1 Department of Medicine, Division of Cardiology, Alpert Medical School of Brown University, Providence, RI 02903, USA
2 Lifespan Cardiovascular Institute, Providence, RI 02903, USA
3 Cardiovascular Medicine, Cleveland Clinic, Cleveland, OH 44195, USA
4 Cardiovascular Medicine, Baylor College of Medicine, Houston, TX 77030, USA
Abstract
Since its food and drug administration (FDA) approval in 2011, transcatheter aortic valve replacement (TAVR) has revolutionized the highly prevalent disease of aortic stenosis. In this review, we present a comprehensive overview of the data and considerations for utilization of TAVR in special populations who were either excluded from or not adequately represented in the seminal TAVR trials, due to high-risk valvular and/or systemic factors. These include nonagenarians, patients with renal dysfunction, chronic thrombocytopenia, bicuspid aortic valve, rheumatic valve disease, patients with failed aortic valve bioprosthesis requiring valve-in-valve intervention and patients with mixed aortic valve disease. In short, TAVR is a feasible therapeutic strategy in high-risk and special populations with mortality benefit and improvement in quality of life. Randomized controlled trials in high-risk populations are recommended to confirm results from observational studies.
Keywords
- aortic valve replacement
- transcatheter aortic valve replacement
- AVR
- bioprosthesis
- special population
Aortic stenosis (AS) is the second most common valvular lesion in the United States, following mitral regurgitation, with a prevalence of about 12.4% in the elderly [1]. In patients above 75 years of age, 2%–4% have severe AS [1, 2, 3]. By the time patients present with severe AS, comorbidities acquired with aging render many of them high risk for surgical aortic valve replacement (SAVR). This created a need for a minimally invasive approach for aortic valve replacement (AVR). In 1990, a patent for transcatheter aortic valve replacement (TAVR) was filed, and granted in 1995 [4]. Subsequent research and development led to the Placement of Aortic Transcatheter Valve (PARTNER) trials from 2010 to 2019 as well as the trials for self-expanding bioprosthesis [5, 6, 7, 8]. These trials established the benefit and efficacy of TAVR leading to the approval of Edwards Sapien valve by the food and drug administration (FDA) in 2011, for high-risk patients with severe AS who were not eligible for SAVR. Subsequently, the valve was approved as an alternative to surgery in high-risk (2012), followed by intermediate (2016), and low-risk patients (2019) with severe AS.
In this paper, we review the safety and efficacy of TAVR in special categories of patients who were minimally represented in or totally excluded from the pivotal TAVR trials due to systemic or valvular features that impart elevated risk for poor outcomes.
Patients with significant renal dysfunction with serum creatinine
Few studies have compared outcomes with TAVR, SAVR, and conservative management
in patients with ESRD. In a large observational study from the US, data from the
National Medicare Provider and Analysis Review (MEDPAR) Part A files were
analyzed to assess outcomes of patients with ESRD on HD who underwent TAVR versus
SAVR and showed improved short- to mid-term outcomes after TAVR as compared to
SAVR (4.6% versus 12.8%, p
A second retrospective observational study from European centers in France and
Belgium showed that moderate and severe chronic kidney disease (CKD) was
associated with a significant increase in all-cause (adjusted HR [95% CI] =1.36
[1.08–1.71], p = 0.009) and cardiovascular mortality (adjusted HR [95%
CI] = 1.39 [1.03–1.88], p = 0.031) [14]. However, propensity-matched
analysis showed that aortic valve replacement (SAVR or TAVR) led to a significant
improvement in all-cause and cardiovascular mortality at 5 years, irrespective of
the CKD stage, relative to conservative management (Cox time varying covariate
p
These findings would indicate that, despite being a higher risk cohort, patients with renal dysfunction may still derive significant benefit from undergoing valve replacement and warrant a shared decision-making to address risks and benefits. In patients who are not yet dialysis dependent, judicious use of contrast during TAVR is critical.
Due to the need for large bore access, there is a risk of major bleeding
associated with TAVR procedure, found to be 10.2% (
Aortic stenosis is a disease of age (barring congenital malformations). In the
PARTNER-I trial, there were 531 patients above 90 years of age; 329 of them
underwent transfemoral and 202 underwent transapical TAVR [5]. This amounted to
50% of the high risk and nonsurgical cohort who were randomized to TAVR versus
conservative management. These patients had improvement in their functional
status from baseline. Furthermore, patients who had undergone transfemoral TAVR
had a slightly higher stroke risk (3.6% versus 2%) but improved 30-day and
three-year mortality as compared to transapical TAVR. Subsequent analysis using
the Transcatheter Valve Therapy registry has shown similar outcomes between
nonagenarians and octogenarian [20]. Utilizing Medicare data, Mentias et
al. [21] demonstrated a temporal trend of improved outcomes in both patients
Bicuspid aortic valve (BAV) is the most common congenital anomaly of the valves
representing about 25% of patients
Although there has been a decline in the prevalence of rheumatic heart disease (RHD) in the developing world, it still represents a significant clinical burden of disease in the low-income countries [27]. Pathologically, RHD is associated with fibrotic changes in the aortic valve anatomy, rather than a purely calcified degeneration which represents a unique challenge in terms of appropriate deployment and anchoring of the TAVR bioprosthesis. Therefore, aortic stenosis due to RHD was excluded from the pivotal randomized control trials for TAVR [5, 8]. However, newer iterations of TAVR valves have been designed to improve anchoring and reduce prosthesis migration and paravalvular leak. New valves (e.g., JenaValve) are being examined in patients with predominant aortic regurgitation and can be used in RHD patients with less degree of annular calcification and concerns about anchoring [28]. In an analysis of Medicare patients from 2015 to 2017, Mentias et al. [29] showed that there was no difference in 30-day and mid-term mortality in patients with rheumatic AS who underwent SAVR versus TAVR (11.2 versus 7.0 per 100 person-year, HR 1.53, 95% CI 0.84–2.79, p = 0.2). Furthermore, TAVR had similar outcomes for patients with rheumatic versus nonrheumatic AS in terms of in-hospital and mid-term mortality, 30-day stroke, heart failure admissions, AKI, and blood transfusion. TAVR was also found to have a favorable intermediate-term outcome in a median follow-up of 19 months, with a lack of need for repeat valve replacement or repair.
Bioprosthetic valves carry the inherent risk of structural valve deterioration (SVD) within 10–20 years [30]. Historically, the only alternative in patients suffering from SVD was redo SAVR which comes with a higher operative risk compared to a primary valve replacement [31, 32]. Valve-in-Valve (ViV) TAVR not only offers a lower risk alternative for patients afflicted with SVD of a bioprosthetic valve but may also have implications on initial selection of mechanical versus biological prosthesis. Over the past two decades, TAVR has been proven to be a viable option for all degrees of surgical risk leading to an increase in TAVR implantation. Inevitably, ViV TAVR has therefore been pursued, with techniques being refined over time for both prior surgical valves as well as TAVR valves. According to the most recent report from the STS-ACC TVT registry, planned ViV TAVR is increasing, the majority of which are performed for failed surgical bioprosthetic valves (TAVR-in-SAVR), with more than 15,000 cases performed between 2012 and 2019, and only 404 TAVR-in-TAVR cases performed over the same period [33].
A meta-analysis of 23 studies, through July 2020, showed no difference in 30-day
and 1-year mortality as well as 30-day stroke risk of ViV TAVR compared with
either redo SAVR or primary TAVR [34]. A more recent systematic review and
meta-analysis of 9 studies, with a total of 9100 patients, showed lower 30-day
mortality associated with ViV TAVR (OR, 0.56; p
Deployment of ViV transcatheter heart valve (THV) has several important concerns and considerations, including coronary ostial obstruction, patient prosthesis mismatch, elevated postprocedural gradients, high-grade atrioventricular block requiring permanent pacemaker placement, and leaflet thrombosis. One of the current challenges is confirming optimal THV expansion during the procedure, which is critical to achieving adequate blood flow restoration, and reducing PVL, and residual gradients. While preprocedural CT scan assessment is the current standard of care to estimate the nominal THV sizing and the required percentage of oversizing, it may not correlate well with the actual THV expansion which is impacted by many factors including the degree of landing zone calcification, 3D anatomy, etc. Currently, there is no validated measure to assess the actual THV expansion in real time intraprocedurally. A case series from Poland published in 2020 described the use of large field intravascular ultrasound to guide and assess TAVR deployment. This may represent a step toward exploring a valid method of optimizing THV deployment and reducing postprocedural transvalvular gradients and PVL. Further studies are needed to assess optimal measures in this regard [36].
A virtual distance of
Mixed aortic valve disease (MAVD) is defined as presence of both severe AS and
moderate to severe aortic regurgitation (AR). This patient population was
excluded from the landmark TAVR trials and the guidelines for management of
valvular heart disease recommended evaluation of individuals and treatment
according to the predominant lesion [5, 6, 7, 8, 42, 43]. The pathophysiology of MAVD
is unique as the left ventricle is faced with both an increased afterload due to
severe AS and volume overload due to AR. Since the seminal TAVR trials, several
observational analyses have demonstrated similar or improved outcomes in patients
with MAVD as compared to pure AS, especially in patients who developed post
procedural AR. In a single center study of 1133 patients, Chahine et al.
[44] demonstrated improved survival and lower 3-year mortality rates in patients
with MAVD versus pure AS (15.3% versus 20.4%; p = 0.02). This effect
was driven predominantly by improved survival in patients who developed post TAVR
AR. Similar findings were demonstrated in a cohort of 622 patients from Cleveland
clinic; although moderate or severe central or paravalvular AR was more common
(15.5% versus 6.7%, p = 0.004) and device success was less prevalent
in MAVD (81% versus 88.9%, p = 0.027), the univariable survival was
better in patients with MAVD as compared to pure AS (71.3% versus 62.6%;
p = 0.02) [45]. Grant et al. [46] published an analysis
utilizing Nationwide Readmissions Database, identifying 100,573 TAVR patients and
3260 patients with MAVD. In this study, in-hospital mortality (2.5% versus
2.6%, p = 0.53) and rates of paravalvular leak (1.0% versus 1.3%,
p = 0.05) were similar in MAVD versus pure AS, MAVD was not a
significant predictor of mortality (adjusted odds ratio [adjOR] 1.25, p
= 0.05), was associated with decreased odds of 30-day (adjOR 0.05, p
The improvement in survival in MAVD patients, especially in the cohort with post procedural AR is proposed to be driven by the preexisting remodeling to accommodate AR as compared to patients who face this challenge de novo post procedurally, i.e., patients with pure AS. Further randomized controlled trials are needed to confirm and expand on these findings, but TAVR appears to be a safe option in patients with MAVD.
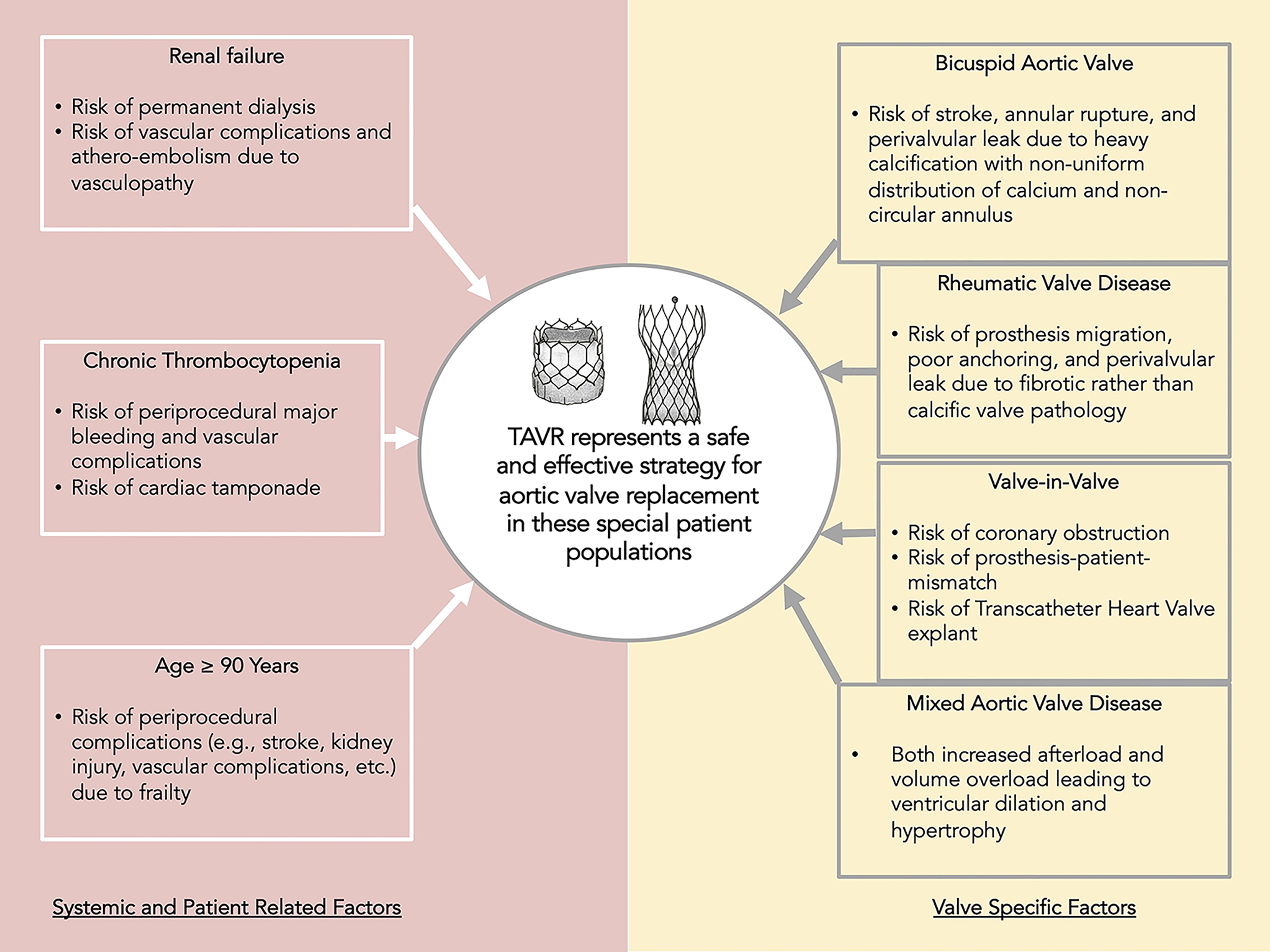
TAVR has effectively revolutionized the treatment of aortic stenosis. Since its inception and adoption, it has been shown to be a cost-effective solution improving both quality of life and mortality in patients across the entire range of surgical risks. Real world data has shown that it is also an effective strategy in patients with special risk conditions; both systemic and pertaining to valvular anatomy (Fig. 1). Further studies to optimize approach and improve outcomes in each of these higher risk conditions are imperative to advance the evolving therapy of TAVR for severe aortic stenosis.
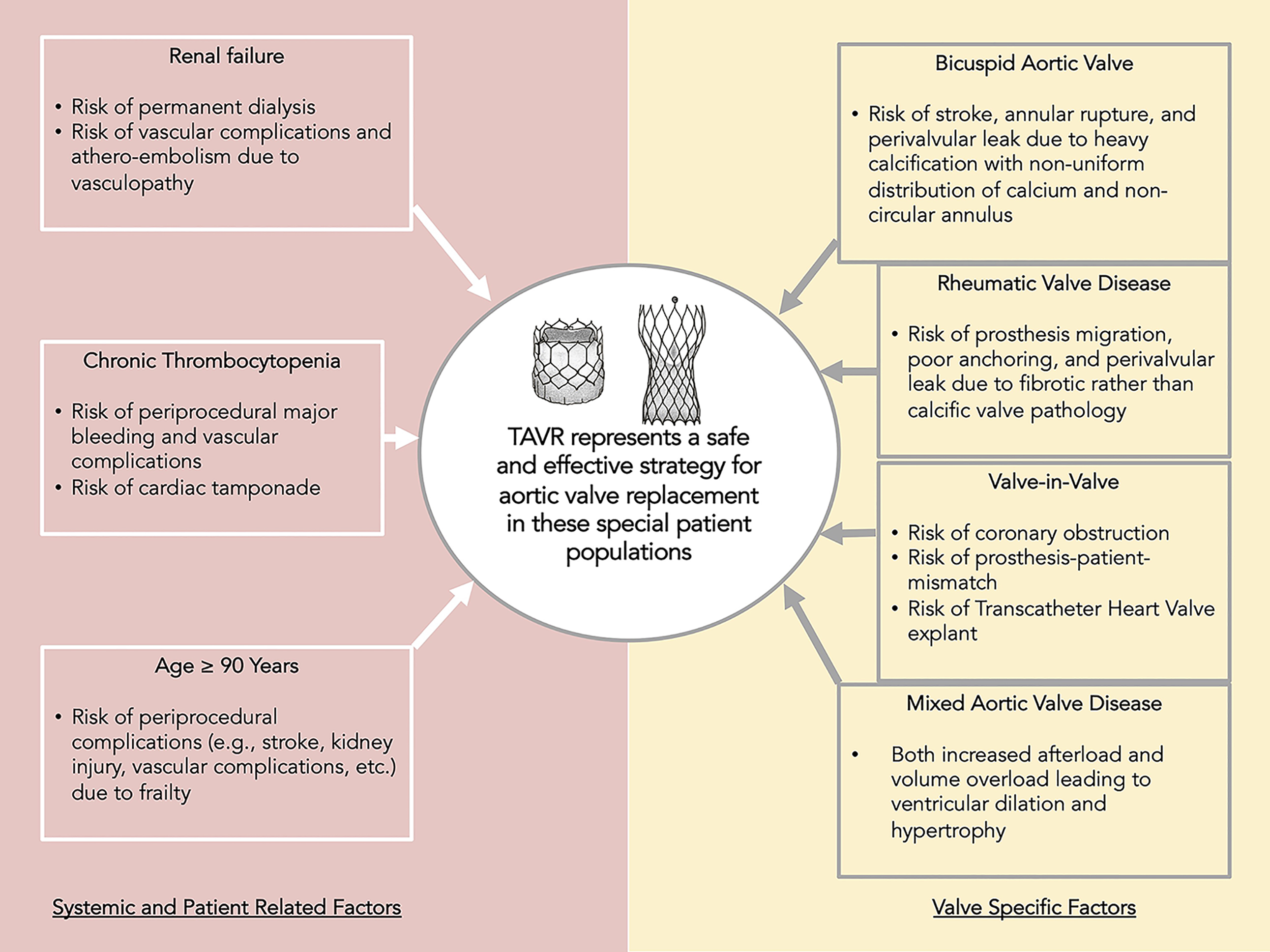 Fig. 1.
Fig. 1.Central Illustration. Despite these considerations of each category of special population of patients, TAVR is an effective therapeutic strategy in severe aortic stenosis which offers improvement in quality of life as well as survival.
KA and MS—conception and design of the work; KA—drafting the manuscript; MS—critical editing and revision for important intellectual content. AM, HI, AE, OH, PG and BS performed critical revision of the work. All authors approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.