1 Department of Cardiology, The Third Affiliated Hospital of Soochow University, 213003 Changzhou, Jiangsu, China
2 Department of Cardiology, Shanghai East Hospital, School of Medicine, Tongji University, 200092 Shanghai, China
3 Department of Anesthesiology and Perioperative Medicine, The First Affiliated Hospital of Nanjing Medical University, 210029 Nanjing, Jiangsu, China
†These authors contributed equally.
Academic Editors: Lee Stoner and Hirofumi Tanaka
Abstract
Heart failure (HF) and depression are both major medical health issues in our society. Currently, an increasing number of studies demonstrate an association between HF and depression. The prevalence of depression is higher in patients with HF, and depression also increases the incidence of HF. Currently, depression has been listed as a major risk factor for heart disease. Patients with HF and comorbid depression have significantly higher rates of hospitalization and mortality, and clinical symptoms manifest as decreased activity tolerance and decreased muscle mass. Enhancement of the muscle function improves the prognosis of patients with HF and depression. Sarcopenia is defined as age-related loss of skeletal muscle mass plus loss of muscle strength and/or reduced physical performance, and its pathogenesis involves malnutrition, physical inactivity, endocrine disorders and chronic inflammation, which are also involved in the pathogenesis of HF with comorbid depression. Therefore, it would be intriguing to explore the linkage between HF, depression and sarcopenia. This review presents an overview of HF with comorbid depression and sarcopenia, elucidates the mechanisms involved in these disorders, and finally summarizes the treatment strategies of HF with comorbid depression and sarcopenia.
Keywords
- heart failure
- depression
- comorbidity
- sarcopenia
- cachexia
Heart failure (HF) is one of the most common chronic diseases and is the final
phase of heart diseases of various etiologies. It has become a major public
health problem owing to the high rates of morbidity, mortality and
rehospitalization associated with it [1]. 2021 European Society of Cardiology
(ESC) guidelines for HF indicate that the prevalence of HF in European adults is
approximately 5/1000 person-years [2]. Given the high prevalence of HF in the
elderly population (
Depression is mainly manifested as a mental state of a depressed mood and an aversion to activity with a core symptom of anhedonia. HF was highly associated with depressive symptoms in a large population study [8]. Similarly, multiple meta-analyses suggested that patients with HF and comorbid depression had a significantly higher risk of mortality and cardiovascular events with the effect sizes remaining significant after adjustment for confounding factors [9]. Contrarily, depression also appears to increase the risk of HF in patients with no previous history of cardiovascular diseases. The Nord-Trøndelag Health Study (HUNT 2), which included 62,567 healthy patients, found that the severity of depressive symptoms was also highly associated with the incidence of HF after 11 years of follow-up [10]. Thus, HF with comorbid depression has a significant impact on the exacerbation and the mortality of both depression and HF. Currently, both the ESC and the American College of Cardiology/American Heart Association (ACC/AHA) recommend the screening and treatment of depression in patients with HF [1, 2]. However, depression overlaps with HF symptoms, and there are difficulties in the determination of depressive symptoms in patients with HF. Decreased activity tolerance is one of the most common manifestations of both HF and depression, and enhancement of muscle function improves the prognosis of patients with HF and depression [11]. In addition, somatic performance in the depression scale was more strongly associated with all-cause mortality in HF than cognitive performance [12]. The muscle hypothesis suggests that left ventricular dysfunction causes reduced peripheral perfusion and decreased exercise capacity and also triggers skeletal muscle myopathy. The above changes further lead to abnormal sympathetic activation, vasoconstriction, and endothelial dysfunction and ultimately exacerbate the deterioration of left ventricular function [13, 14]. Thus, muscle function may play a significant role in HF combined with depression.
Skeletal muscle accounts for 40%–50% of the total body weight and is an important component of the human body [15]. Under physiological conditions, muscle mass declines by an average of 0.47% and 0.37% per year in men and women, respectively. Such a decline can reach 1%–2% per year with aging [16]. In this regard, I. H. Rosenberg coined the term sarcopenia to describe skeletal muscle loss [17]. Sarcopenia refers to a form of muscle atrophy that occurs with aging and is characterized by a degenerative loss of skeletal muscle weight, mass and strength independent of weight gain or loss [18, 19]. Overall, 5%–13% of people aged more than 60 years have sarcopenia, whereas the prevalence in those above 80 years old exceeds 50% [19].
Sarcopenia is defined as low appendicular skeletal muscle mass (ASM), which
means that the sum of the muscle mass of the extremities divided by the square of
the height is less than two standard deviations from the reference value for
young people of the same sex. Asian Working Group for Sarcopenia (AWGS) 2019
consensus defined sarcopenia as age-related loss of skeletal muscle mass plus
loss of muscle strength and/or reduced physical performance [20]. In most cases,
ASM is measured via dual-energy X-ray absorptiometry (DEXA); however, in
some cases, bioelectrical resistance can be employed as an alternative. A cutoff
value of 7.0 kg/m
Sarcopenia is independently associated with several cardiovascular diseases and their associated risk factors, including myocardial infarction, congestive HF, atrial fibrillation, and atherosclerosis [22, 23]. Sarcopenia may accelerate the progression of chronic diseases such as cancer and HF. Indeed, sarcopenia can be found in 20–50% of patients with heart failure with reduced ejection fraction (HFrEF) and is commonly associated with increased morbidity and mortality [2]. The prevalence of sarcopenia in patients with heart failure with preserved ejection fraction (HFpEF) was found to be 19.7% in the SICA-HF study (European multicenter study) [24, 25]. In turn, chronic HF can worsen the adverse outcomes associated with sarcopenia, including osteoporosis, falls, cachexia, frailty, rehospitalization, and death, which may be associated with HF-related malnutrition, altered hormone levels, inflammation, oxidative stress, autophagy, and apoptosis [26]. Bekfani et al. [27] have showed recently in skeletal muscle biopsies elevated levels of atrophy genes and proteins in patients with HFpEF compared to HFrEF and healthy controls. Furthermore, patients with HF showed distorted fatty acid oxidation, glucose oxidation and mitochondrial number and function. Patients with reduced muscle function showed elevated levels of inflammatory parameter and reduced fatty acid oxidation.
Very recently, an association between sarcopenia and depression has gradually attracted attention. Depression leads to alterations in neurological, immune, and endocrine functions, which are significantly associated with physical fitness. A meta-analysis including 10 studies revealed that sarcopenia remained significantly positively associated with depression after adjustment for potential confounders, such as age, gender, cognitive performance, and physical activity [28]. In China, a cohort study demonstrated that sarcopenia was also significantly associated with depressive symptoms after adjustment for confounders and that new-onset depressive symptoms were associated with muscle mass, suggesting that sarcopenia is an independent risk factor for depressive symptoms [29].
Unlike sarcopenia, cachexia is defined as complex metabolic syndrome associated
with underlying illness and characterized by loss of muscle with or without loss
of fat mass. Its major clinical feature is a
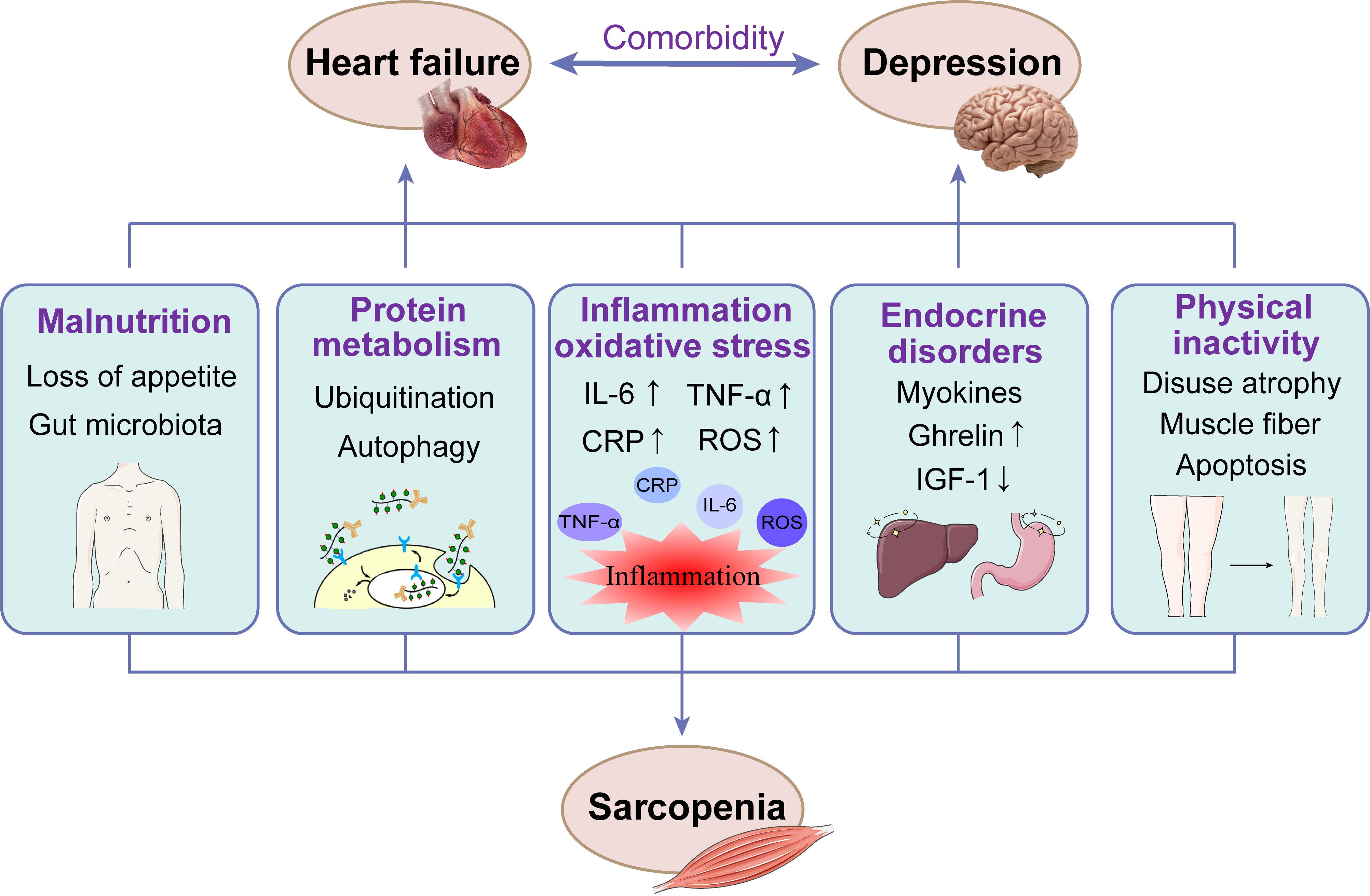
Recent studies have found that sarcopenia and HF with comorbid depression seem to share some common risk factors, such as malnutrition, chronic inflammation, dysregulation of the hypothalamic–pituitary–adrenal axis (HPA axis), and physical inactivity [5, 32]. The mechanisms involved in the pathogenesis of sarcopenia, HF and depression are presented in Fig. 1.
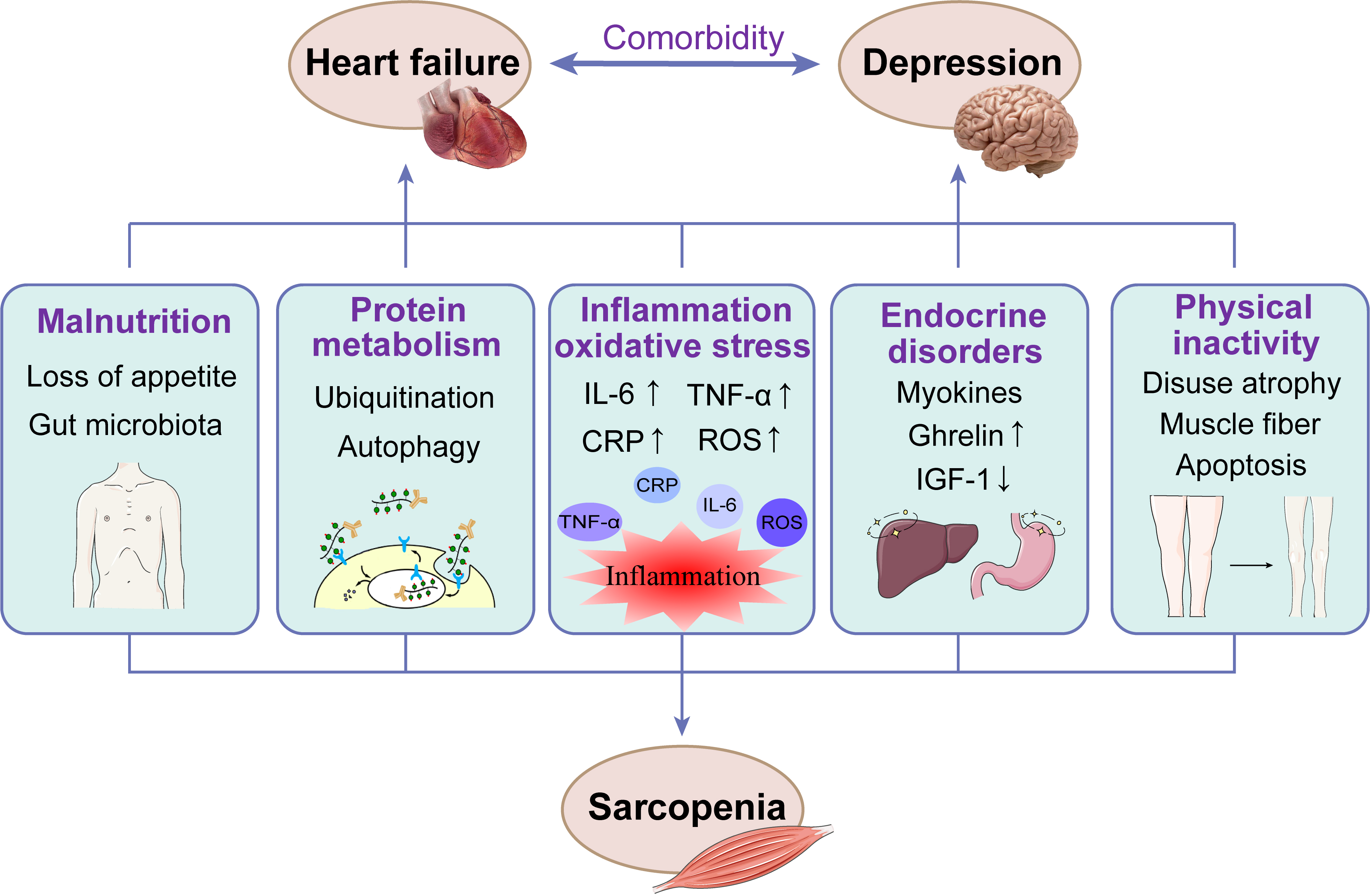 Fig. 1.
Fig. 1.The mechanisms involved in the pathogenesis of sarcopenia, HF
and depression. CRP, C-reactive protein; IL-6, interleukin 6; IGF-1,
Insulin-like growth factor 1; ROS, reactive oxygen species; TNF-
A study conducted by Chen et al. [33] on the relationship between sarcopenia, depression, and cognitive function demonstrated that subjects with sarcopenia were significantly more malnourished than those without, and there was also a significant difference in nutritional scores between subjects with depressive symptoms. The most direct cause of malnutrition in patients with HF and depression is loss of appetite, which is often associated with symptoms such as dyspnea and nausea but also with side effects of standard drug therapy for HF (such as digoxin, angiotensin-converting enzyme inhibitors, beta-blockers, and diuretics). Patients with severe HF often suffer from intestinal edema associated with loss of taste, nausea, and gastrointestinal disorders, which can lead to loss of appetite and malabsorption [34]. It is worth noting that the gut microbiota, which is known as the “second largest genome”, is widely involved in nutrient absorption, regulating intestinal epithelial function and influencing local or systemic immune inflammatory responses in the intestine; intestinal metabolites can also be released into the blood to play a systemic regulatory role [35]. In addition, blood redistribution and depression in patients with HF can lead to the upregulation of zonulin 2 precursor expression and the downregulation of zonula occludens-1 (ZO-1) expression, resulting in increased intestinal permeability and impaired intestinal epithelial barrier function. This could, in turn, lead to bacterial translocation and endotoxin release into the blood, further aggravating cardiomyocyte and myocyte injury [36]. It is worth noting that loss of appetite is a long-standing problem, and fatigue, depressive symptoms, and low quality of life are independent predictors of appetite decline over time [37].
The proteasome is a protein complex that labels and degrades unwanted or damaged
proteins with the help of a small protein called ubiquitin. The entire system of
ubiquitination and proteasome degradation is known as the ubiquitin–proteasome
system (UPS). Excessive activation of the UPS leads to increased proteolytic
metabolism, resulting in an imbalance in myogenic fibronectin levels and causing
muscle atrophy [38]. Monoubiquitinated target proteins are degraded by lysosomes,
whereas the degradation of target proteins by UPS requires polyubiquitination. E3
ubiquitin–protein ligase is the rate-limiting enzyme for the polyubiquitination
of protein substrates, and its most relevant to sarcopenia are tripartite
motif-containing 63 (TRIM63) and F-box only protein 32 (FBXO32)
[39, 40, 41]. TRIM63 and FBXO32 are mainly regulated by the
transcription factors nuclear factor-
Autophagy is another important mechanism of sarcopenia in which the lysosomal protease histone L plays a major role. Under normal conditions, autophagy is considered to be a nonselective degradation pathway of unnecessary or nonfunctional cellular components, such as damaged organelles and protein polymers [44]. Thus, autophagy under physiological conditions plays a significant role in maintaining normal muscle function. However, excessive autophagy exacerbates muscle atrophy, and excessive accumulation of autophagic vesicles occurs in almost all myopathies. Autophagy is usually increased through the FOXO and adenosine monophosphate-dependent protein kinase pathways, leading to myofiber atrophy. Studies have demonstrated that both UPS and autophagy are induced in the skeletal muscle early in the course of HF [45]. The above suggests that although autophagy is involved in the maintenance of muscle function, it may be detrimental when induced under catabolic conditions.
HF is a disease characterized by chronic low-level inflammation. Inflammation
not only affects cardiovascular function but also has a persistent effect on the
skeletal muscle. Furthermore, chronic inflammation is thought to be associated
with the pathogenesis of depression, with inflammation and inflammatory diseases
causing mood disorders and poor mental health [46]. A cross-sectional study by
Visser et al. [47] demonstrated that high levels of interleukin 6 (IL-6)
and TNF-
The abnormal release of reactive oxygen species (ROS) due to oxidative stress (OS) is a major cause of cellular senescence and apoptosis and often manifests as metabolic abnormalities. OS is extensively involved in diseases associated with aging, including sarcopenia and HF [51]. Studies have demonstrated elevated levels of OS-related markers in patients with HF and an association with reduced exercise tolerance and poor prognosis, which may be related to the high rate of anaerobic metabolism imposed on the organism by a low cardiac output and skeletal muscle hypoxia due to endothelial dysfunction [52]. In addition, excessive ROS can lead to mitochondrial dysfunction, which results in cytochrome c release and activation of caspase 3 and caspase 9, accelerating skeletal muscle injury and degeneration, especially by disrupting the excitation–contraction coupling structure of the muscle [53, 54].
Cardiac and skeletal myocytes could secrete different myokines
that are released into the circulation in an autocrine, paracrine, or endocrine
manner to regulate the body’s energy metabolism, insulin sensitivity, lipolysis,
free fatty acid oxidation and glycogen metabolism [55]. Myostatin have been shown
to be elevated in skeletal muscle in patients with HFpEF or HFrEF [27]. Some of
the myokines exert metabolic regulatory effects related to the prevention of
cardiovascular diseases, such as myostatin, irisin, apelin, and brain-derived
neurotrophic factor (BDNF) [56]. Myostatin, also known as growth differentiation
factor 8 (GDF8), is a member of the transforming growth factor
Ghrelin expressed in the gastric fundus stimulates the secretion of growth hormone (GH), cortisol, aldosterone, catecholamines and prolactin [62]. The body’s anabolic and catabolic activities are closely related to the aforementioned hormones. For example, ghrelin induces the secretion of GH, which, in turn, exerts anabolic effects directly or indirectly through insulin-like growth factors. Plasma ghrelin levels reflect the nutritional status of the individual and the level of body fat storage. Ghrelin has been found to be significantly increased in patients with cardiac cachexia [63].
Insulin-like growth factor 1 (IGF-1) is a hormone with a molecular structure similar to that of insulin and plays an important anabolic role in the adult body [64]. Contrarily, diminished IGF-1 function is an important component of the sarcopenia process and is not associated with the downregulation of IGF-1 or IGF-1 receptor expression [65]. Mechanistically, upon binding of IGF-1 to its receptor, insulin receptor substrate 1 (IRS1) is activated by phosphorylation and subsequently activates the phosphoinositide 3-kinase (PI3K)-protein kinase B (PKB/AKT)-mammalian target of rapamycin (mTOR) signaling pathway. The PI3K-AKT-mTOR pathway inhibits FOXO and glycogen-synthase kinase 3 (GSK3) and suppresses the activation of SMAD2 by myostatin, leading to increased protein synthesis [66]. Moreover, GH facilitates amino acid delivery to the skeletal muscle and inhibits protein hydrolysis via downstream signaling from IGF-1. However, the number of GH receptors decreases with age, and GH deficiency and lower levels of IGF-1 are associated with an increased risk of endothelial dysfunction and cardiovascular events [67]. In addition, Gold et al. [68] found increased secretion of corticotropin-releasing hormone (CRH) in depressed patients, which leads to increased secretion of epinephrine and norepinephrine in the body and induces an inflammatory response. Epinephrine and norepinephrine can lead to endothelial dysfunction, blood flow heterogeneity, and reduced capillary density, which are associated with the development of sarcopenia [69].
Patients with depression have sedentary lifestyles. A retrospective study by Burton et al. [70] demonstrated that depressed patients exhibited reduced daytime activity compared with healthy controls. Patients with HF also show a lack of exercise due to physical limitations. From a neurological point of view, physical inactivity also means a reduction in the activity of motor units. Unused or rarely used neurons undergo disuse degeneration, which, in turn, leads to further disuse degeneration of their synaptic junctional cells [71]. Thus, physical inactivity due to disease or sedentary behavior can cause disuse atrophy of the muscles.
In addition, the skeletal muscle is composed of different types of fibers. Among them, type I fibers are rich in mitochondria, muscle protein content and associated capillaries and have low ATPase, creatine kinase and glycolytic activities. Therefore, type I fibers efficiently use oxygen for sustained and slow muscle contraction [72, 73]. Contrarily, type II fibers have high ATPase, creatine kinase and anaerobic glycolytic activity, so type II fibers primarily use anaerobic metabolism for rapid contraction [74]. Muscle fiber types can change in response to external stimulation. Aging-induced loss of peripheral motor neurons, reduced number of motor units, altered neuromuscular connectivity and selective denervation of type II fibers all lead to atrophy of fast-contracting type II fibers [75]. Contrarily, the fiber-type shift in patients with severe HF tends to occur before muscle atrophy, with a lower percentage of type I fibers and a higher percentage of type II fibers [76]. In addition, apoptosis of the skeletal muscle cells correlates with the severity of HF and is accompanied by decreased levels of the antiapoptotic factor B-cell lymphoma 2 (BCL-2) and increased levels of the pro-apoptotic factor BCL-2-associated X protein (BAX) [77].
Aging is strongly associated with impairments in cardiovascular function, muscle
strength and cognitive performance. Mechanistically, aging- associated
mitochondrial dysfunction, decreased levels of protein synthesis and peroxisome
proliferator-activated receptor
In patients with HF, decreased skeletal muscle blood flow due to reduced cardiac output affects the oxygen supply to that organ, which leads to decreased muscle function. The skeletal muscle is an important organ of the body involved in glucose metabolism, and reduced muscle mass affects glucose homeostasis in the body. Several studies have revealed a relationship between blood glucose and depressive symptoms [80, 81].
In addition, visceral fat accumulation is a risk factor for chronic degenerative diseases, such as cardiovascular disease, dementia and depression. Another morphological aspect of aging skeletal muscle is the infiltration of fat into muscle tissue components. Fat can be contained in both adipocytes and deposited in muscle fibers, which is an important factor contributing to reduced blood flow and decreased muscle mass [82]. Patients with sarcopenia may be involved in muscle loss due to increased adipose tissue through increased proinflammatory cytokines and decreased release of muscle factors, leading to the development of cardiovascular disease [83].
As to clinical studies on the pharmacological treatment of HF combined with
depression, sertraline and paroxetine reduced depressive symptoms with fewer side
effects. However, significant difference hadn’t been observed between sertraline
and control group. Nonetheless, related studies revealed that sertraline and
escitalopram did not achieve significant efficacy over placebo in the treatment
of depression and HF [2]. Contrarily, the drug sacubitril/valsartan for HF
improved depressive symptoms in patients with heart failure with reduced ejection
fraction and depression [84]. The aforementioned studies suggest that
antidepressant treatment in patients with HF and comorbid depression may improve
their depressive symptoms but not their HF symptoms and prognosis and that
effective HF treatment may improve the comorbid depressive symptoms. Thus, the
treatment modality of HF with comorbid depression still needs to be explored, and
probably, HF treatment should be the main focus. However, HF and body wasting
should be detected early in the process of muscle loss in patients. The loss of
body mass often begins with the loss of functional muscle. Although it is still
not too late to start treatment in patients who have already experienced weight
loss, an important therapeutic window might have been missed. It is important to
first define the drug regimen for patients with HF, including maximum tolerated
doses of angiotensin converting enzyme inhibitors (ACEI),
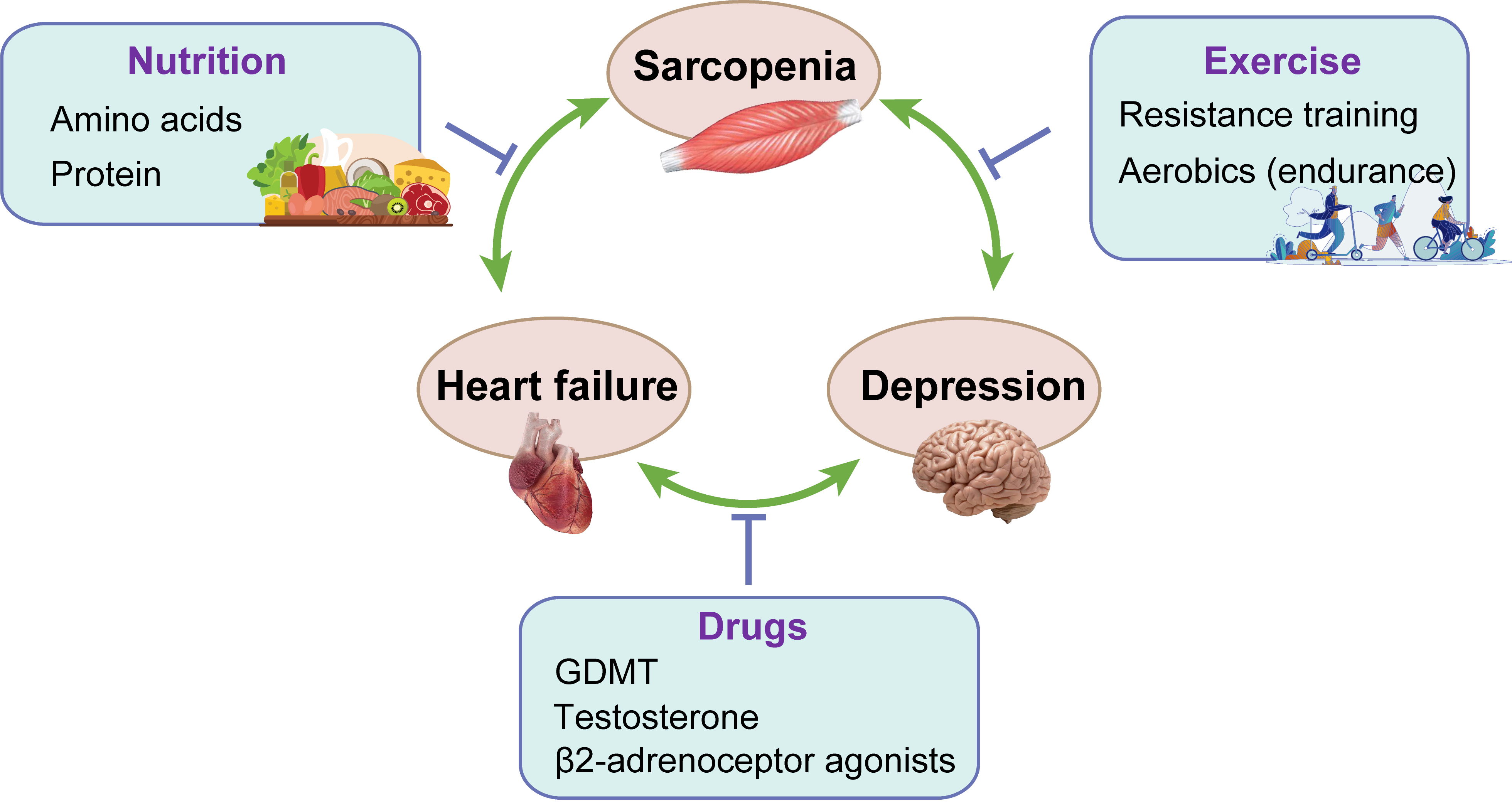
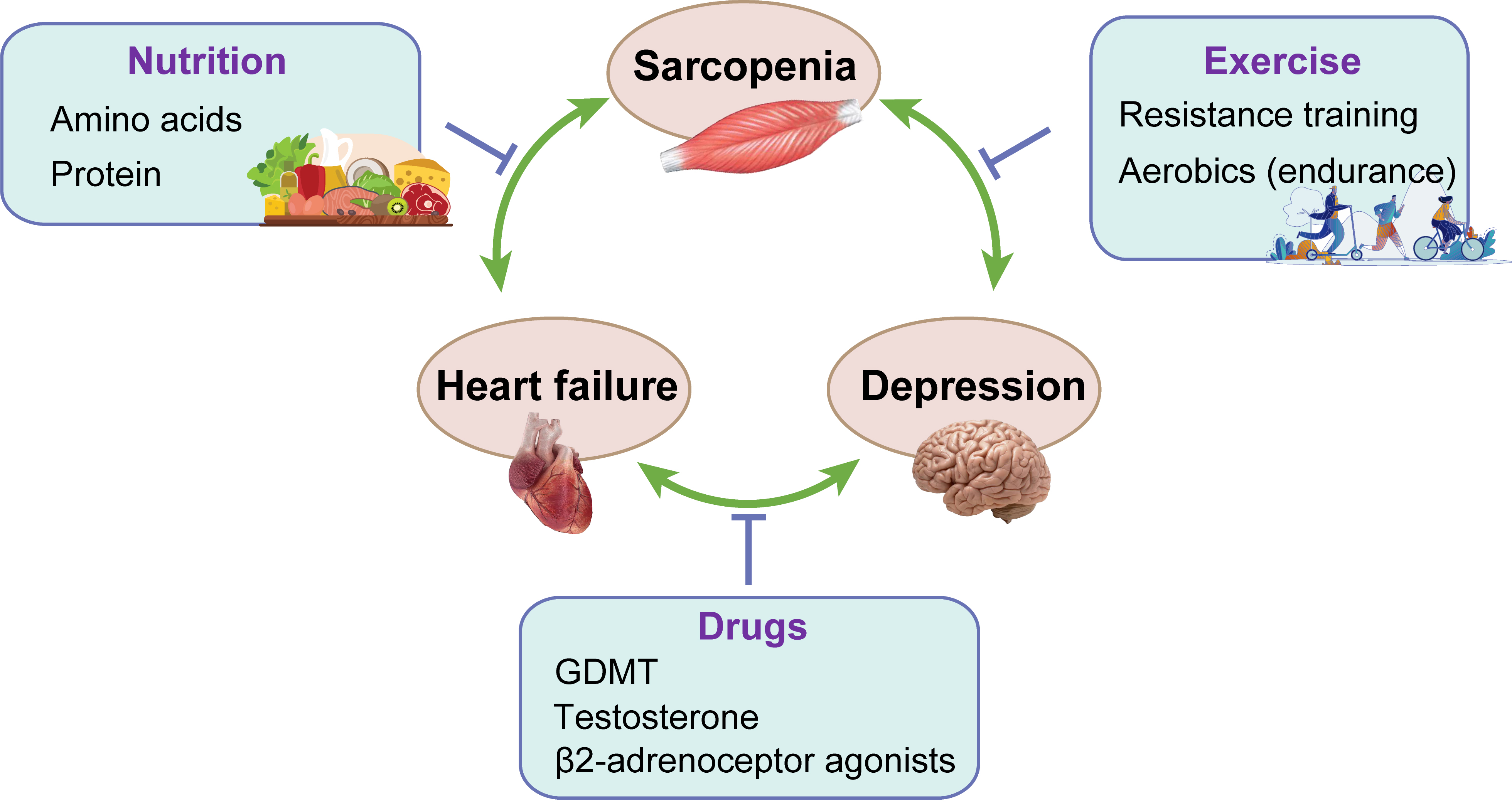 Fig. 2.
Fig. 2.The treatment of sarcopenia, HF and depression. GDMT, guideline-directed medical therapy.
Both HF and sarcopenia are associated with anorexia, and patients with both
conditions have low scores in nutritional assessment, suggesting that patients
with HF and sarcopenia are more predisposed to malnutrition [85]. In addition,
although nutritional assessment is currently performed less frequently in
patients with depression, the pathogenesis of nutritional status of patients with
depression remains unclear [28]. Similarly, anorexia nervosa is very common in
patients with cachexia. Although cachexia cannot be reversed by improving
nutritional intake, the beneficial effects may be achieved by increasing calorie,
protein, or amino acid intake [14]. Saitoh et al. [86] have showed that
Inflammation, use of loop diuretics, and cachexia are associated with an
increased likelihood of anorexia in patients with HF, and patients with anorexia
showed impaired functional capacity and poor outcomes. Some scholars have
suggested that all patients with HF should take micronutrient supplements and
avoid excess salt (
Although the level of evidence for amino acid supplementation still falls short of clinical guidelines, there may also be some beneficial effects from nutritional intake of certain proportions of essential amino acids. Among them, branched-chain amino acids isoleucine, leucine and valine may be the major amino acid components of the skeletal muscle. Oral supplementation with these amino acids may enhance protein synthesis and inhibit protein hydrolysis in the muscle tissue, an effect that is most pronounced with leucine. Mechanistically, leucine promotes insulin signaling and glucose uptake in the skeletal muscle via the PI3K-AKT-mTOR pathway [90]. A double-blind, randomized, controlled study that included 38 patients with HF revealed improvements in peak oxygen consumption and 6-min walk distance after 2 months of oral administration of an essential amino acid blend [91]. Similarly, another double-blind, randomized, controlled trial revealed a slight improvement in peak oxygen consumption in elderly patients with chronic HF on oral supplementation with amino acids for 30 days [92].
A large part of the muscle mass is determined by protein synthesis and catabolism. The recommended intake of protein, in general, is 0.8 g/kg per day, and in patients with both HF and sarcopenia, a higher intake may be required [14]. Studies have demonstrated that after 6 weeks of high protein intake (20 g/day) and high-calorie (600 kcal) supplementation in patients with sarcopenia, these patients exhibited decreased plasma TNF levels and improved weight and quality of life [93]. Observational studies on sarcopenia support 1.0–1.2 g/kg protein per day as the optimal dietary protein intake to prevent sarcopenia [94].
Exercise training is a nonpharmacological, low-cost, effective and safe
treatment that is capable of improving depressive symptoms regardless of the
patient’s psychiatric diagnosis, which makes it an excellent treatment strategy
for depression [95]. Aerobic exercise activates peroxisome proliferator-activated
PGC1-
Regular and appropriate physical activity can promote physical and mental health. As demonstrated in the HF-ACTION study, exercise can moderately reduce depressive symptoms in patients with HF [101]. However, excessive exercise can lead to emotional instability, reduce the body’s immunity, and affect physical health [102, 103]. Regular aerobic exercise that causes mild or moderate shortness of breath is recommended for patients in the ESC guidelines (Class I, Level A evidence) [2]. Similarly, regular physical activity is recommended in the ACC/AHA guidelines as a safe and effective way of improving body function (Class I, Level A evidence) [1]. Resistance exercise training may be a good strategy to improve the muscle structure in patients with sarcopenia. A randomized controlled trial revealed that after a 10-week period of high-intensity resistance training performed three times a week, patients exhibited an increase in muscle strength and endurance [104]. Also, Tieland et al. [105] found that protein supplementation combined with resistance exercise training for 24 weeks resulted in significant improvements in muscle mass and strength, suggesting that nutritional supplementation combined with exercise therapy may be more efficient.
A large number of medications may be beneficial to patients with HF and wasting. Potential drugs include anabolic steroids, anti-myostatin antibodies and ghrelin receptor agonists (such as anamorelin), anti-inflammatory drugs, appetite enhancers, proteasome inhibitors, and beta-adrenergic agonists.
GDMT offers a beneficial role in combating physical wasting in addition to
treating HF. For example, ACEI can improve mitochondrial function, increase IGF-1
levels, promote skeletal muscle glucose uptake and help treat sarcopenia [106].
Data from the SOLVD trial comparing the ACEI enalapril treatment group with the
placebo group revealed that patients receiving enalapril had a 6% lower risk of
weight loss than those receiving placebo [31]. In addition, in a small study of
the
In addition to being an anabolic steroid, testosterone is a vasodilator in the coronary vasculature and pulmonary vasculature. Low testosterone levels are common in patients with HF and exacerbate cardiac dysfunction by altering peripheral vascular resistance, increasing cardiac afterload, and decreasing cardiac output [109, 110]. Decreased testosterone levels are also associated with decreased muscle mass and functional impairment [111]. In contrast, in patients with HF, low levels of testosterone are independently associated with exercise intolerance [112]. A study evaluating testosterone therapy in patients with HF revealed that testosterone therapy was associated with a relative increase in cardiac output and a decrease in systemic vascular resistance [110], with significant improvements in the Minnesota Living with HF Questionnaire scores in the testosterone-treated group. There was also a significant improvement in walking distance and grip strength in the testosterone-treated group [113].
Several studies have demonstrated that
Sarcopenia can occur at any stage of HF with comorbid depression, and it is not only a complication of HF and depression but also a risk factor for both. Mechanistically, there are many similarities and potential links between the pathogenesis of all three. In terms of treatment, the current therapy is focused on the treatment of HF, supplemented by the prevention and treatment of sarcopenia. Given that studies on amino acids and proteins are small studies, supplementations of amino acids and proteins are not part of the recommended therapies for these patients. Thus, larger randomised studies are still needed in the future. Because of the multiple pathogeneses involved, a single treatment cannot meet the clinical needs of the patients. As such, a combination of modalities is required, and the treatment is based on increasing physical activity and supplementation [118]. Improved nutritional status and exercise tolerance in patients with HF have the potential to improve HF and its comorbid depressive symptoms. Enhancement of the muscle function improves the prognosis of patients with HF and depression. The 2021 ESC guidelines for HF also recommend that all patients with chronic HF who are moderately active participate in exercise to improve their quality of life and reduce the hospitalization rate. For patients with severe comorbidities or frailty, supervised exercise may be considered as the basis for a cardiac rehabilitation program [2]. In addition, it is important not to neglect the issue of patient education to improve patient compliance. Furthermore, aside from making patients aware of the complexity of the disease, it is important to popularize multiple treatment modalities and lifestyles, such as supplementation with multiple nutrients along with reasonable physical exercise. Given the complex mechanisms of HF combined with depression and sarcopenia, more clinical studies involving all three diseases are needed in the future to gain a deeper understanding of the relationship between the three diseases and to optimize treatment.
CY and LY designed the topic. RW, JD, WL, KH and ZC searched for references. RW and JD prepared original manuscript and figures. WL, KH and ZC provided help and advice on the manuscript. CY and LY reviewed and proofread the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This work was supported by the National Natural Science Foundation of China, grant number 82070405 and 81974171, and Innovative and Entrepreneurial Team of Jiangsu Province, grant number JSSCTD202144.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.