1 Department of Cardiology and Internal Medicine, Collegium Medicum, Nicolaus Copernicus University, 85-094 Bydgoszcz, Poland
2 Clinic of Cardiology and Cardiac Care Unit Department, Provincial Polyclinic Hospital, 87-100 Toruń, Poland
3 Department of Cardiology and Department of Cardiological Intensive Care, Provincial Hospital, 82-300 Elbląg, Poland
4 Department of Cardiology and Internal Medicine, University of Warmia and Mazury, 10-082 Olsztyn, Poland
5 Department of Cardiology, Regional Specialist Hospital, 10-082 Olsztyn, Poland
Academic Editors: Domenico D’Amario and Mattia Galli
Abstract
Antiplatelet treatment is one of the pillars of contemporary therapy in acute coronary syndromes. It is based on dual antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12 receptor inhibitor. Antiaggregatory treatment reduces ischemic events, but at cost of increased bleeding rates. As a result of irreversible inhibition of platelet P2Y12 receptors, the antiplatelet action of clopidogrel and prasugrel is prolonged for the lifespan of thrombocytes and lasts up to 7 days. The antiaggregatory effect of ticagrelor may persist up to 5 days despite its reversible nature of P2Y12 receptor inhibition. These pharmacodynamic properties may prove problematic in patients requiring immediate reversal of antiplatelet effects due to severe or life-threatening bleeding, or in presence of indications for an urgent surgery. The current review summarizes available knowledge on different strategies of restoring platelet function in patients treated with ticagrelor. Non-specific methods are discussed, including platelet transfusion, human albumin supplementation and hemadsorption. Finally, bentracimab, the first specific antidote for ticagrelor, and in fact against any antiplatelet agent, is described.
Keywords
- antidote
- bentracimab
- MEDI2452
- PB2452
- platelet transfusion
- ticagrelor
Antiplatelet treatment is one of the pillars of contemporary therapy in acute coronary syndromes (ACS). It substantially improves the clinical outcomes in ACS, mainly due to reduction of ischemic events. Notwithstanding, antiplatelet treatment increases the incidence of bleeding, which can range from nonsignificant and not requiring medical contact to fatal hemorrhages [1].
Antiaggregatory therapy in ACS is based on dual antiplatelet therapy (DAPT) consisting of aspirin and a P2Y12 receptor inhibitor [2]. Standard duration of DAPT after ACS is 12 months, unless contraindications or excessive bleeding risk exist. Duration (shortening or extension) and type of antiplatelet treatment following ACS should consider individual ischemic and bleeding risks [1]. In general ACS population potent P2Y12 receptor inhibitors, ticagrelor or prasugrel, are recommended over clopidogrel [2, 3, 4]. Both of these agents provide stronger and more predictable antiaggregatory effect, and in result improved clinical outcomes, compared with clopidogrel [2]. However, in the landmark trials both ticagrelor and prasugrel were related with greater incidence of bleeding compared with the latter [5, 6]. Additionally, non-adherence to DAPT after ACS, and especially early discontinuation of antiplatelet treatment, is associated with significantly increased risk of major adverse cardiovascular events [7].
The antiplatelet effect of oral P2Y12 receptor antagonists extends to at least several days after intake of the last dose. Ticagrelor provides stronger platelet inhibition than clopidogrel even in reduced doses [8]. Due to irreversible inhibition of platelet P2Y12 receptors, the antiaggregatory action of clopidogrel and prasugrel is prolonged for the lifespan of thrombocytes and lasts up to 7 days [1, 9]. The antiplatelet effect of ticagrelor may persist up to 5 days, despite its reversible nature of P2Y12 inhibition [10]. These pharmacodynamic properties may prove problematic in patients requiring immediate reversal of antiplatelet effects due to severe or life-threatening bleeding, or in presence of indications for an urgent surgery. Detailed data are not available, but it is estimated that up to 25% of patients undergoing coronary stenting may require non-cardiac surgery within 5 years after percutaneous coronary intervention (PCI) [11]. Thus, feasibility of prompt restoration of platelet function is of great importance in patients receiving antiplatelet agents.
Currently there are no commercially available specific antidotes for any of oral P2Y12 receptor antagonists. Due to irreversible nature of adenosine diphosphate (ADP) receptor binding, antidotes for thienopyridines (clopidogrel, prasugrel) are not very likely to be developed in the near future. Conversely, because of reversible P2Y12 inhibition yielded by ticagrelor, recovery of platelet function in patients receiving this antiplatelet agent should be more feasible.
The aim of this review was to summarize available data on non-specific and specific methods of platelet function recovery in patients receiving ticagrelor.
Ticagrelor is a P2Y12 receptor inhibitor that belongs to cyclopentyl-triazolo-pyrimidine group and binds to platelet P2Y12 receptors in a reversible and noncompetitive manner. It is an active drug, but it also undergoes hepatic metabolism and is transformed into 10 metabolites, of which one exerts antiplatelet potency equal to one of the parent drug (Fig. 1). Ticagrelor is expeditiously absorbed after oral intake and is characterized by rapid onset of antiaggregatory effect. In stable setting time to maximal concentration usually does not exceed 2 hours [10]. However, in patients with ACS both absorption and antiplatelet action of ticagrelor can be reduced and delayed for few hours, especially if morphine is administered [12, 13, 14]. Elimination half-time of ticagrelor is 7.7–13.1 hours and duration of platelet inhibition lasts for 3–5 days [10]. One of the most important differences between thienopyridines and ticagrelor is the reversibility of P2Y12 receptor inhibition by ticagrelor. Subsequently, recommended time of P2Y12 receptor antagonist discontinuation prior to a non-emergent cardiac or non-cardiac surgery is 3, 5 and 7 days for ticagrelor, clopidogrel and prasugrel, respectively [1, 2].
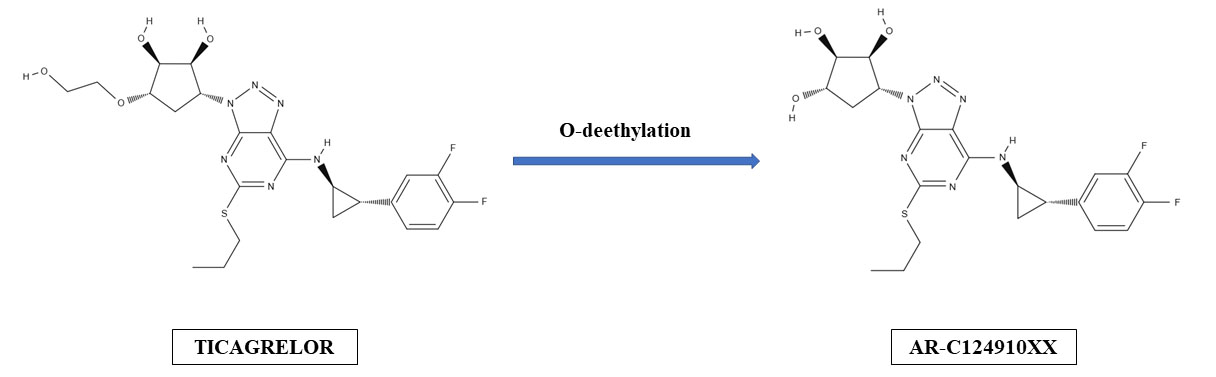 Fig. 1.
Fig. 1.Hepatic formation of ticagrelor’s active metabolite (AR-C124910XX).
Ticagrelor exerts more potent antiplatelet effect compared with clopidogrel,
while its antiaggregatory action is comparable to one observed with prasugrel
[15, 16, 17]. Clinical superiority of ticagrelor over clopidogrel has been proven in
the Platelet Inhibition and Patient Outcomes (PLATO) study [5]. This was a
double-blind, randomized trial, which included 18,624 patients with ACS. Patients
receiving ticagrelor had a significant reduction in occurrence of composite of
death from vascular causes, myocardial infarction (MI), or stroke, compared with
patients on clopidogrel (9.8% vs. 11.7%, p
Ticagrelor can be used in numerous clinical scenarios. In the majority of ACS patients ticagrelor is preferred over clopidogrel. Treatment with ticagrelor ought to start with 180 mg loading dose and should be followed by 90 mg twice daily, usually for 12 months [2, 3]. Ticagrelor has class I recommendation in both ST-elevation myocardial infarction (STEMI) and non-ST-elevation ACS (NSTE-ACS). In contrast to prasugrel, it can be administered in patients treated conservatively. In patients with MI at high ischemic risk who have tolerated DAPT without bleeding for 12 months, ticagrelor in reduced maintenance dose of 60 mg twice daily may be preferred over thienopyridines [2]. Notably, according to the Swedish Web-system for Enhancement and Development of Evidence-based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry, ticagrelor is used in patients with MI over 17 times more often than prasugrel [24].
Clopidogrel remains the P2Y12 receptor inhibitor of choice in patients with chronic coronary syndrome undergoing elective stenting. However, ticagrelor may be considered in specific high-risk situations, such as procedural characteristics associated with high risk of stent thrombosis, complex left main stem, or multivessel stenting, or if DAPT cannot be used because of aspirin intolerance [25]. In elective stenting patients with moderate to high risk of stent thrombosis and indication for chronic anticoagulation, ticagrelor with oral anticoagulant may be considered as a substitute for triple therapy with anticoagulant, aspirin, and clopidogrel [25].
In the PLATO trial use of ticagrelor was associated with significantly higher rate of major and minor bleeding events compared with clopidogrel (16.1% vs. 14.6%, p = 0.008) [5]. Patients receiving ticagrelor more frequently suffered from major bleeding not related to coronary-artery bypass grafting (CABG) (4.5% vs. 3.8%, p = 0.03), nonintracranial fatal bleeding (0.1% vs. 0.3%, p = 0.03) and fatal intracranial bleeding (0.1% vs. 0.01%, p = 0.02). Additionally, 8.9% of patients receiving ticagrelor-based DAPT experienced bleeding requiring red-cell transfusion, 5.8% suffered a life-threatening or fatal bleeding, and 7.4% had CABG-related major bleeding [5].
Ticagrelor-related bleeding appears to be dose-dependent and leads to premature interruption of treatment even in up to 7.8% of patients receiving standard maintenance dose (90 mg twice daily). Although bleeding seems to occur less often during treatment with reduced maintenance dose of 60 mg twice daily, it still leads to discontinuation of treatment in 6.2% of patients [26]. This emphasizes a great need for clinical introduction of methods allowing to promptly overcome ticagrelor’s antiplatelet action in case bleeding control or reduction of hemorrhagic risk before urgent surgery is needed.
New ticagrelor-based de-escalation strategies have been proposed recently to reduce the incidence of bleeding in patients receiving ticagrelor. The Ticagrelor with Aspirin or Alone in High-risk Patients after Coronary Intervention (TWILIGHT) trial showed that monotherapy with ticagrelor following 3 months of DAPT after PCI reduces rates of clinically relevant bleeding without increasing the risk of ischemic events [27]. This was also true for patients with NSTE-ACS [28]. Still, as many as 4% of patients from the monotherapy arm experienced the Bleeding Academic Research Consortium (BARC) type 2, 3 or 5 bleeding, compared with 7.1% in the DAPT arm [21]. The pre-specified analysis revealed that patients at high bleeding risk had larger absolute risk reduction in major bleeding than non-high bleeding risk patients [29]. Significant reduction of bleeding risk is also expected from a novel approach currently under investigation in the Evaluation of Safety and Efficacy of Two Ticagrelor-based De-escalation Antiplatelet Strategies in Acute Coronary Syndrome (ELECTRA-SIRIO 2) study. This trial evaluates the impact of monotherapy with reduced maintenance dose of ticagrelor (60 mg twice daily) on bleeding and ischemic events in ACS patients [30, 31].
Restoration of thrombocyte function by external supplementation of platelets initially appeared to be both uncomplicated and economical method of overcoming the therapeutical effect of antiplatelet agents [32].
In theory, to achieve maximal reversal of platelet function and to prevent inhibition of transfused thrombocytes, platelet transfusion should occur after the active compounds have been eliminated from circulation [33, 34]. This has been confirmed for prasugrel, in case of which supplemented platelets were partially inhibited by its active metabolite up to 2 hours after prasugrel loading dose, while no significant inhibition was observed starting from 6 hours after a loading dose [35]. Time-dependent effect of platelet transfusion may be even more expressed in subjects receiving ticagrelor. A study by Hansson et al. [36] shows that influence of ex vivo platelet supplementation on platelet aggregability in blood samples from patients receiving ticagrelor 2 hours prior is limited and lower than in those treated with clopidogrel. Scharbert et al. [37] have examined the impact of plasma obtained from P2Y12 receptor antagonist-treated patients on platelet function of subjects not receiving antiplatelet agents. The plasma was collected 3 hours after administration of thienopyridines or ticagrelor. Clopidogrel had no and prasugrel had only mild effect on platelet function of healthy volunteers, as their active metabolites were mostly bound or vanished by the time of assessment. Ticagrelor completely abrogated ADP-mediated platelet activation, and even at low concentrations, it has substantially inhibited platelet aggregation [37]. Platelet rich plasma obtained at 4 hours after the last ticagrelor’s dose also reduces ADP responsiveness of platelets in ticagrelor-naïve patients. This supports the concept that plasma- or platelet-bound ticagrelor and its active metabolite can decrease platelet reactivity of supplemented thrombocytes. Of note, this was not observed with clopidogrel or prasugrel [38]. The Antagonize P2Y12 Treatment Inhibitors by Transfusion of Platelets in an Urgent or Delayed Timing After Acute Coronary Syndrome or Percutaneous Coronary Intervention Presentation-Acute Coronary Syndrome (APTITUDE-ACS) study evaluated influence of ex vivo autologous platelet transfusion 4 hours after P2Y12 receptor antagonist loading dose in patients with ACS or undergoing coronary stenting on the restoration of platelet reactivity [39]. In patients receiving clopidogrel transfusion led to a significant 34% relative increase of platelet reactivity according to the vasodilator-stimulated phosphoprotein phosphorylation (VASP) assay (p = 0.0008) compared to baseline. In pooled population of patients treated with prasugrel or ticagrelor a 24% relative increase was not statistically significant (p = 0.22) [39].
A trial by Kruger et al. [40] showed that supplementation of an
equivalent of six apheresis platelet units produces a 50% relative reversal of
ticagrelor-induced platelet inhibition at 10 hours after the last maintenance
dose. The same amount of transfused platelets could lead to reversal exceeding
90% at 24 hours after the last ticagrelor dose [40]. Similar study by Zahar
et al. [41] confirmed that restoration of platelet function in
ticagrelor-treated patients is time-dependent regarding the last dose intake.
In vitro addition of concentrated platelets at 4 or 6 hours after
administration of ticagrelor loading or maintenance dose produced at most 35% of
baseline aggregation. Depending on the amount of supplemented platelets,
transfusion at 24 hours post-ticagrelor dose generated 59–79% of baseline
reactivity, which increased to
These pharmacodynamic data have not been verified in an appropriately sized clinical trial so far. Single case reports indicated clinical inefficacy of platelet transfusion for management of major bleeding in ticagrelor-treated patients [43, 44]. Although it appears that platelet supplementation is not very likely to be useful in urgent scenarios (bleeding, need for immediate surgery), it should be considered that currently available studies evaluated surrogate endpoints only.
Schoener et al. reported potential usefulness of human albumin
supplementation in reversal of ticagrelor antiplatelet action. In their
in vitro study, they have attempted to overcome antiaggregatory effect
of ticagrelor in patients with ACS using several different strategies [45].
Supplementation of pooled platelets has not improved platelet function according
to the VASP assay, while addition of platelet rich plasma managed to
significantly increase platelet aggregation (14.8%
An alternative strategy to restore platelet function in patients treated with
ticagrelor is to remove the drug from circulation using sorbent hemadsorption
[47]. In their study, Angheloiu et al. [47] used CytoSorb, a styrene
copolymer with bead diameters of 425 to 1000 mm and surface area 850 m
Several substances with mechanism of action different from enhancement of ADP-dependent platelet aggregation have been proposed for improvement of hemostasis in patients receiving ticagrelor [48, 49, 50, 51]. In a study with healthy volunteers desmopressin shortened ticagrelor-induced bleeding time, but it was not statistically significant and was not considered clinically relevant. As expected, inhibition of platelet aggregation by ticagrelor was not affected by co-administration of desmopressin [48]. Activated recombinant human factor VII, as well as recombinant human prothrombin were shown to reduce blood loss and bleeding time in ticagrelor-pretreated mice [49]. Recombinant activated factor VII, fibrinogen concentrate and factor XIII concentrate were shown to partly compensate ticagrelor-induced bleeding by acting on fibrin formation and fibrinolysis in an in vitro study. Simultaneously, they had no impact on ticagrelor-induced platelet inhibition [50]. There is a case report available on recombinant activated factor VII administration in a patient treated with ticagrelor who required urgent neurosurgery for an intracranial hematoma. Platelet inhibition remained unchanged, but thromboelastometric clotting time was reduced and patient had improved hemostasis. No bleeding complications of surgery occurred, but the patient developed pulmonary embolism secondary to recombinant activated factor VII administration [51].
Bentracimab, formerly known as MEDI2452 or PB2452, is the first specific antidote for ticagrelor, and in fact against any antiplatelet agent. It is an antigen-binding fragment (Fab) that displays 100-fold greater affinity for ticagrelor and its active metabolite (AR-C124910XX) than for their target, platelet P2Y12 receptor [46]. The antidote is highly specific and does not bind to substances of structure similar to ticagrelor or AR-C124910XX, such as adenosine, ADP or adenosine triphosphate. Bentracimab binds to both ticagrelor and its active metabolite in 1:1 ratio, and reverses their antiplatelet effect in a concentration-dependent manner (Fig. 2). Bentracimab also reduces concentration of free ticagrelor, which inversely correlates with recovery of ADP-induced platelet aggregation [52]. This antidote is not expected to be effective against clopidogrel or prasugrel, which are irreversible P2Y12 receptor inhibitors.
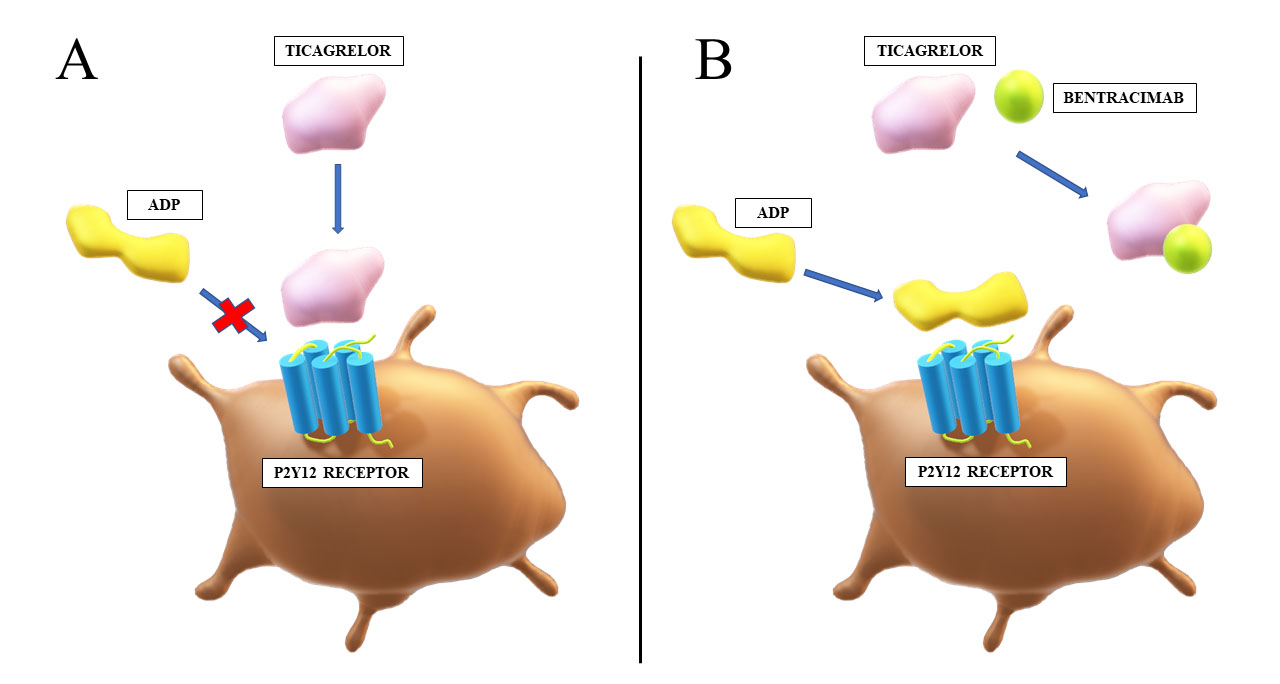 Fig. 2.
Fig. 2.Mechanism of action of bentracimab. (A) Ticagrelor reversibly binds to the P2Y12 receptor and inhibits ADP-mediated activation of thrombocytes. (B) Bentracimab binds to ticagrelor with high affinity and specificity. This prevents inhibition of ADP signaling through the P2Y12 receptor by ticagrelor, which restores platelet activity. ADP, adenosine diphosphate.
In murine model bentracimab enables a 94% in vivo reversal of ticagrelor-induced platelet inhibition, with a full onset of reversal occurring 30 minutes after administration of the antidote. This leads to reduction of blood loss and shortens bleeding time to levels similar to ticagrelor-naïve mice [46]. In a porcine model bentracimab dosing leads to complete clearance of free ticagrelor and AR-C124910XX within 5 minutes of administration [53]. This produces platelet function recovery, however with some delay, as 60 minutes are needed to restore ADP-mediated platelet aggregation in ticagrelor-treated pigs. These pharmacokinetic and pharmacodynamic effects translate into numerical increase in survival and reduction of blood loss in pigs with induced major bleed (excision of a liver lobe), however, without a statistical significance. Still, bentracimab substantially reduced the rate of mean arterial pressure decrease over time in these animals [53].
Safety, efficacy, and pharmacokinetic profile of bentracimab in healthy
volunteers pretreated with ticagrelor for 48 hours, were evaluated in a
single-center, randomized, double-blind, placebo-controlled, phase 1 trial
performed by Bhatt et al. [54]. A total of 64 volunteers were randomized
to placebo (n = 16, mean age: 34.0
Abovementioned study evaluated several dosing regimens of bentracimab. The
greater were the bolus and duration of infusion, the more rapid and sustained
ticagrelor reversal was. With maximal bolus of 6 g followed by a 12-hour or
16-hour infusion up to total dose of 18 g, bentracimab provided reversal of
ticagrelor-induced platelet inhibition within 5 minutes after initiation of the
infusion, that was maintained for 16 to 24 hours [54]. These pharmacodynamic
effects in healthy volunteers were documented with three different platelet
function tests. Reversal of platelet aggregation was
Consistent results were recently made available from a phase 2b trial assessing
safety and efficacy of bentracimab in reversing the antiplatelet effect of
ticagrelor [55]. Peer-reviewed results of this trial are not available yet,
however, main findings have been made public. In this study 205 healthy
volunteers pretreated with ticagrelor for 48 hours were randomized in a 3:1 ratio
to either bentracimab (n = 154) or placebo (n = 51). Study participants were
older than in the previous phase 1 trial (mean age: 61 years), half of them were
female, and 59% had mild and 9% moderate chronic kidney disease. Volunteers
allocated to bentracimab had significantly lower platelet inhibition according to
the VerifyNow assay within the first 4 hours of the antidote infusion compared
with volunteers receiving placebo (p
Clinical evaluation of bentracimab is currently under investigation in the Bentracimab in Ticagrelor-treated Patients With Uncontrolled Major or Life-Threatening Bleeding or Requiring Urgent Surgery or Invasive Procedure (REVERSE-IT) study. It is a phase 3, open-label, single arm trial including on-ticagrelor patients with uncontrolled major or life-threatening bleeding, or requiring urgent surgery or invasive procedure [56]. The trial is expected to include target 200 participants by the end of year 2023.
It is not possible to precisely estimate the number of patients who may benefit from bentracimab. It is highly probable that its common use may be restricted due to limited availability and resources. This issue has been observed with idarucizumab and andexanet alfa, specific antidotes for dabigatran, and rivaroxaban and apixaban, respectively. According to the Premier Healthcare Database accounting approximately 25% of all inpatient admissions in the United States, among 550,663 patients hospitalized due to life-threatening bleeding between May 2018 and June 2019, only 407 received idarucizumab and 151 were administered with andexanet alfa [57].
Currently there are no commercially available methods to reverse ticagrelor-induced platelet inhibition when timely reversal is required in case of a severe hemorrhage or a need for an urgent surgery (Table 1). Non-specific methods aiming to overcome antiplatelet effect of ticagrelor are ineffective or have not been verified in an adequate clinical trial. Bentracimab, the first specific antidote against ticagrelor, provides rapid and sustained restoration of platelet aggregation, but its clinical efficacy and safety are yet to be confirmed in a phase 3 trial, that is currently ongoing.
| Strategy | Specificity for ticagrelor | Key points |
| Platelet transfusion | No | |
| Human albumin supplementation | No | |
| Hemadsorption | No | |
| Bentracimab | Yes | |
With the shortest time between the last dose and offset of antiplatelet effect, a specific antidote being under advanced development, and very strong recommendations from the cardiac societies, ticagrelor should be considered as P2Y12 receptor inhibitor of choice in the majority of ACS patients. Nevertheless, novel strategies to reduce bleeding burden in patients on ticagrelor are urgently needed, as clinical verification of its first antidote.
PA and JK contributed to conception and design of the review. PA and JK conducted the literature search and assembled data. GS, TH and AK provided help and advice on manuscript preparation. PA wrote the draft of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
This work origins from representatives of the ELECTRA-SIRIO 2 study group. The ELECTRA-SIRIO 2 is a phase 3, randomized, multicenter, double-blind, placebo-controlled, investigator-initiated study evaluating clinical efficacy and safety of novel ticagrelor-based antiplatelet therapy de-escalation strategies.
This research received no external funding.
PA and JK received lecture honoraria from AstraZeneca. TH received honoraria from AstraZeneca for participation in a clinical trial. The remaining authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


