1 Cardiology Division, Department of Medicine, University of Verona, 37126 Verona, Italy
2 Nuclear Medicine, ASST Spedali Civili Brescia, 25123 Brescia, Italy
3 Department of Nuclear Medicine, University of Brescia, 25123 Brescia, Italy
4 Cardiovascular and Imaging Departments, CNR Research Area, Fondazione CNR/Regione Toscana Gabriele Monasterio, 56124 Pisa, Italy
Abstract
Myocardial perfusion single photon emission computed tomography (SPECT) is widely used in assessing coronary artery disease (CAD) owing to its proven efficacy in extensive clinical experience. Like other functional tests, myocardial SPECT is recommended for the diagnosis of obstructive CAD, risk stratification assessment, and treatment decision making. Besides quantifying left ventricular volume, global and regional function by electrocardiography (ECG)-gated acquisition, myocardial SPECT can identify myocardial ischemia, scars, stunning, and viable hibernating myocardium. It provides comprehensive functional data across the spectrum of CAD and a cost-effective strategy in patients with intermediate pre-test probability of CAD or with a history of ischemic cardiomyopathy. With ongoing advances in cardiovascular prevention and risk factor management many patients referred for testing now have a low-to-intermediate probability of CAD. Besides, CAD has become a chronic condition resulting from novel therapeutic strategies. Against this background, approaches combining anatomical and functional tests in sequence or simultaneously include coronary artery calcium score integrated with perfusion imaging or fusion SPECT/coronary computed tomography angiography (CCTA). In this review we summarize current indications for myocardial perfusion SPECT and integration of SPECT with other imaging techniques to improve diagnostic performance, patient management, and outcome prediction in CAD.
Keywords
- nuclear imaging
- myocardial perfusion scintigraphy
- coronary artery disease
- multimodality imaging
Myocardial perfusion imaging (MPI) constitutes a milestone in diagnosis and management of coronary artery disease (CAD). By virtue of its ability to detect stress-induced myocardial perfusion defects, single photon emission computed tomography (SPECT) is a primary tool for diagnosis of obstructive CAD. It is also useful in risk stratification of patients with suspected or known CAD [1, 2, 3]. Finally, functional SPECT data on total ischemic burden, ischemia site, extension and severity can inform treatment decisions [4].
The shared view of the “ischemic issue” was embodied by the ischemic cascade [5] and the principle that greater coronary stenoses trigger more severe ischemia. Perfusion abnormalities were thought to occur soon in the ischemic cascade and derive from stenoses of borderline significance, giving reason for the high sensitivity of SPECT in detecting obstructive CAD. Furthermore, the degree and the extent of ischemia was assumed to be directly related to event risk. Later evidence showed, however, that, because of the effect of atherosclerotic plaque and coronary microvasculature features on myocardial perfusion, interaction between atherosclerosis, stenosis, and ischemia is not linear [6]. In addition, the prognostic implications of anatomical and functional abnormalities vary widely. The results from trials like FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) [7], ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) [8], and ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) [9] have changed our perspective on myocardial ischemia and its implications for prognosis and clinical decision-making.
With the present review we summarize indications for myocardial perfusion SPECT in patients with suspected or known CAD and potential applications of MPI in multimodality imaging.
Since cardiac metabolism is predominantly aerobic, it depends on continuous
extraction of oxygen from coronary blood flow. Normal coronary flow to the left
ventricle under basal conditions is 0.6–1 mL/min/g but it can increase up to six
fold during increased demand. Cardiac arterioles and capillaries control blood
flow according to metabolic needs and are the main determinants of coronary flow
resistance. In detail, small coronary arteries (
In the diagnosis of ischemia, there are two techniques to detect stenosis of the
epicardial coronary arteries by increasing coronary flow: exercise or
pharmacological stressors that increase oxygen consumption or flow nonuniformity
through vasodilatation [12]. The physiological method to increase coronary flow
is physical effort. Pharmacological agents (mainly the vasodilators dipyridamole,
adenosine, and regadenoson and the sympathomimetic dobutamine) can be used in
patients unable to adequately exercise. In this setting, MPI with SPECT are
applied to evaluate coronary blood flow in stress and rest conditions by means of
radioactive tracers ([
| Myocardial state | Description | Perfusion SPECT | Gated SPECT |
|---|---|---|---|
| Normal | Normal myocardial perfusion and function | Normal stress and rest perfusion | Normal function |
| Ischemia | Reversible perfusion defect with or without myocardial functional abnormalities due to transient reduction in coronary blood flow | Reduced stress and normal rest perfusion | Normal or reduced during stress |
| Stunned myocardium | Myocardial dysfunction during stress which persists at rest despite restored normal resting myocardial blood flow; it can occur after stress-induced reversible ischemia or in myocardial infarction after restoration of coronary flow in a previously occluded vessel; myocardial function recovers after a variable amount of time | Reduced stress and normal rest perfusion | Reduced during and after stress, improved at rest |
| Hibernating myocardium | Dysfunctional but viable myocardium with mildly reduced resting myocardial blood flow resulting from repetitive ischemia and leading to chronic myocardial ischemia | Reduced stress and rest perfusion | Reduced function |
| Scar | Severely dysfunctional and not viable myocardium with severely reduced resting myocardial blood flow | Severely reduced stress and rest perfusion | Reduced function |
Reduced blood flow to the myocardium during stress and subsequent ischemia can
impair left ventricular contractility and regional or global function eventually.
One of the advantages of cardiac SPECT is its utility in functional assessment
via electrocardiography (ECG)-gated acquisition. Left ventricular ejection
fraction, volume, mass, myocardial systolic wall thickening, and contraction
dyssynchrony can be quantified [18, 19], thus providing detailed information
beyond those characteristic of myocardial perfusion imaging. ECG-gated
acquisition in the stress phase of SPECT can detect myocardial stunning [6, 20].
During ischemia, a regional perfusion deficit may be associated with impaired
contractility in the same region. After blood flow is restored, abnormal cardiac
function can persist for hours to days despite restoration of normal perfusion.
Since SPECT acquisition occurs at least 15 minutes after radiopharmaceutical
injection as per protocol, evidence of stress-induced regional perfusion
impairment associated with a persistent mild alteration in motion or thickening
in the same region is suggestive of stunned myocardium (Figs. 1,2). Evidence of
stunning in
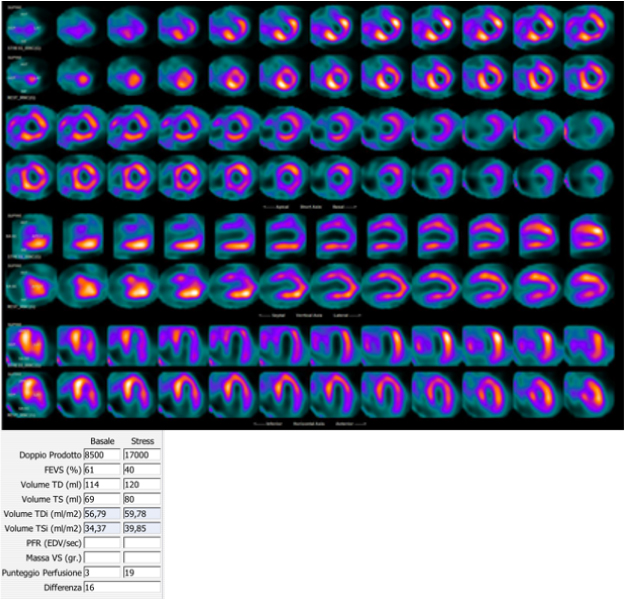 Fig. 1.
Fig. 1.99mTc-tetrofosmin cardiac SPECT with cadmium, zinc, tellurium (CZT) technology images of a 72-year-old woman with multiple cardiovascular risk factors symptomatic for typical effort angina. Perfusion SPECT revealed a reversible defect in the apex, septal, and anterior walls. Functional data showed an increase in left ventricular end-diastolic volume and end-systolic volume after stress compared to rest (120 versus 114 mL and 80 versus 69 mL, respectively); global systolic function was reduced after stress normal at rest (ejection fraction 40 versus 61%), compatible with myocardial stunning.
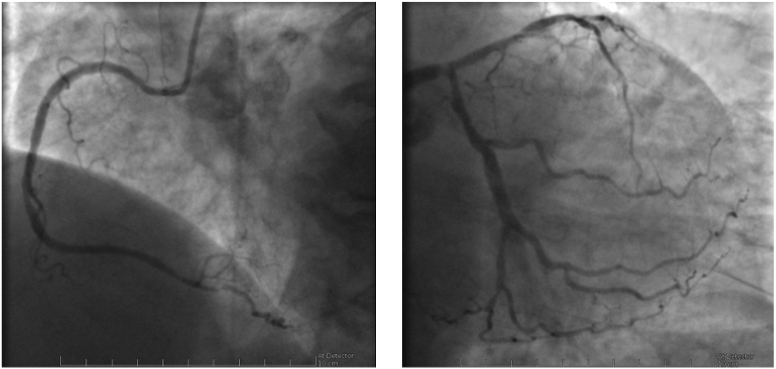 Fig. 2.
Fig. 2.Coronary angiography images of the case described in Fig. 1 showing diffuse CAD, with severe stenosis of the left main artery. Left: right coronary artery; Right: left main, anterior descendent and circumflex arteries.
The assessment of MPI at rest is essential for evaluating cardiac viability and
for differentiating impaired retention of perfusion tracers found in myocardial
stunning from decreased uptake due to decreased perfusion in hibernation or scar
[23, 24]. In patients with known CAD and prior infarction, the SPECT protocol
includes tracer injection after nitrate infusion to improve detection of the
viable hibernating myocardium [25]. The acute administration of nitrate increases
radiopharmaceutical uptake in dysfunctional territories which show functional
recovery after revascularization [26]. Furthermore, the combination of metabolic
and perfusion imaging via
Coronary artery disease is the leading cause of death worldwide and a major public health problem. The age-adjusted prevalence of CAD is approximately 7% in males and 4% in females [27]; the prevalence is 25% and 16%, respectively, in adults aged 60 to 79 years and is even higher in those 80 years or older [28]. Early detection of CAD is a primary strategy to reduce the burden of cardiovascular disease and mortality. As a perfusion imaging technique, myocardial SPECT provides a non-invasive tool for diagnosing obstructive CAD in symptomatic or asymptomatic patients at intermediate or high cardiovascular risk. Clinical referral for SPECT has increased in Western countries [29] and myocardial SPECT is contemplated by guidelines for the management of patients with known or suspected CAD, including risk stratification, treatment decision making, and prognosis.
There is international consensus on performing myocardial SPECT in at least four clinical scenarios: suspected or known stable CAD, suspected acute coronary syndrome, before non-cardiac surgery, and heart failure.
American and European guidelines recommend the use of myocardial perfusion SPECT as a diagnostic tool in chronic chest pain and suspected or known CAD [30, 31]. Besides its use in diagnosis, SPECT provides prognostic information and can guide patient management.
In patients with suspected CAD, non-invasive imaging modalities are the preferred method for diagnosing CAD [30], except in those with a high pretest probability in which coronary angiography is indicated instead (Class of Recommendation [COR] I, Level of Evidence [LOE] B [30], COR IIa, LOE C [32]) [31]. The application of myocardial SPECT or stress echocardiography is recommended in symptomatic patients with intermediate pre-test probability (PTP) of disease (arbitrarily defined between 15% and 85% estimated according to age, sex, and nature of symptoms [33]), especially in patients unable to exercise and with abnormalities at resting electrocardiography (COR I, LOE B). In patients with an intermediate PTP and an interpretable ECG and can adequately exercise (5 metabolic equivalents (METs) or more), exercise ECG may be considered an alternative according to the 2021 American guidelines (COR IIa, LOE B) [31]. However, the diagnostic utility of exercise ECG is lower than that of imaging testing. Most recent European guidelines [30] suggest that exercise electrocardiography be performed as a diagnostic tool only when other imaging modalities are unavailable (COR IIb, LOE B).
Non-invasive functional imaging to detect ischemia and anatomical imaging by coronary CT angiography (CCTA) are recommended in symptomatic patients. SPECT, because of its high rule-in power, is the preferred technique in patients at intermediate-high risk. SPECT has a diagnostic sensitivity of 82–88% for exercise and 88–91% for pharmacological stress testing and a specificity of 70–88% and 75–90%, respectively [5, 34]. Initial evaluation by stress MPI resulted in less downstream non-invasive and invasive testing in decision-making processes. In one study cohort [35], initial SPECT findings were abnormal in 29% of patients compared to 56% in those undergoing CCTA, and a high proportion of patients underwent a functional test following positive CCTA. Symptomatic patients with suspected CAD but normal SPECT findings have a favorable prognosis (0.6% event rate according to pooled analyses), similar to the general population risk [36]. Differently, abnormal findings may be suggestive of an increased event risk, in which the event rate is related to patient risk profile, left ventricular function, and ischemic burden [37].
Non-invasive tests may also be considered in patients with a PTP between 5% and
15%, especially when the clinical likelihood is greater due to risk modifiers
such as cardiovascular risk factors, electrocardiographic abnormalities, left
ventricular dysfunction, and coronary calcifications on CT findings [38, 39, 40, 41]. Data
from the PROMISE trial showed a prevalence of CAD in 7% of patients with low CAD
probability (
In patients with known CAD, the utility of myocardial SPECT relies on its ability to define the site and severity of ischemia. In this population, guidelines-directed medical therapy is key to slowing disease progression, improving symptoms, and preventing acute atherothrombotic events, as well as relief from angina and prognosis improvement. In patients with stable chest pain, guidelines currently recommend an ischemia-based strategy to guide revascularization, with the use of stress imaging to estimate disease severity and identify target lesion(s), especially in moderate coronary stenoses at angiography and in lesions of uncertain functional significance. Applied in stress testing, myocardial SPECT can help identify the site and the extent of inducible perfusion defects, and the anatomical distribution of a hemodynamically significant coronary stenosis (Fig. 3).
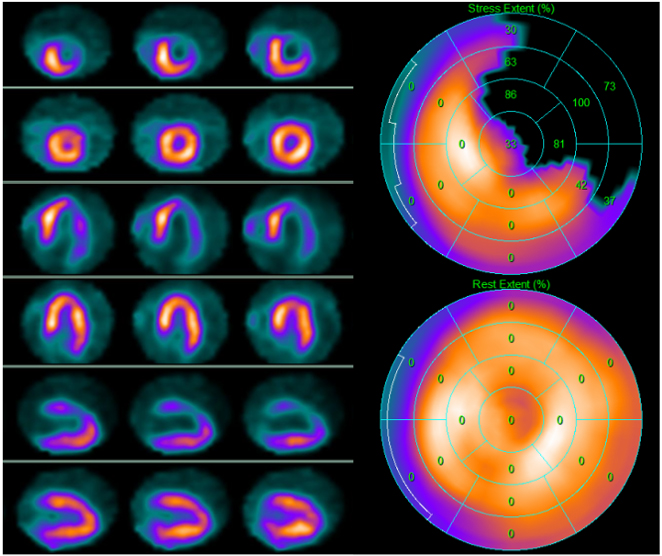 Fig. 3.
Fig. 3.99mTc-tetrofosmin cardiac single photon emission computed tomography (SPECT) images in a 74-year-old woman with arterial hypertension, diabetes mellitus, and a history of obstructive CAD without myocardial infarction treated with triple coronary artery bypass surgery five years earlier. Perfusion imaging was ordered to assess ischemia due to persistent atypical angina after medical therapy optimization. SPECT revealed a large reversible defect in the anterior and the lateral walls showing severe myocardial ischemia. Subsequent angiography revealed complete occlusion of the left main artery, occlusion of the saphenous vein graft anastomosed to the diagonal branch of the left anterior descendent coronary artery, and patency of the other two bypass grafts. Medical therapy was optimized. Left side top row: stress-rest short axis; stress-rest horizontal long axis; stress-rest vertical long axis; right side: polar map of stress (upper image) and rest perfusion (lower image).
Revascularization is indicated when large areas of ischemia are detected
(
Perfusion imaging identifies not only ischemia as reversible perfusion defects
but also myocardial scars as fixed perfusion defects. In a study involving 3216
patients with prior myocardial infarction undergoing SPECT, 70% presented scars
which were judged clinically significant in 25% of cases (
Functional testing after coronary revascularization is routinely performed in
clinical practice. According to observational data, 1 out of 2 to 3 patients
undergoes a stress test within two years after percutaneous revascularization
[50, 51, 52]. In a study involving 1848 patients undergoing revascularization mainly
for acute coronary syndromes, 1 out of 8 underwent an imaging stress test even if
asymptomatic. This approach led to repetition of revascularization in only
The risk for major adverse cardiac events (MACE) on follow-up was reported to be
proportional to the magnitude of residual ischemia and that a 5% reduction in
ischemia had a significant prognostic benefit [45]. The observational BASKET LATE
IMAGING study [58] reported that abnormal SPECT findings 5 years after
revascularization were frequent regardless of symptoms and predictive of adverse
events. While re-evaluation of risk may be useful to guide intensification of
medical therapy in selected high-risk patients, perfusion imaging is rarely
appropriate unless symptoms or a change in clinical status occurs, especially if
it is performed less than 2 years after PCI or less than 5 years after coronary
artery bypass graft (CABG) surgery. Patients in which myocardial SPECT is
strongly recommended have previous obstructive CAD (myocardial infarction or
coronary revascularization, COR I, LOE B) or known non-obstructive CAD (COR IIa,
LOE C) but only when stable chest pain persists despite optimal medical therapy
[31, 54]. Guidelines recognize the role of perfusion imaging for risk assessment
after percutaneous or surgical revascularization in patients with incomplete
revascularization, left main artery or proximal left anterior descending disease,
diabetes or other high-risk factors [59, 60]. In patients with limiting angina and
insufficient response to optimized medical therapy or significant anatomical or
functional CAD (
Perfusion imaging has demonstrated its utility in the emergency department for triage decision making. Early resting SPECT has become common practice in the United States [61]. In the early assessment of acute chest pain, resting SPECT can be appropriately performed in patients with ongoing symptoms or symptom resolution within 3 hours after evaluation and in the absence of other findings suggestive of ACS like ECG abnormalities or elevated first troponin levels [62]. In this setting, normal resting perfusion SPECT showed a high negative predictive value for acute infarction and short-term cardiac events, so that these patients can be safety discharged [63]. Acute resting SPECT proved a cost-effective approach [64, 65]. If symptoms have resolved hours before evaluation in the emergency department (3 hours or more in clinical trials), rest SPECT may be performed but the test could result insensitive to perfusion defects. When ACS is still suspected after serial ECG and troponin result negative or borderline for ACS, and the patient does not qualify for ‘rule-out’ or ‘rule-in’, non-invasive imaging using stress testing targeting myocardial ischemia or CCTA is recommended before deciding on an invasive approach (COR I, LOE B) [66, 67]. In such cases, stress-rest SPECT or a stress-only protocol is considered appropriate and safe to rule-out ACS [18, 25, 62]. In patients with known CAD and new-onset or worsening symptoms, stress testing is indicated (COR IIa, LOE B) since rest SPECT cannot distinguish between chronic and acute ischemia. Direct invasive coronary angiography (ICA) is recommended only when there is previously documented significant left main or proximal left anterior descending or multivessel CAD or in patients with previous coronary revascularization (COR I, LOE A).
The role of stress testing in preoperative risk assessment of patients before noncardiac surgery has been extensively demonstrated [68, 69, 70]. Moderate to severe myocardial ischemia is a sensitive marker of increased risk of perioperative MACE. Normal SPECT findings portend a high negative predictive value for perioperative cardiac events, while detection of scars has a low positive predictive value. Due to the underlying CAD, however, long-term prognosis is worse in these patients (Fig. 4).
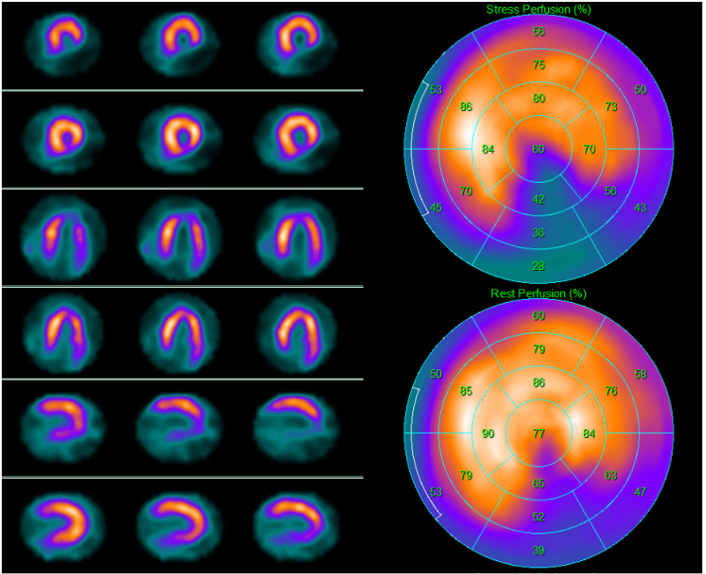 Fig. 4.
Fig. 4.A 69-year-old asymptomatic man with multiple cardiovascular risk factors underwent 99mTc-tetrofosmin cardiac single photon emission computed tomography (SPECT) screening for myocardial ischemia before kidney transplantation. SPECT revealed a partially reversible inferior defect. Subsequent invasive coronary angiography revealed severe stenosis of the posterior descending artery, which was treated with angioplasty. Left side top row: stress-rest short axis; stress-rest horizontal long axis; stress-rest vertical long axis; right side: polar map of stress (upper image) and rest perfusion (lower image).
In a meta-analysis of ten studies with pharmacological stress SPECT, the 30-day MACE rates were 1% in patients with normal test results, 7% and 9% in those with fixed and reversible perfusion defects, respectively, with a higher event rate in those with at least two reversible defects [71].
According to American guidelines, recommendations for stress imaging are limited
to patients with elevated surgical risk, performing exercise stress imaging in
patients with excellent (
CAD is a major contributor to heart failure worldwide [75]. Tests detecting CAD help clinicians understand the etiology of heart failure and guide patient management in relation to symptoms and prognosis improvement (COR IIa, LOE B) [76]. New or worsening symptoms of heart failure may be associated with myocardial ischemia (Fig. 5). Severely dysfunctional but viable myocardium (hibernating myocardium) is associated with poor outcome, but appropriate revascularization may ameliorate the prognosis [77, 78, 79]. The recently published results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) showed that patients with ischemic heart disease who underwent CABG surgery had a better prognosis than those who received medical therapy alone at 10-year follow-up [80]. Stress imaging (SPECT or positron-emission-tomography [PET], stress echocardiography, cardiac magnetic resonance) may be considered in patients with CAD who are eligible for coronary revascularization in which the aim should be the detection of myocardial ischemia and viability (COR IIb, LOE B) [76, 81]. Debate continues to surround the management of ischemic left ventricular dysfunction because ischemia, hibernation, viability, scar, and remodeling are variably involved, and it is unclear how to identify the patients who may gain benefit from revascularization in terms of prognosis.
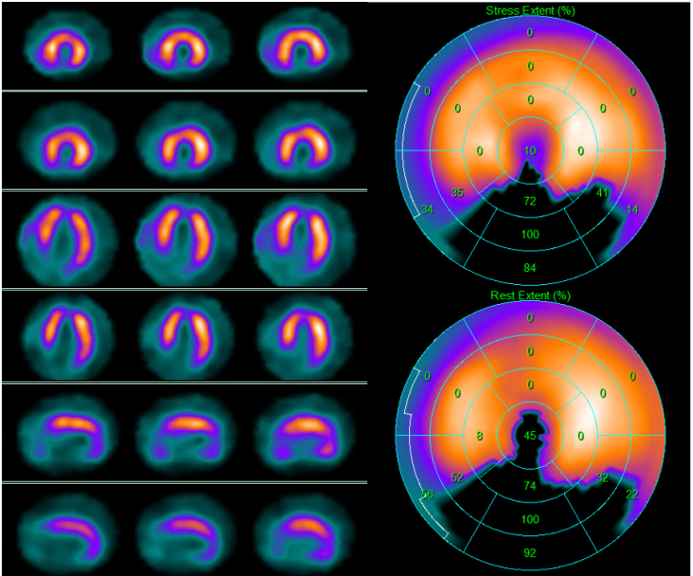 Fig. 5.
Fig. 5.99mTc-tetrofosmin cardiac single photon emission computed tomography (SPECT) images in a 67-year-old man with dyspnea on exertion and new-onset left ventricular dysfunction with inferior wall akinesia at resting echocardiography. SPECT revealed on the inferior wall a scar due to a previous silent myocardial infarction. Left side top row: stress-rest short axis; stress-rest horizontal long axis; stress-rest vertical long axis; right side: polar map of stress (upper image) and rest perfusion (lower image).
In clinical practice, the evidence for myocardial viability is necessary, given
that superior survival rates afforded by revascularization strategy over medical
therapy have been reported only in patients with hibernating myocardium
[77, 82, 83]. In the STICH trial, however, the degree of left ventricular systolic
dysfunction and remodeling and the number of stenotic coronary arteries appeared
to be stronger determinants of the benefit of revascularization than myocardial
viability [84]. In the prespecified viability sub-study of the STICH trial,
myocardial viability was associated with a modest improvement in left ventricular
systolic function, irrespective of treatment, albeit not associated with a
long-term benefit over surgical revascularization at 10-year follow-up [85]. In
the PPAR-2 trial, assessment of ischemia was associated with incremental benefit
over viability, especially in patients with mild to moderate CAD [86]. The
stress-rest SPECT study mentioned above revealed that the extent of ischemic
myocardium (
In nuclear medicine, both SPECT and PET modalities can be used to evaluate myocardial perfusion for diagnosing CAD. A higher pooled mean sensitivity for significant CAD has been reported for PET in comparison to SPECT, but the respective value of SPECT and PET in terms of specificity is less defined. The EVINCI study showed higher sensitivity and specificity of PET compared to SPECT in the detection of coronary stenosis (81% versus 73% and 89% versus 67%, respectively) in patients with an intermediate likelihood of CAD [87]. In the PACIFIC study, patients with suspected CAD underwent CCTA, PET, and SPECT, followed by ICA with fractional flow reserve (FFR) measurements [88]. Surprisingly, SPECT resulted noninferior to PET in specificity for significant CAD. On comparison of the three modalities, CCTA showed the highest sensitivity (90%), whereas SPECT and PET showed higher specificity compared to CCTA (94% and 84% versus 60%, respectively); PET showed the highest diagnostic accuracy overall (85%), whereas CCTA and SPECT showed similar accuracy (74% and 77%, respectively). In the PACIFIC 2 study, patients with a history of myocardial infarction or percutaneous revascularization underwent SPECT, PET, and cardiac magnetic resonance, followed by ICA with FFR. PET had the highest sensitivity for hemodynamically significant CAD, whereas specificity and diagnostic accuracy did not differ between the three imaging modalities [89].
In addition to quantifying perfusion defects and ventricular function, PET MPI is the gold standard for the non-invasive evaluation of absolute myocardial blood flow (MBF) and myocardial blood flow reserve (MFR) (Fig. 6). MFR (the ratio between maximal hyperemic flow and resting myocardial flow) reflects the global hemodynamic effect of CAD, including coronary artery stenosis and microvascular dysfunction. PET-derived MFR estimation has been strongly associated with prognosis and can help in guiding treatment strategies in patients with CAD [90]. The ability to assess MBF and MFR is a considerable advantage of PET over SPECT. With the introduction of the new cadmium, zinc, tellurium (CZT) technology to SPECT, dynamic acquisition can be performed to assess quantitative flow indices (MBF and MFR) similar to dynamic PET study [2]. Supporting evidence is growing [91], which will probably make MFR study with SPECT technology more available due to the widespread use and lower cost of SPECT compared to PET. In this setting, assessment of absolute MBF and MFR with SPECT is promising and may also fill the gap for the proper diagnosis of multivessel disease, which has been historically considered a main limitation of SPECT [19, 92]. More data are needed before the technique becomes routine use; nonetheless, the available data are highly encouraging.
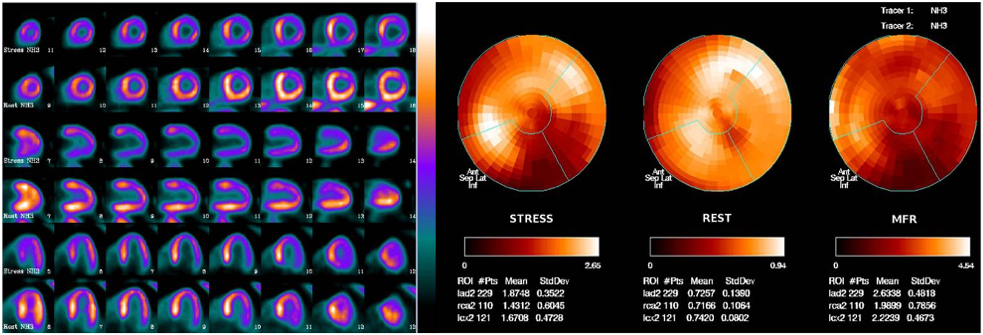 Fig. 6.
Fig. 6.Myocardial perfusion PET with
Diagnosis of CAD relies on the detection of atherosclerosis extension and severity and of myocardial ischemia, the anatomical and functional substrates of CAD, respectively. CT has become a primary tool for CAD detection by coronary artery calcium (CAC) quantification and direct coronary artery visualization and stenosis quantification by CCTA, whereas SPECT can quantify the hemodynamic consequences of anatomic CT findings. Since the correlation between CAC, coronary artery stenosis, and myocardial perfusion is not linear [93, 94, 95], these approaches are considered complementary rather than mutually exclusive. SPECT combined with CT has gained growing interest in the concept of hybrid imaging.
CT is routinely performed in combination a perfusion study to manage attenuation of SPECT photons in the body and improve perfusion imaging quality. This procedure is called CT-based attenuation correction and is recommended by European Association of Nuclear Medicine (EANM) guidelines [16]. It provides a map of the attenuation coefficients based on the Hounsfield unit of a low voltage CT scan. The additional patient effective dose is very low, so these CT scans can be used to approximate the extent of coronary calcification [96]. Alternatively, CT for CAC scoring can be performed [97] and then used for attenuation correction [16]. Furthermore, SPECT and CCTA datasets can be fused to create a single fused hybrid image that integrates anatomical and functional information.
Although myocardial ischemia is a potent predictor of cardiac events, most events occur in patients with a normal functional test. Combining a patient’s CAC score with perfusion imaging findings increases risk stratification efficacy in patients with and without myocardial ischemia [98, 99] (Fig. 7).
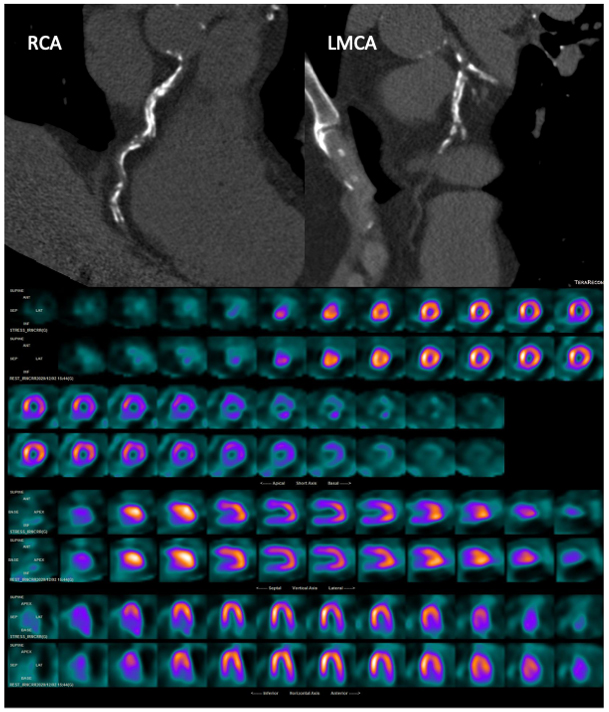 Fig. 7.
Fig. 7.A 77-year-old asymptomatic man with multiple cardiovascular risk factors underwent CT with CAC scoring and subsequent 99mTc-tetrofosmin cardiac SPECT. CT images (top) showed a very high CAC score (2129 HU) in the three main vessels, whereas myocardial perfusion SPECT (bottom) showed a partially reversible defect in the basal and middle anterior segments and in the basal and middle inferior segments. RCA, right coronary artery; LMCA, left main coronary artery. Myocardial perfusion SPECT images: stress-rest short axis; stress-rest horizontal long axis; stress-rest vertical long axis.
The CAC score is a low-radiation and low-cost non-invasive estimate of coronary
atherosclerotic plaque burden, though it does not reflect obstructive CAD or
ischemia. A zero CAC score demonstrated very high sensitivity to rule out
obstructive CAD and to predict very low events rates in asymptomatic and
symptomatic patients with suspected CAD [43, 100, 101, 102]. Differently, a positive CAC
score is proportionally related to the frequency of obstructive CAD and inducible
ischemia, also when calcifications are not directly associated with the degree of
luminal or functional coronary artery stenosis. CAC score was found equal to CCTA
to predict MPI alterations [103]. A meta-analysis [104] of studies involving
asymptomatic and symptomatic patients reported a prevalence of ischemia of 6.6%
for CAC-zero patients, 8.5% for CAC score 1 to 100, 10.5% for CAC score 100 to
399, and 23.6% for CAC score
Given the strengths and the limitations of CAC evaluation and SPECT MPI (i.e., high sensitivity of CAC score for detecting CAD and high specificity of functional tests for obstructive CAD), a combined strategy dictates that a zero CAC score may rule out patients, especially those with low-intermediate PTP and atypical chest pain, whereas MPI or other functional tests may identify patients with a positive CAC score at higher risk of events [43]. CT for CAC scoring is now recommended in patients with stable symptoms categorized as low-risk (COR IIa, LOE B) [31].
In patients with suspected CAD, the complementary use of CAC score and SPECT-MPI
may: improve the assessment of PTP of CAD in initial diagnostic work-up and help
in selecting patients for stress testing; improve cardiac risk stratification,
since risk increases in patients with abnormal MPI findings who also have CAC
abnormality [106, 107] and in patients with normal SPECT findings and a CAC score
The CAC score is not a diagnostic tool for obstructive CAD but rather a cardiovascular risk modifier. In diabetic patients, MPI abnormalities are more strongly associated with CAC score than with traditional risk factors [110]. For example, symptomatic patients with non-calcified obstructive plaque may have a zero CAC score but spotty or microcalcifications associated with high-risk plaques. Several studies reported that the relationship between CAC score and the likelihood of CAD is influenced by the overall clinical risk of the population, the patient’s clinical presentation and PTP [111, 112, 113, 114, 115, 116]. In patients at high risk for CAD, abnormal MPI findings were more frequent than in patients at low or intermediate risk also in those with a low CAC score [117, 118].
Finally, cardiac event risk was significantly higher in asymptomatic patients
with normal SPECT-MPI when the CAC score was elevated (
The synergistic combination of myocardial perfusion SPECT imaging with CCTA offers contrast-mediated visualization of the coronary artery lumen and detects anatomical abnormalities and their functional consequences in a single setting (Fig. 8). CCTA has an excellent negative predictive value (NPV) to exclude CAD, whereas less robust is the positive predictive value (PPV) for the identification of hemodynamically significant lesions [119, 120], since CCTA tends to overestimate stenosis severity due to coronary calcification or artifacts. CCTA can also document multivessel disease, which is viewed as a weak point of SPECT imaging due to possible balanced ischemia.
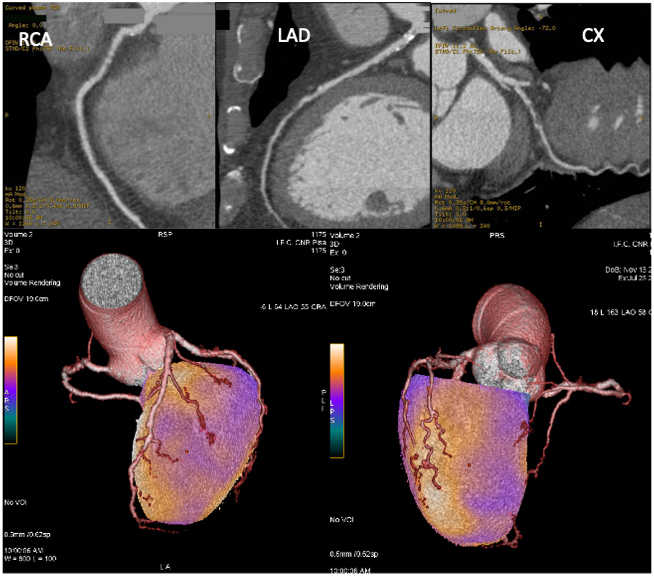 Fig. 8.
Fig. 8.Coronary CT angiography and hybrid myocardial perfusion SPECT/coronary CT angiography imaging of the patient described in Fig. 6. Upper images: CCTA showed non-calcific subcritical plaques in the right coronary artery (RCA; left side) and left anterior descendent coronary artery (LAD) and a stenotic non-calcific plaque in the left circumflex artery (CX; right side). Lower images: fusion of CCTA and SPECT datasets allows the integration of functional and anatomical data, clearly indicating the relationship between the atherosclerotic plaques and ischemic areas.
Hybrid cardiac SPECT/CCTA provides superior diagnostic data compared to either stand-alone or side-by-side interpretation of SPECT and CT data sets by virtue of the spatial co-localization of a myocardial perfusion defect with a subtending coronary artery. There is substantial inter-individual variability in coronary artery anatomy and actual segmental assignment to coronary artery territories differs from that expected in more than half of patients [121]. This hybrid imaging creates a panoramic three-dimensional view by integrating volume-rendered CT data with the perfusion information from SPECT to identify the culprit lesion with higher sensitivity and specificity and optimize the final ruling of intermediate lesions and equivocal perfusion defects [122]. Since a sizeable proportion of obstructive stenosis doesn’t induce ischemia [122] and coronary stenosis of 50% to 70% may be associated with coronary flow reduction [123], the complementary information provided by the two techniques can help to exclude patients with normal coronary arteries, diagnose subclinical atherosclerosis, define the ischemic burden of specific coronary lesions, detect multivessel disease, and identify the culprit lesion [124]. In a population with a high pre-test likelihood of CAD, hybrid imaging analysis improved the PPV to 96% (versus 85% and 77% of SPECT and CCTA, respectively) and the NPV to 95% (versus 89% and 96% of SPECT and CCTA, respectively) versus a reference standard of fractional flow reserve (FFR) measurement [125].
Hybrid imaging techniques may be useful also in follow up after CABG surgery, in which coronary physiology is completely altered. When ischemia is present, identification of a single coronary artery or graft as the culprit lesion poses a great challenge. A high prevalence of perfusion defects in territories supplied by patent grafts has been reported [126]. Integrating anatomical and perfusion data through hybrid imaging is a promising tool to visualize post-operative anatomy, localize vessel stenosis and occlusions, and gain additional insights into the mechanism of ischemia [127]. Also, it has been suggested that complementary approaches may improve event prediction in patients after CABG surgery [128].
The disadvantages of additional radiation exposure and cost and time investment related to fusion SPECT/CCTA limit its routine application. Sequential use of SPECT and CCTA in selected patients may be an attractive alternative [129, 130, 131, 132]. The wider availability of image fusion software may allow the use of CCTA from external sources to create fusion images [133].
CT myocardial perfusion (CTP) imaging has gained interest as a diagnostic tool alternative to perfusion SPECT. The CTP protocol includes rest and stress scanning phases, as in other functional imaging techniques. CTP demonstrated good diagnostic performance in detecting ischemia defined by FFR [134] or invasive coronary angiography [135], also in patients with previous percutaneous coronary angioplasty or with severe coronary calcification [136]. The results so far are encouraging. If corroborated in studies with larger patient samples, CTP may compensate the weaknesses of CCTA. CCTA and stress CTP may be integrated to assess both anatomical and functional features; however, radiation exposure is very high and iodinated contrast agents are injected.
Another emerging CT-based technique for evaluating ischemia is CT-derived FFR. A greater prognostic benefit over angiography has been demonstrated when revascularization is guided by invasively assessed FFR [137, 138], so that a non-invasive method to measure FFR seems attractive. Data extracted from resting CCTA can be used to calculate CT-derived FFR and to directly determine the significance of a CCTA lesion. Since no additional radiation or contrast agent dose is required, it’s becoming the gold standard for the identification of flow-limiting coronary artery stenosis. The drawback is that CCTA image quality need to be very high, limiting the diagnostic accuracy in severe calcifications or previous percutaneous or surgical revascularization [139]. Further evidence from randomized clinical trials is needed before extensive use of CT-derived FFR can be recommended for clinical practice. Finally, cost-effectiveness analyses and the availability of this advanced CT technology need to be taken into account.
SPECT-MPI has been a preferred method for evaluating patients with known or suspected CAD. With recent advances in the management of cardiovascular risk factors and acute coronary syndromes, CAD has become a chronic condition. Because many patients referred to SPECT have a low PTP, the diagnostic accuracy of MPI for obstructive CAD tends to decline. A considerable proportion of patients undergoing ICA after stress imaging has normal coronary arteries or non-obstructive coronary stenosis [140], while most cardiac events occur in patients in which inducible ischemia has not been detected. A guideline-directed selection of patients to refer to SPECT-MPI is essential to increase test diagnostic accuracy and the benefit of testing.
It is currently assumed that the relationship between atherosclerosis, degree of coronary artery stenosis, and ischemia is dynamic across levels of lesions severity and influenced by plaque stability and microvascular and endothelial dysfunction. Consequently, alternative approaches that combine anatomical and functional studies in sequence or during the same session have been developed, including CT for CAC scoring +/– SPECT MPI in the initial evaluation of patients with low-to-intermediate PTP of CAD, or fusion SPECT/CCTA imaging to define the ischemic burden of coronary lesions, detect multivessel disease or follow-up patients after CABG surgery. The synergistic performance of CAC or CCTA and SPECT MPI has demonstrated excellent diagnostic accuracy and outcome prediction of CAD. Finally, CZT technology applied to SPECT allows assessment of quantitative flow indices similar to those provided by PET studies, further supporting the value of SPECT MPI in the multimodality imaging era.
CM and RG gave a major contribution in conceiving the manuscript; CM, AG, FLR and FD wrote and edited the manuscript; all authors approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.








