level of the lncRNA MHRT was increased in mice with MI or cardiac
fibrosis and treated with TGF-
| LncRNA | Stimulation | Target gene | Function | Reference |
| H19 | MI, AF | miR-22-3p/KDM3A | Anti | [47, 56] |
| miR-29a/b-3p, VEGFA | ||||
| 554 | MI | TGF- |
Anti | [49] |
| Gpr19 | MI | miR-324-5p, Mtfr1 | Anti | [50] |
| Wisper | MI | TIAI | Anti | [53] |
| NRON | Ang II | miR-23a | Anti | [57] |
| Chast | TAC, AS | Pleckstin | Anti | [59] |
| SOX2OT | HF | TGF- |
Anti | [60] |
| KCNQ1OT1 | DOX | FUS | Anti | [61] |
| MHRT | TGF- |
miR-3185 | Pro | [48] |
| TUG1 | MI | miR-133b/CTGF | Pro | [51] |
| XIST | AMI | miR-155-5p | Pro | [52] |
| PVT1 | AF | miR-128-3p/TGF- |
Pro | [54] |
| NEAT1 | AF | miR-320/NPAS2 | Pro | [55] |
| MALAT1 | HBP | SMA | Pro | [58] |
| ROR | VMC | C-myc, IL-6 | Pro | [62] |
| FUS, fusion-type sarcoma; Gpr19, G protein-coupled receptor 19; KCNQ1OT, KCNQ1 opposite strand/antisense transcript 1; KDM3A, lysine-specific demethylase 3A; MALAT1, Metastasis-associated lung adenocarcinoma transcript 1; Mtfr1, mitochondrial fission regulator 1 Antibody; NEAT1, nuclear-enriched abundant transcript 1; PVT1, plasmacytoma variant translocation 1; TUG1, taurine upregulation gene 1; XIST, X-inactive specific transcript; Wisper, Wisp2 super-enhancer-associated RNA. | ||||
LncRNAs, important regulators of atrial fibrosis that can increase CF
proliferation and collagen expression leading to excessive RCM deposition, can
promote the formation of atrial fibrosis in AF by competing with endogenous
miRNAs. These lncRNAs have been found in pathways and are involved in the
regulation of atrial fibrosis. For example, the lncRNA PVT1 and the lncRNA NEAT1
were increased in atrial muscle tissue of AF patients and positively correlated
with collagen I and III [54, 55]. PVT1 overexpression facilitated
TGF-
In addition, lncRNAs play important regulatory roles in other diseases. For
example, Li et al. [58] found that overexpression of the lncRNA MALAT1
increased arterial smooth muscle cell activity and caused severe myocardial
fibrosis in spontaneously hypertensive rats. The lncRNA Chast was specifically
upregulated in hypertrophic myocardial tissue of mice with aortic coarctation and
patients with aortic stenosis and negatively regulated protein family M member
one of the pleckstrin homologous structural domains (in the strand opposite to
that carrying Chast), impeding cardiomyocyte autophagy and hypertrophy,
suggesting that Chast may be a potential target for preventing cardiac remodeling
[59]. HF is the end-stage manifestation of cardiovascular disease. SOX2OT
knockdown reduced myocardial injury and collagen in HF mice, and the expression
of collagen I,
CircRNAs are novel endogenous ncRNAs that show high conservation and stability. CircRNAs can be divided into three categories depending on the source: intron (circular intronic RNA, ciRNA); exon (exonic circular, ecRNA); and exon and intron (exon–intron circular RNA, EIciRNA). Target genes are regulated by sponging miRNAs, interacting with proteins and regulating the degradation and stability of mRNAs. An increasing number of studies have reported that circRNAs are emerging as regulators of pathophysiology in many diseases. However, the expression and function of circRNAs in cardiac fibrosis remain largely unknown.
Zhang et al. [63] found that in inducing CF activation with
TGF-
| CircRNA | Stimulation | Target gene | Function | Reference |
| BMP2k | TGF- |
miR-455-3p/SUMO1 | Anti | [63] |
| LAS1L | AMI | miR-125b/SFRP5 | Anti | [64] |
| CELF1 | AMI | miR-636/DKK2 | Anti | [66] |
| Fndc3b | MI | FUS | Anti | [67] |
| Yap | TAC | TPM4, ACTG | Anti | [68] |
| NIgn | DOX | H2AX | Anti | [69] |
| Foxo3 | HT | miR-433, miR-136 | Anti | [70] |
| FSCN1 | HT | tDCs | Anti | [71] |
| Ube3a | AMI | miR-138-5p/Rhoc | Pro | [65] |
| 000203 | Ang II | miR-26b-5p | Pro | [72] |
| BMP2K, BMP-2 inducible kinase; CELF1, gugbp Eeav-like family member1; Foxo3, forkhead box O3; FSCN1, fascin actinbundling protein 1; Fndc3b, fibronectin type III domain-containing protein 3B; LAS1L, LAS1-like; SUMO1, small ubiquitin-like modifier-1; SFRP5, secreted frizzled related protein 5; tDCs, tolerogenic dendritic cells; TPM4, tropomyosin-4; Ube3a, ubiquitin protein ligase E3A; Yap, Yes-associated protein. | ||||
Age exacerbates mortality from cardiovascular disease in elderly individuals.
During cardiac aging, the accumulation of senescent cells and the deposition of
ECM with collagen lead to a progressive decline in cardiac functions. A 3%
increase in ECM volume and a 50% increase in the risk of all-cause mortality
have been reported [73]. Studies have shown that ncRNA expression correlates with
aging. For example, miR-1468-3p expression has been shown to be increased in
healthy elderly hearts and to promote cardiac fibrosis by enhancing
TGF-
Viral myocarditis (VMC) is local or diffuse damage to the myocardial parenchyma
or interstitium caused directly by viral infection or indirectly by immune system
dysfunction and is accompanied by cardiomyocyte destruction, reparative fibrosis
and ultimately HF. NcRNAs regulate the viral life cycle and immune and
inflammatory responses by targeting viral or host genes. MiR-155, lncRNA AK085865
and lncRNA MEG3 regulated macrophage polarization and reduced myocardial injury,
which was correlated with a reduction in the development of myocardial fibrosis
[35, 74, 75]. Studies in mice with autoimmune myocarditis revealed that silencing
miR-21a-5p resulted in a significant reduction in TNF
In recent decades, the mechanisms of drug-induced cardiotoxicity, mainly DNA
damage, excessive reactive oxygen species production, mitochondrial dysfunction,
endoplasmic reticulum-mediated apoptosis, and disruption of calcium homeostasis
have been studied. With the deepening of research, it has been shown that ncRNAs
play key roles in the mechanisms of cardiotoxicity induced by drugs such as
doxorubicin (DOX) [78, 79]. For example, silencing miR-34a upregulated B
lymphocytoma-2, and sirtuin 1 attenuated DOX-induced cardiotoxicity, reducing the
apoptosis rate, attenuating the inflammatory response, and inhibiting senescence
and fibrosis in rats [32]. MiR-133b attenuated polypyrimidine tract
bundle-binding protein 1 and transgelin 2 to regulate apoptosis and mediate
cardiac fibrosis induced by adriamycin, implying that miR-133b may be a potential
biomarker of adriamycin-induced cardiac injury [33]. Endogenous miR-495-3p
protects cells against DOX-induced cardiotoxicity by activating the AKT pathway
in vivo and in vitro [36]. In addition, the expression of the
lncRNA KCNQ1OT1 downregulated fusion-type sarcoma in oocytes and reduced the
myocardial fibrosis area in DOX-treated mouse models [61]. Increasing the
expression of circ-Nlgn decreased cardiac function and induced cardiac fibrosis
by upregulating Gadd45b, Sema4C and RAD50 and activating p38 and pJNK in circNlgn
transgenic mouse hearts. Silencing circ-NIgn prevented DOX-induced expression of
fibrosis-associated molecules. The Nlgn173 protein translated by circ-NIgn can
bind and activate H2AX and the production of
Heart transplantation (HT) is the best treatment option for end-stage HF. However, HT can cause cardiac ischemia reperfusion injury. In a mouse model of ectopic HT, 59 miRNAs were dysregulated in transplanted hearts with ischemia reperfusion injury compared to undamaged transplanted hearts [80]. In contrast, circ-Foxo3 is a newly discovered molecular regulator that protects cardiac grafts from prolonged ischemia reperfusion injury during HT. MircFoxo3 also indirectly affects miR-433 and miR-136 expression [70]. In addition, many ncRNAs can be used as noninvasive detection indicators, such as for the assessment of myocardial injury after HT. MiR-133b has been found to be an important marker of myocardial injury and to be associated with hemodynamic changes evident early after transplantation [34]. Circ-FSCN1 silencing produced tolerogenic dendritic cells, which prevented alloimmune rejection in HT, prolonged patient survival and reduced myocardial fibrosis [71]. However, whether functional interventions based on specific ncRNAs can block the development of long-term fibrosis after HT and thus improve survival after transplantation still needs to be explored in the future.
With the rapid development of the bioinformatics technologies, such as RNA sequencing and genomics, the role of ncRNAs in cardiovascular diseases has been extensively studied. NcRNAs are important regulators of cardiovascular disease through transcriptional regulation, posttranscriptional regulation and epigenetic level regulation of gene expression, especially in relation to the development of myocardial fibrosis. The construction of a cardiac fibrosis-specific ceRNA regulatory network could help further elucidate the molecular mechanism of the cardiac fibrosis process and provide plausible target genes for future research in this field. However, due to the limitations of the current experimental technologies, ceRNA regulatory networks are mainly confirmed by bioinformatics techniques to confirm their molecular mechanisms, large sample of population studies are still needed to explore the diagnosis, target therapy and prognosis of cardiovascular diseases. And the further study also is needed to confirm the molecular mechanisms of ncRNAs by constructing animal and cellular models of cardiovascular diseases. In addition, interspecies variability makes it more difficult to explore the the mechanisms of action of homologous ncRNAs through in vivo experiments. Finally, there is lack of a ceRNA network that can show regulator actions between coding RNAs and non-coding RNAs.
CYW and SLB designed the study, and wrote the manuscript. RJL, HS and YZP provided help and advice on images, tables and manuscript development. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
The authors thank Figdraw because the figures were created with figdraw.com.
This study was support by the National Nature of Science Foundation of China (82160439) and Basic Research Plan Project of Yunnan Provincial Science and Technology Department (202001AY070001-028).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.