1 Emory Women’s Heart Center and Emory Clinical Cardiovascular Research Institute, Division of Cardiology, Emory University School of Medicine, Atlanta, GA 30322, USA
2 J. Willis Hurst Internal Medicine Residency Program, Emory University, Atlanta, GA 30322, USA
3 Division of Preventive Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02215, USA
Academic Editor: Brian Tomlinson
Abstract
Cardiovascular disease (CVD) remains a major health threat in women. While traditional CVD risk factors such as hypertension, hyperlipidemia, diabetes, and smoking have been recognized for over 50 years, optimal control of these risk factors remains a major challenge. Unique sex-specific risk factors such as adverse pregnancy outcomes, premature menopause and low estrogen states, and chronic autoimmune inflammatory disorders also contribute to increased CVD risk in women. In addition, psychological risk factors such as stress, depression, and social determinants of health may have a disproportionately adverse impact in women. An improved understanding of traditional and emerging sex-specific CVD risk factors and management of modifiable factors is critical for clinicians who provide care for women. Early recognition and treatment of risk factors may alter the trajectory of adverse CVD events. A multi-disciplinary approach with team-based care involving multiple specialists and improved, targeted educational efforts are needed to reduce CVD risk factors and its adverse consequences in women.
Keywords
- heart disease
- sex-specific risk factors
- women
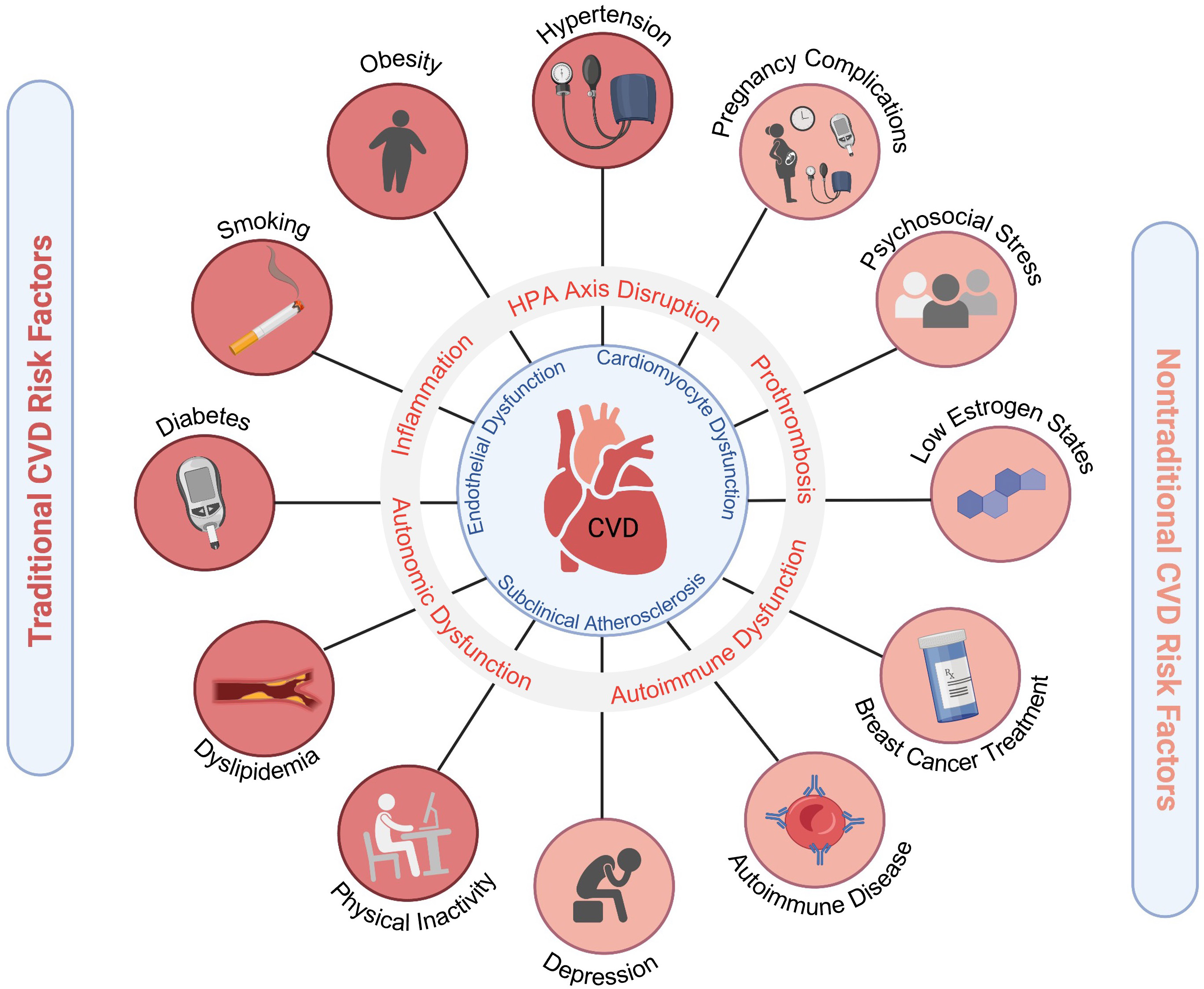
Among cardiovascular diseases (CVD), ischemic heart disease remains the leading cause of morbidity and mortality in both men and women, followed by stroke [1]. While ischemic heart disease typically presents a decade later in women compared to men, women have less favorable outcomes with higher mortality, and increased angina, and health-related morbidities [2, 3]. This disparity is in part due to increased comorbid risk factors in women, delays in presentation and treatment, and less use of guideline-based therapies [2, 4]. Women have higher rates of traditional risk factors such as hypertension (HTN), diabetes, and obesity. In addition to the traditional CVD risk factors that are common in both men and women, there are also sex-specific risk factors that are either more prevalent or unique to women. These factors contribute to CVD risk via mechanisms of inflammation, autonomic dysregulation, and hypothalamic-pituitary-adrenal (HPA) hormonal axis disruption. This results in a pro-atherogenic and pro-thrombotic milieu which increases the incidence of myocardial infarction, heart failure, stroke, and CVD death (Fig. 1). Over the past three decades it has also become increasingly apparent that there are sex differences in the pathophysiology of heart disease [5]. For example, there are certain conditions that predominate in women such as ischemia with no obstructive coronary artery disease (INOCA), myocardial infarction with no obstructive coronary artery disease (MINOCA), spontaneous coronary artery dissection (SCAD), Takotsubo syndrome, and heart failure with preserved ejection fraction (HFpEF). Mortality from heart disease among young women (less than age 55 years) is increasing and is associated with more risk factors and comorbidities [6, 7, 8].
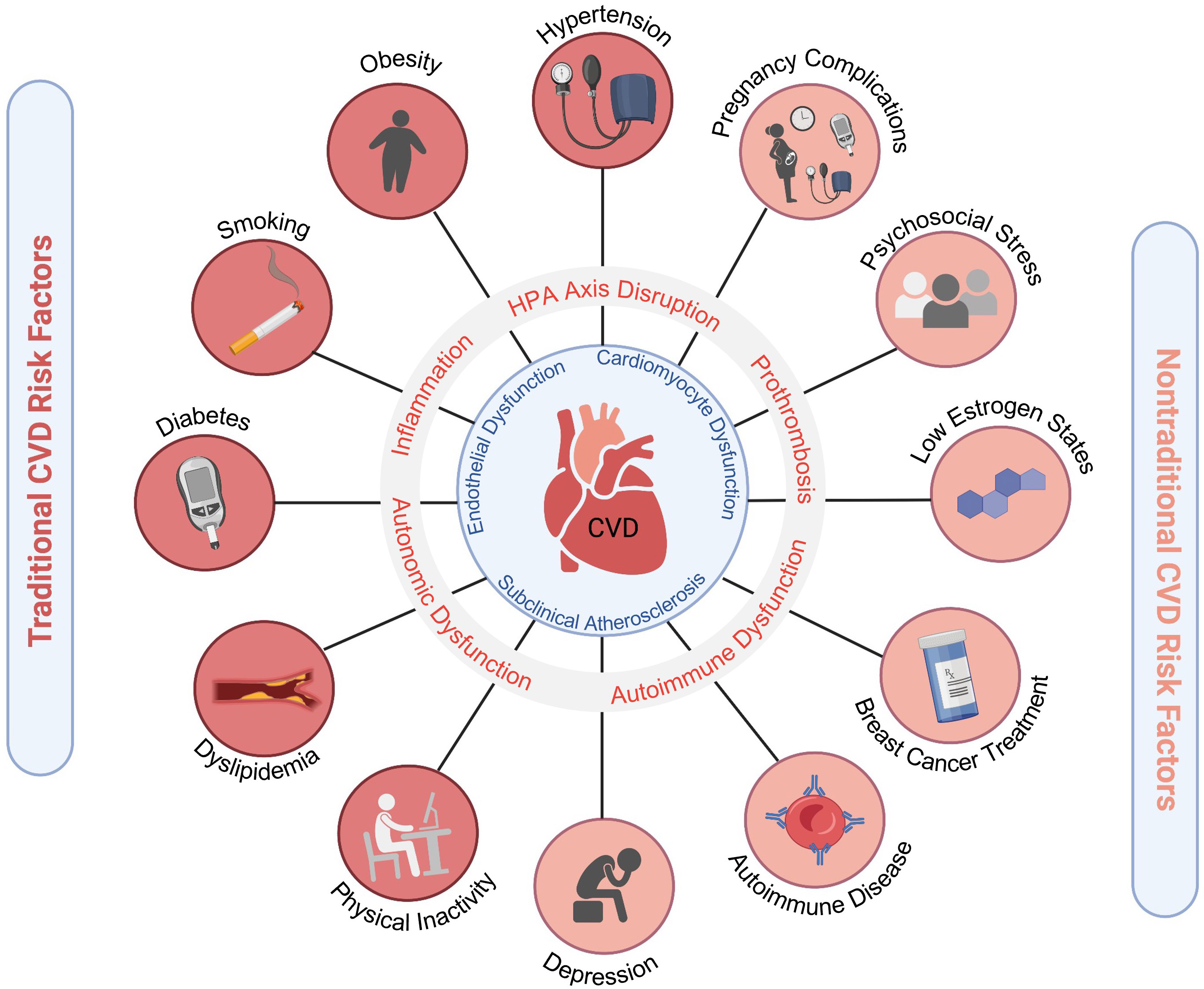 Fig. 1.
Fig. 1.Cardiovascular risk factors in women. Traditional and emerging risk factors contribute to pro-atherosclerotic, pro-inflammatory, pro-thrombotic states that ultimately lead to increased CVD morbidity and mortality in women. Adverse pregnancy outcomes include hypertensive disorders of pregnancy, gestational diabetes, and pre-term birth. Figure created using https://Biorender.com.
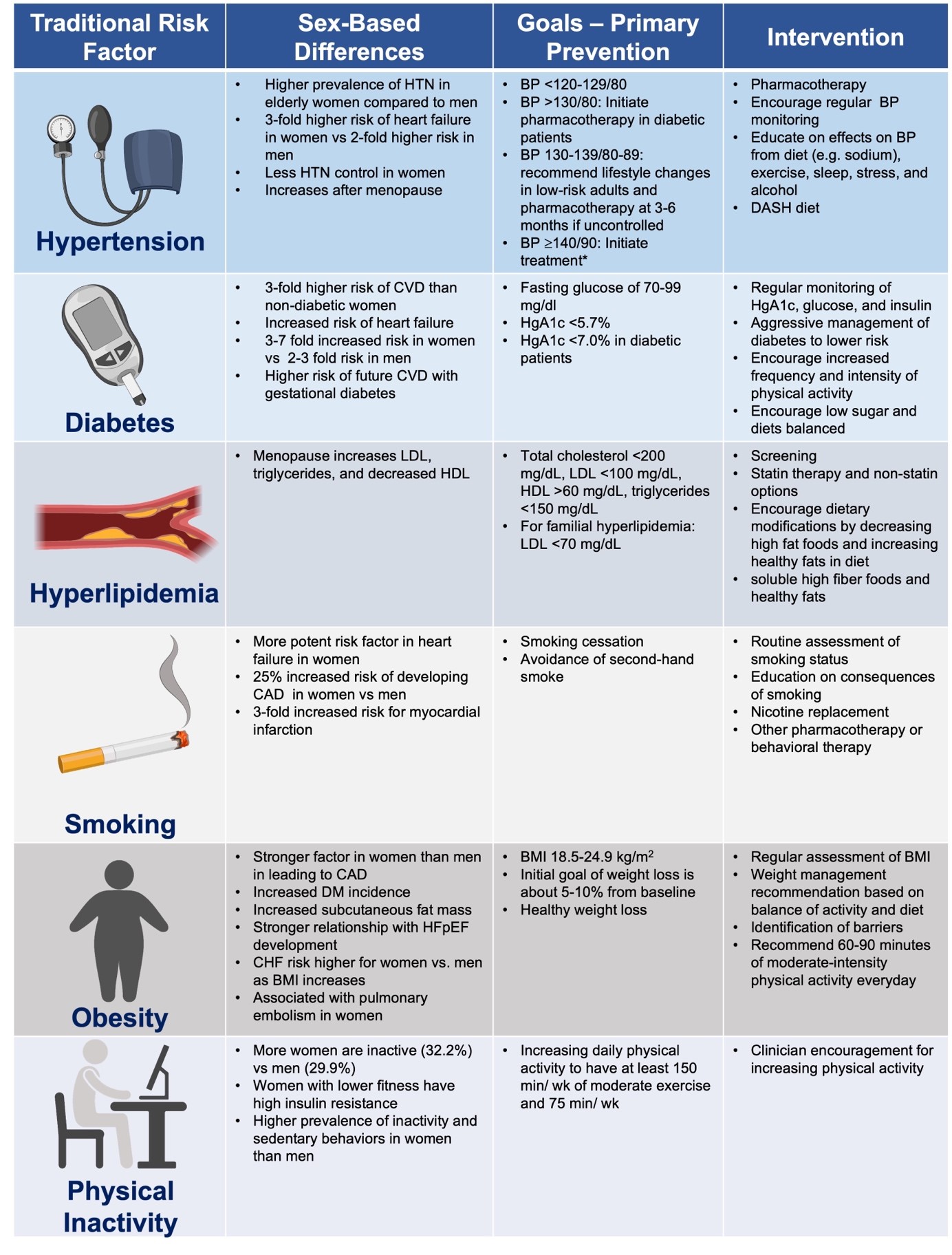
A summary of updated recommendations for primary prevention of CVD in women was recently published [9]. In primary prevention, CVD risk is estimated based on various risk scores such as the ACC/AHA ASCVD pooled cohort equation risk calculator, Framingham risk score, Reynold’s risk score, and others, but none of these tools incorporate emerging/novel sex-specific markers. Whether incorporation of these novel risk factors would improve CVD risk prediction remains uncertain. Coronary artery calcium scoring is another powerful tool that can be helpful in borderline risk patients to help determine how aggressive to target primary prevention strategies such as statins [10]. In order to reduce CVD-related morbidity and mortality, it is imperative to improve access to healthcare, identify CVD risk factors earlier, promote heart-healthy lifestyle choices, and implement pharmacologic treatment expeditiously when indicated. This review highlights traditional (Table 1, Ref. [11]) and emerging risk factors for CVD that can be treated to reduce morbidity and mortality in women.
|
| *AHA recommendations for management of stage 1 hypertension in low-risk adults [11]. Family history of premature coronary heart disease is a risk factor that should also be considered in decision-making. |
HTN is a highly prevalent and powerful CVD risk factor in both women and men that is associated with myocardial infarction, heart failure, stroke, atrial fibrillation, peripheral vascular disease, and chronic kidney disease. Systolic blood pressure begins to increase around the time of transition to menopause in women and becomes more prevalent after 65 years of age in women compared to men. In addition, studies have found that the risk of HTN is higher in women than men [12, 13, 14]. Young women with HTN are at greater risk of end-organ damage compared to age-matched men [15]. Arterial stiffness, autonomic dysfunction, generalized endothelial dysfunction, and upregulation of the renin-angiotensin system all contribute to these increases in blood pressure in the setting of declining estrogen levels [16]. African American (AA) women are more likely to have HTN diagnosed at younger ages and have more severe HTN compared to white women [17]. In addition, AA women have nearly twice the age-adjusted death rate attributable to HTN, although this and other CVD risk factors are often undertreated in AA women. A recent community-based CVD risk factor screening study in 945 AA women found that elevated blood pressure and obesity were prevalent at younger ages [18]. Older and AA women are particularly susceptible to increased risk from heart failure, stroke, and renal disease due to HTN [19]. Women are also more likely to have anxiety-related and “white coat” HTN compared to men [20, 21]. Current HTN guidelines recommend avoiding the use of oral contraceptives in reproductive-age women with uncontrolled HTN seeking contraception and instead recommend using low dose ethinyl estradiol, progestin only formulation, or alternative methods such as an intrauterine device [14].
Diabetes mellitus remains one of the most prevalent diseases in the United States with an estimated one in nine women having this condition [22, 23]. The inflammation and atherogenesis associated with diabetes results in an increased risk of CVD [24, 25, 26]. Diabetic women have a 3–7 times increased risk of developing heart disease compared to a 2–3 times increased risk in diabetic men [27, 28, 29, 30]. The greater impact of diabetes on women may be partially due to increased adiposity [31]. Women are more likely than men to progress from prediabetes to diabetes [32]. In the original cohort of the Framingham Heart Study, there was a strong, independent association between HgA1c and CVD in women, but not in men. For every 1% increase in HgA1c, the relative odds for CVD increased by 1.39 in women [33]. The strong association in women between hyperglycemia and CVD remained significant even when women who were classified as diabetics were excluded [33]. In a meta-analysis, the rate of fatal coronary heart disease was higher among patients with diabetes compared to those without diabetes (5.4% vs. 1.6%) [27]. However, the difference was more pronounced in diabetic vs. non-diabetic women (7.7% vs. 1.2%) compared to diabetic vs. non-diabetic men (4.5% vs. 2.0%) [27]. Women with diabetes had a higher relative risk of fatal coronary heart disease compared to diabetic men (3.5 vs. 2.0 respectively) [27]. Menopause results in additional risk factors for CVD [27]. Although there does not seem to be a correlation between the onset of menopause and higher glucose levels, an increased risk of metabolic syndrome emerges after the transition to menopause, beyond the risk attributable to age alone [34, 35, 36]. Early diagnosis of diabetes is essential, particularly in racial/ethnic groups at high risk for diabetes such as AA, Hispanics, American Indians, and Pacific Islander Americans. While glucose-lowering therapy reduces microvascular complications, randomized trials have not shown a benefit of intensive glucose lowering on CVD outcomes in patients with long-standing diabetes [37, 38].
Hyperlipidemia is a well-established modifiable risk factor for CVD, with a
direct relationship between low-density lipoprotein (LDL) cholesterol and ASCVD
events. Studies have shown that lowering LDL is associated with decreased risk
for CVD in both primary and secondary prevention populations which have included
women [39]. The INTERHEART trial was a large case control study that sought to
quantify the risk of various modifiable risk factors for CVD. Smoking and an
abnormal lipid profile (defined as an elevated ApoB/ApoA1 ratio) were the two
strongest risk factors for myocardial infarction, with abnormal lipid profiles
being the highest of all the risk factors studied [40]. The 2013 ACC/AHA
Guideline on the Treatment of Blood Cholesterol recommends initiation of statins
in both women and men with an LDL-C
Cholesterol levels may fluctuate throughout a woman’s life-span, from young adult to pregnancy to the transition to menopause, and treatment should be tailored accordingly. Women are at increased risk of hyperlipidemia, with the postmenopause period being a particularly vulnerable time [45]. Following menopause, women have higher total cholesterol, triglycerides, and LDL, and reduced HDL, which places them at higher risk for CVD [46]. High non-HDL-C and triglyceride levels are more important CVD risk factors in women than men, especially women with diabetes [47, 48].
An elevated lipoprotein(a) level is considered a risk factor that is
pro-thrombotic, pro-inflammatory, and pro-atherogenic in both men and women and
is associated with a higher risk of CVD events [39]. In the Multi-Ethnic Study of
Atherosclerosis (MESA), Lp(a) was associated with increased CVD risk when there
was evidence of systemic inflammation as determined by higher C-reactive protein
levels (
| Recommendations for ASCVD Secondary and Primary Prevention in Women | ||
| YES | NO | |
| Aspirin |
||
| Statins |
||
| * Clinician-patient discussion to consider aspirin if low risk for bleeding and
high ASCVD risk in those 40 to 59 ( ** Could consider statin if at age 40 to 75 years with borderline risk (5% to *** USPSTF Recommendations [53]. # ACC/AHA Blood Cholesterol Guidelines [39]. | ||
Cigarette smoking is the leading behavioral contributor for CVD mortality, with an estimated 30.8 million adults in the United States currently smoking cigarettes [54]. It is associated with multiple forms of CVD, including coronary heart disease, cerebrovascular disease, and peripheral arterial disease [55]. While men are more likely to smoke cigarettes (14.1% vs. 11%), women are disproportionately affected by the risk of CVD [54, 56]. The surgeon general in a weekly morbidity and mortality report in 2002 wrote, “Like their male counterparts who smoke, women smokers are at increased risk of cancer, cardiovascular disease, and pulmonary disease, but women also experience unique risks related to menstrual and reproductive function…it is tragic that an entirely preventable factor continues to claim so many women’s lives” [57]. Huxley et al. [56] conducted a meta-analysis of cohort studies that included 2.4 million individuals and found that compared to non-smokers, women who smoke have a 25% greater relative risk of coronary heart disease than male smokers, after adjusting for other risk factors. All patients should be asked about tobacco use at each office visit; and smokers should be advised to quit and given the tools to do so [58]. Furthermore, AHA/ACCF guidelines also recommend that patients should be advised to avoid environmental tobacco exposure [59].
Electronic cigarettes (e-cigarettes) have been growing in popularity since their introduction in 2007 and contain hazardous compounds [60]. One study found that within one month of switching from tobacco smoking to e-cigarettes, endothelial function assessed by flow mediated dilation (FMD) and vascular stiffness (measured by pulse wave velocity) improved in both men and women, but women had more improvement compared to men [61]. However, other studies have found impaired vascular function with both tobacco cigarettes and e-cigarettes [60]. In a retrospective study of 96,000 participants, those who smoked e-cigarettes were more likely to have myocardial infarction (odds ratio: 1.56) and stroke (odds ratio: 1.3) compared to non-e-cigarette users [62]. Whether e-cigarettes pose a relatively higher risk in women compared to men has not been studied. In a study with over 400 pregnant women, 6.5% were using e-cigarettes during pregnancy and 65% stated that e-cigarettes were safer than tobacco cigarettes. Future studies are needed to address the safety of e-cigarettes on women and fetuses [63].
An elevated body mass index (BMI) is associated with increased CVD risk in both
women and men, but there are important sex differences in fat distribution
(visceral vs. subcutaneous) [64]. Women predominantly accumulate subcutaneous
fat, while men accumulate more visceral fat. However, with menopause, women have
increased visceral fat compared to premenopausal women of similar ages, which
contributes to insulin resistance and inflammation [65]. Cardiometabolic
biomarkers such as serum adiponectin levels also predict CVD risk and mortality
in both men and women [66, 67]. Several CVD risk factors are increased in
patients with greater adiposity and obesity. For example, HTN is associated with
overweight (OR = 2.1) and obesity (OR = 5.2) in women, and diabetes is associated
with abdominal obesity (OR = 3.9) in women [68]. Weight reduction is associated
with improvements in cholesterol levels, lower blood pressure, and a lower risk
of developing type 2 diabetes [69]. Overweight and obese individuals and women
with waist circumference of
Physical activity is one of the most important modifiable risk factors for CVD.
In one study, the risk of heart disease with physical inactivity was higher
compared to other traditional CVD risk factors [72]. The AHA defines adequate
physical activity as 150 minutes/week of moderate intensity or 75 minutes/week of
vigorous intensity exercise. Physical activity is also part of the AHA’s Life’s
Simple 7, created in 2010, which outlines and defines modifiable risk factors
that contribute to cardiovascular health [73]. Physical activity levels are lower
among women compared to men, especially in AA and Hispanic adults [13]. Women
undergo several events throughout their lives that reduce their amount of
physical activity compared to men, including pregnancy and parenting [74]. A
recent study analyzing the lifetime risk of coronary heart disease based on
genetic factors and/or lifestyle modifications (defined in accordance with AHA’s
Life’s Simple 7) of nearly 10,000 participants found that lifestyle factors
affect overall freedom from coronary heart disease more than genetic factors
[75]. Lifestyle factors also have a greater effect on women than men for future
CVD events [75]. In a Swedish cohort study, total physical activity time was
inversely associated with a risk of myocardial infarction only in women [76].
There may be a dose-dependent risk of CVD in older women (
Exercise training leads to direct improvements in vascular function, diastolic function, and beneficially alters autonomic tone [78, 79, 80, 81, 82, 83, 84]. In a meta-analysis of 48 randomized trials of cardiac rehabilitation (CR) vs. usual care in ischemic heart disease patients, CR was associated with reduced cardiac mortality (OR = 0.74; 95% CI: 0.61 to 0.96) and all-cause mortality (odds ratio = 0.80; 95% CI: 0.68 to 0.93) [85]. Despite its beneficial effects on morbidity, mortality, functional capacity, and quality of life, CR is unfortunately grossly underutilized in women [86, 87, 88]. A comprehensive cardiac rehabilitation program includes not only aerobic and strength training exercises, but also nutrition counseling, education on tobacco cessation strategies, and psychological evaluation [89].
Eliciting a family history of CVD is important, as this risk factor
increases CVD risk. A family history of premature CVD in a first-degree relative
(defined as
Hypertensive disorders of pregnancy are a major cause of maternal morbidity and
mortality [94]. Women with pre-eclampsia have not only an increased risk but also
an earlier onset of CVD risk factors including HTN, diabetes, and hyperlipidemia
[95]. A 2021 Swedish cohort study with over 2 million women and 4 million
pregnancies tracked CVD after pre-eclampsia pregnancy complications. CVD
mortality rates were higher among those with pre-eclampsia or eclampsia at a
hazard ratio (HR) of 2.10, which was the third highest predictor, after
stillbirth (3.14) and gestational diabetes (3.03) [96]. The elevated rate of CVD
mortality for women with a history of pre-eclampsia was especially pronounced in
the 4th post-partum decade [97]. In addition, other adverse pregnancy outcomes
(APOs) such as gestational diabetes, preterm birth (
Though the pathogenesis is under active investigation, there appears to be an element of chronic inflammation after a complicated pregnancy, resulting in a long-term increase in CVD risk factors [103, 104]. Although pregnancy complications are established CVD risk predictors, knowledge gaps remain as to how these factors should be incorporated to inform decision-making regarding CVD preventive strategies. Women who have experienced an APO should be routinely screened for CVD risk factors, including HTN, diabetes, hyperlipidemia, smoking cessation, and obesity, with counseling and pharmacotherapy when appropriate. It is recommended that women with adverse pregnancy outcomes undergo CVD risk screening within 3 months post-partum [9].
Endogenous estrogen generally has favorable effects on the vasculature in
premenopausal women and is anti-inflammatory, anti-thrombotic, and
athero-protective. Declining estrogen levels during the transition to menopause
and a shift in the ratio of estrogen/testosterone levels contributes to
endothelial dysfunction and vascular aging, resulting in an increased incidence
of CVD in women after menopause [105]. Premature menopause (menopause before age
40, either natural or surgical) is a risk factor for early CVD [106, 107]. CVD
risk factors such as HTN, hyperlipidemia, and weight gain also become more
prevalent after menopause. There is a shift in the lipid profile resulting in an
increase in more atherogenic lipoproteins such as LDL and triglycerides and a
lowering of the protective HDL. However, while prior observational studies
indicated that hormone therapy during menopause may have some benefit, the two
large, randomized, controlled Women’s Health Initiative (WHI) trials (conducted
in women with a mean age of 63) showed that hormone therapy (0.625 mg conjugated
equine estrogen + 2.5 mg medroxyprogesterone acetate) vs. placebo was associated
with a higher risk of CVD events compared to placebo in the overall group with an
intact uterus [108, 109]. In the WHI-E-alone trial, 0.625 mg of conjugated equine
estrogen was compared to placebo in those women without a uterus, and showed no
benefit in the reduction of CVD [108]. In stratified analyses by age and time
since menopause, results for younger women were generally more favorable than for
older women, providing support for the “timing hypothesis” [110, 111, 112]. Moreover,
the ELITE trial, which directly tested the timing hypothesis, found that oral
estradiol (1 mg/day), with or without progesterone vaginal gel, was associated
with less progression of carotid-intima media thickness (CIMT) at a 5-year median
follow-up if initiated early (within 6 years postmenopause) compared to placebo
[113]. However, in those women who were
Bilateral oophorectomy (BSO) induces a “surgical menopause” and is performed
for several indications including risk reduction for women at increased risk of
hereditary cancer syndromes such as BRCA gene carriers [120]. Observational
studies have reported on the association of BSO and increased CVD risk. The
Nurses’ Health Study found an increased risk of coronary heart disease in women
with hysterectomy + BSO compared to those with hysterectomy and ovarian
conservation, with the risk elevated in those undergoing BSO at ages
PCOS is a common endocrine disorder in young women, that is associated with hyperandrogenism, ovulatory dysfunction, and insulin resistance. Cardiac risk factors such as HTN, diabetes, and obesity are prevalent in women with PCOS, and it is recommended that women with PCOS are regularly screened for CVD risk factors. Weight management and regular physical activity may improve their risk profile. Women with PCOS had a higher coronary artery calcium score compared to controls with normal ovulatory cycles [124].
Atherosclerosis is a pathologic, inflammatory process in the vascular wall that is triggered by risk factors such as diabetes, dyslipidemia, and smoking, but also may accelerate due to immune dysregulation from chronic infections such as human immunodeficiency virus (HIV) [125, 126, 127, 128, 129, 130]. Sex hormones play an important role in adaptive and innate immune responses and systemic inflammation in women. Moran et al. [130] published a review of mechanisms involving immune dysregulation which contribute to inflammation and increase the risk of CVD in women.
C-Reactive Protein (CRP) is a marker of inflammation and has been shown to be a
risk factor for cardiovascular events. It is incorporated in the Reynolds Risk
Score for improved risk prediction in women [131]. Inflammatory cytokines are
thought to stimulate the liver to produce CRP, which is an acute phase reactant
[132]. Elevated high sensitivity CRP (hsCRP) levels are predictive of CVD events
[133, 134]. Statins, a mainstay of CVD management and prevention, have been shown
to reduce hsCRP levels [135, 136]. Thus the benefit of statins in reducing
cardiovascular risk is thought to be in part due to their anti-inflammatory
properties. Modulating the inflammatory response to reduce CVD risk has been
studied in several clinical trials using agents such as canakinumab and
colchicine [137, 138, 139]. The Canakinumab Anti-Inflammatory Thrombosis Outcomes Study
(CANTOS) demonstrated that in patients with a history of an MI and high
sensitively CRP
Rheumatologic and autoimmune disorders, such as systemic lupus erythematosus (SLE), rheumatoid arthritis, psoriasis, and systemic sclerosis are more prevalent in women, and are associated with increased CVD, including CAD, valvular heart disease, arrhythmias, and pericardial disease [6, 9]. Ischemic heart disease is a leading cause of morbidity and mortality in SLE patients, and AA women are much more likely to be diagnosed with SLE compared to white women. Similarly, rheumatoid arthritis is associated with a two-to-threefold higher risk of ischemic heart disease [142]. This increased risk is attributed to not only an augmented systemic inflammatory response leading to endothelial dysfunction and rupture of vulnerable plaques, but also to microvascular dysfunction [143, 144]. Treatment for autoimmune disorders with corticosteroids may also increase CVD risk, due to weight gain, the development of the metabolic syndrome, and premature atherosclerosis [145]. Coronary artery calcium scores may be more predictive of CVD risk than Framingham risk scores in women with SLE and rheumatoid arthritis [146].
In addition to the above risk factors, several studies have shown that psychological risk factors such as anxiety, work and marital stress, depression, low socioeconomic status and loneliness, are associated with CVD [147, 148, 149, 150, 151, 152, 153, 154]. In the Stockholm Female Coronary Risk (FemCorRisk) study of community women with CAD, marital stress predicted a poor prognosis with three times the increased risk of coronary events after controlling for other risk factors [155]. In 300 young and middle-aged patients (ages 18 to 60 years) with a history of a recent MI, chronic stress burden with self-reported early-life trauma was also found to be an independent risk factor for adverse CVD outcomes in both men and women (66% AA and 50% women) [156]. In another large, prospective study of 23,196 men and women (ages 20 to 54 years), childhood adversities were more powerful predictors of CVD in women compared to men [157].
While conditions such as post-traumatic stress disorder are being increasingly recognized as risk factors for CVD [158], the link between depression and its impact on heart disease is already well established [159]. Depression is associated with autonomic nervous system dysregulation as well as inflammation [152, 160]. Patients who are depressed are also less likely to make heart-healthy choices, have uncontrolled cardiac risk factors, lower medication compliance, and medical follow-up. In the Women’s Health Initiative (WHI), depression was an independent predictor of cardiovascular death [161]; similarly, in the Nurses’ Health Study, depression was associated with an increased incidence of adverse cardiac events [162].
Several studies have looked at how neurobiological mechanisms and emotions may influence stress physiology and lead to heart disease [154, 163]. In patients with stable CAD, even transient endothelial dysfunction, measured by flow-mediated dilation of the brachial artery during mental stress tasks, was a prognostic marker associated with adverse outcomes (composite endpoint of CV death, MI, revascularization, and health failure hospitalization) after adjusting for medical history and sociodemographic risk factors [164]. Women, in particular, seem to be more at risk for adverse consequences of mental stress. For example, young women with a history of an MI have more ischemia with mental stress compared to young men with an MI [165, 166]. Among 918 patients with stable CAD, those with mental stress ischemia (16% of the cohort) had increased cardiovascular death or non-fatal MI compared to those with no mental-stress ischemia at a median of 5 years of follow-up. Furthermore, compared to young men, young women with CAD had a greater inflammatory response with interleukin-6 to mental stress [167]. Sex-differences in vascular reactivity to mental stress have also been documented, with women having more microvascular vasoconstriction in response to laboratory-induced mental stress. Similarly, post-menopausal women are more susceptible to takotsubo syndrome after a significant emotional or physical stressor [168].
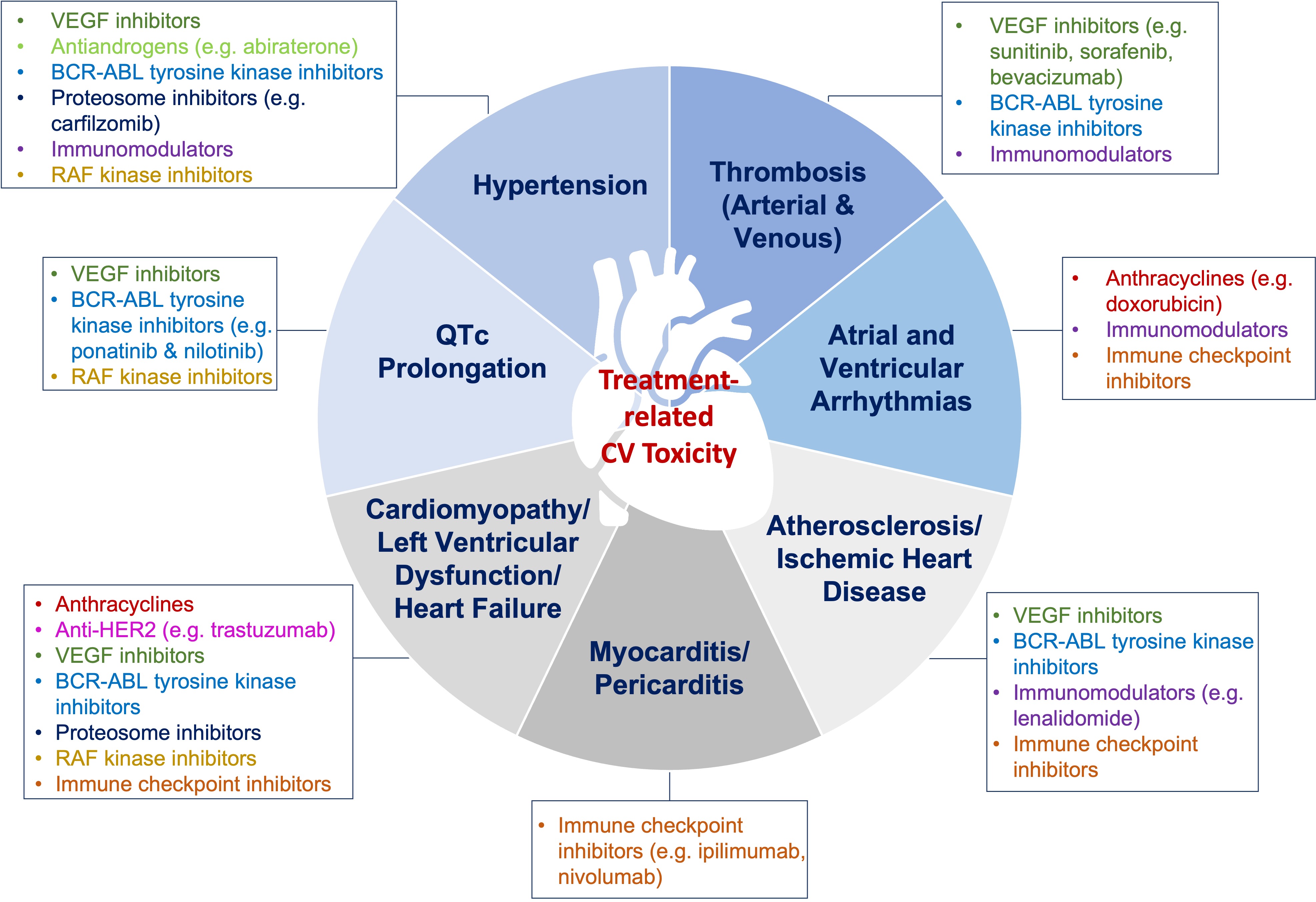
While anti-neoplastic therapy is necessary to combat cancer, some types are associated with cardio-toxic effects and increase CVD risk. Over the past decade the field of cardio-oncology has emerged to address consequences of cancer therapy not only in the short-term, but also long-term in cancer survivors. A consensus statement defining cardiovascular toxicities from cancer was recently published by the International Cardio-Oncology Society (IC-OS) [169]. Anthracycline-based therapy has been known to be associated with cardiomyopathy, and newer agents such as tyrosine kinase inhibitors that target vascular endothelial growth factor (VEGF) (e.g., bevacizumab, sunitinib, sorafenib); are associated with HTN and left ventricular dysfunction (Fig. 2) [169, 170]. Baseline cardiovascular risk assessment in patients who are undergoing chemotherapy can be helpful; since as the number of CVD risk factors increase, the risk of cardiotoxicity also increases [171]. A comprehensive position statement on risk assessment tools to address baseline risk in patients who are starting chemotherapy has been recently published by the European Society of Cardiology and ICOS [171]. While a comprehensive review of cardiotoxic effects related to specific cancers and chemotherapy is beyond the scope of this manuscript, we have focused on breast cancer since it is the most common cancer in women.
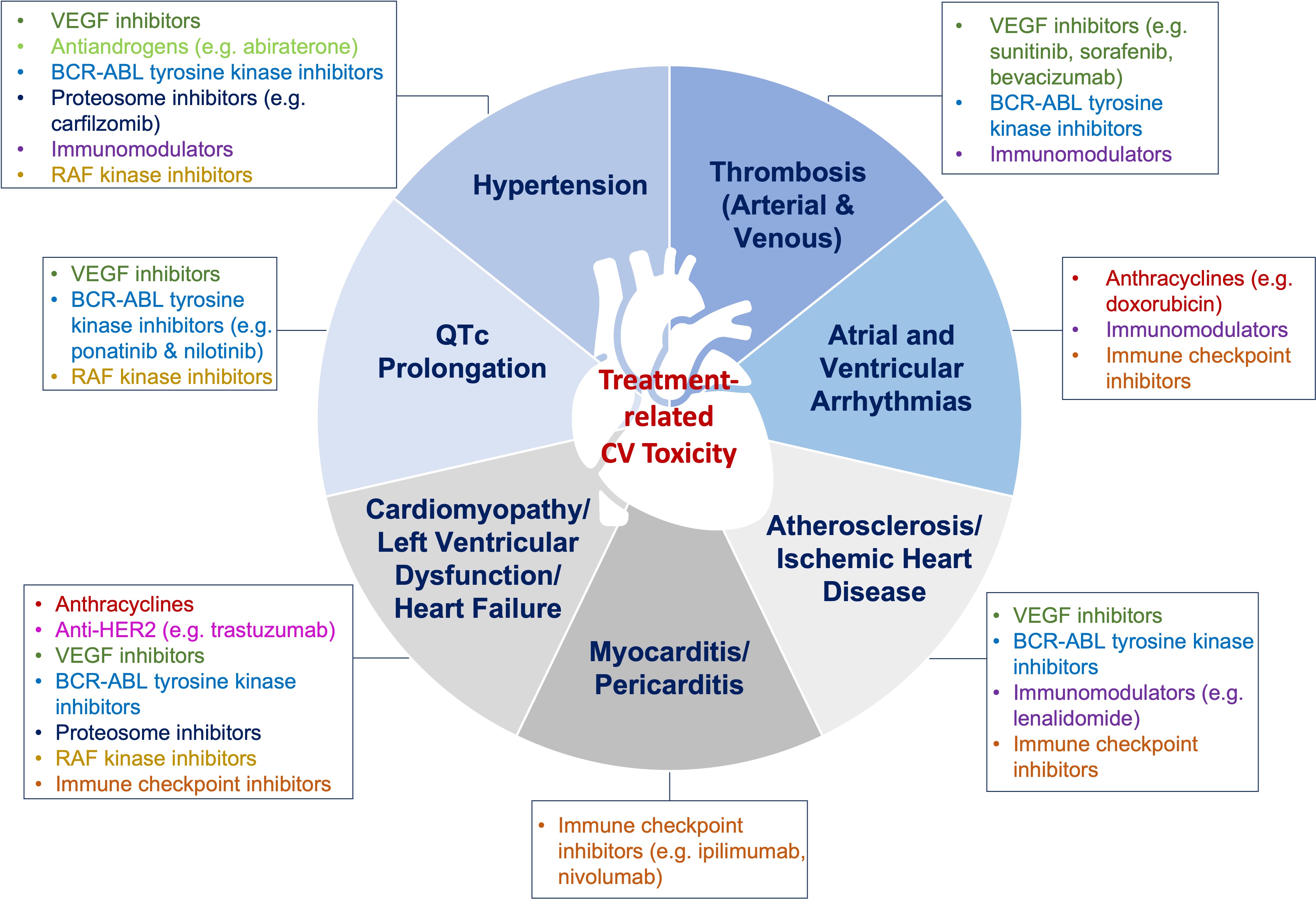 Fig. 2.
Fig. 2.Cardiotoxic effects of chemotherapy. Chemotherapeutic agents are associated with various cardiotoxic effects. Timely cardiovascular assessment prior to chemotherapy initiation, and close monitoring, as well as surveillance post-therapy, are recommended. VEGF, Vascular endothelial growth factor.
While advances in early detection and targeted treatment have led to declining
death rates in breast cancer, some therapies have been associated with
significant adverse cardiac events [172, 173, 174, 175, 176]. There are an estimated 3.8 million
breast cancer survivors in the U.S. [177]. Adverse events, including myocardial
ischemia, heart failure, venous thromboembolism, and bradycardia may result from
breast cancer treatment, among which anthracyclines carry the highest risk of
cardiotoxicity [178]. Following long-term therapy, a large percentage of patients
exposed to anthracycline-based therapy develop abnormalities in cardiac structure
and function, placing them at five-times the risk of heart failure compared to
patients treated with non-anthracycline-containing chemotherapy [173]. Women who
have a higher baseline burden of CVD risk factors are particularly vulnerable to
adverse consequences following cancer chemotherapy [179]. The cardiotoxic effects
of doxorubicin are dose-dependent, with heart failure noted in 26% of patients
given the maximum lifetime cumulative dose of 550 mg/m
Chest wall or mediastinal radiation for treatment of malignancies such as Hodgkin’s lymphoma and breast cancer is associated with coronary atherosclerosis, as well as pericardial and valvular disease. The risk of radiation-induced heart disease increases in the presence of risk factors. An elevated risk for CVD starts within the 5 years of radiation exposure, and the rate of events increases by 7.4% per gray of radiation [189]. Left-breast radiation doses to the heart are higher than right-sided breast radiation and is associated with atherosclerosis in both the left and right coronary arteries [190]. It is important to continue to screen for CVD risk factors and atherosclerosis to guide the management in patients exposed to cardio-toxic chemotherapy or radiation therapy.
Our understanding of CVD continues to expand with discoveries of new and
potentially targetable risk factors. The gut microbiome is an emerging risk
factor implicated in CVD. There may be sex-specific differences in intestinal
dysbiosis that if corrected, could improve CVD risk, especially in women
[191, 192, 193]. Gut microbiome and intestinal barrier dysfunction has been linked to
HTN [193]. In a pilot study, Zonulin, a gut epithelial tight junction protein
regulator, was found to be elevated in those patients with HTN compared to
controls, and significantly correlated with elevated systolic blood pressure
(R
Another novel biomarker under investigation for early detection of arterial
stiffness and HTN is marinobufagenin (MBG) [197]. This is a
Na
There is a substantial lack of awareness, especially among young and racial/ethnic minority women, that CVD is a major health threat in women, and targeted educational efforts are needed [201]. Multi-specialty and team-based care to improve CVD risk factor control is needed to make progress in the battle against CVD in women [202]. Moreover, an improved understanding of how to practically incorporate socio-cultural factors and social determinants to guide care in women is essential [1, 203]. Several questions regarding optimal risk factor assessment and deployment of pharmacotherapy remain: Does early treatment of cardiac risk factors in women with adverse pregnancy outcomes lead to a significant reduction in mortality? Should preventive strategies such as statins and aspirin be recommended to all patients with underlying chronic autoimmune inflammatory disorders? Should young women with autoimmune inflammatory disorders or adverse pregnancy outcomes undergo coronary artery calcium scoring to help guide primary prevention, and if so, starting at what age? Does early initiation of menopause hormone therapy, especially transdermal formulations, during the transition to menopause in younger women under the age of 60, attenuate the cardiometabolic and hypertensive risk associated with declining estrogen levels?
Adverse drug reactions (ADRs) can range from mild, simple rash, to severe and life-threatening conditions such as angioedema. There has been a historic lack of comparisons of the rate of adverse reactions between men and women in clinical trials [204]. There are sex-specific differences in the incidence of drug reactions among common cardiovascular medications, with women experiencing adverse events more often than men [205]. For example, an increased incidence of ADRs has been observed with ACE-I (enalapril) (odds ratio 1.30) and particularly a higher risk of cough in women compared to men (odds ratio: 2.38) [206]. In a meta-analysis comparing the effects of statins on cardiovascular outcomes, no differences in adverse drug reactions were found in women compared to men; however, data for myopathy and new-onset diabetes were limited [207].
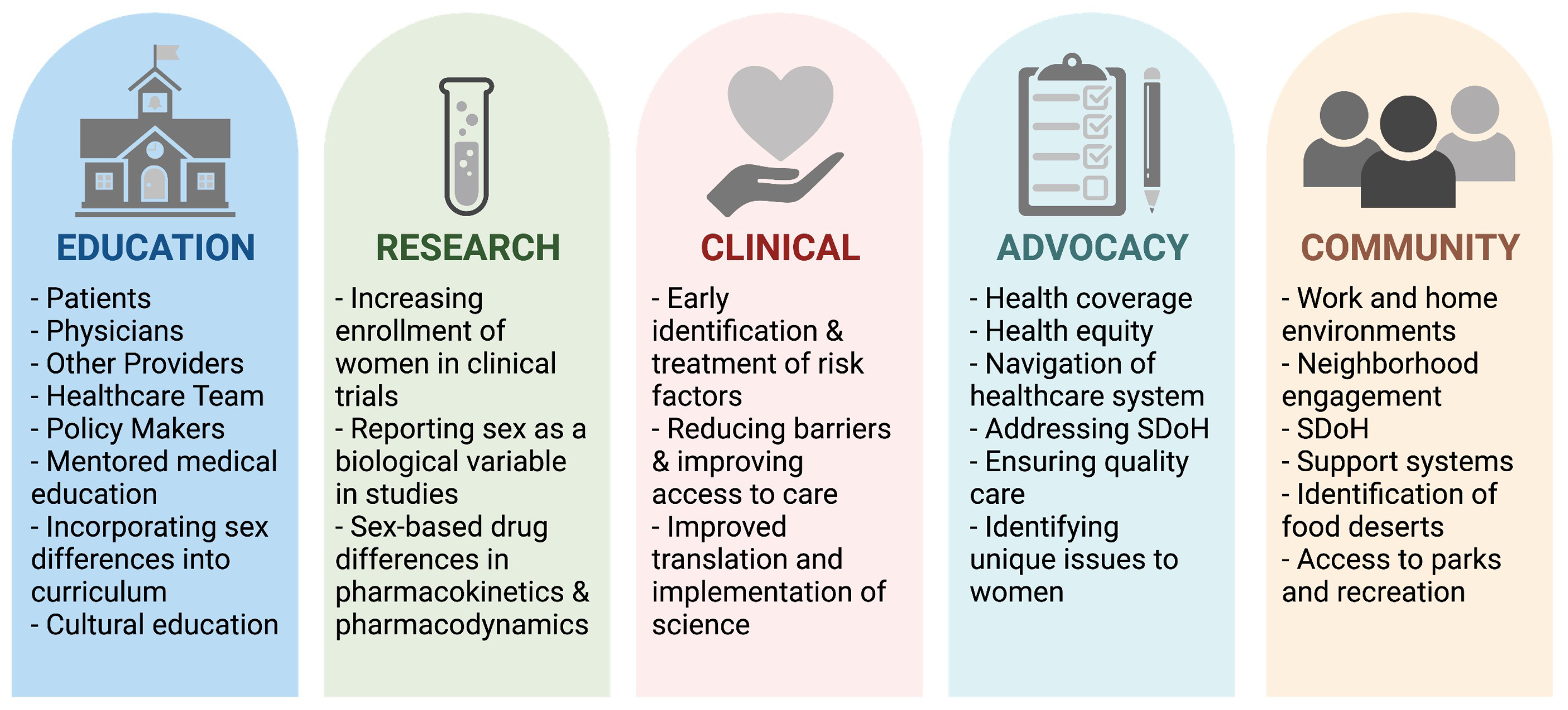
What are the main barriers that prevent optimal CVD risk assessment care in young women and what system level changes can be optimized to reduce the sex-based disparities in CVD care? Questions related to pathobiological differences in CVD in women vs. men should also continue to be investigated [208]. Improved education, screening, identification, and treatment (pharmacologic and lifestyle-based approaches) of CVD risk factors in women require engagement across all levels and a systems-based approach (Fig. 3) [209].
 Fig. 3.
Fig. 3.Pillars for integrated cardiovascular risk factor care in women. Optimizing and improving CVD care for women requires efforts at all levels. SDoH, Social Determinants of Health. Figure created using https://Biorender.com.
In addition to traditional CVD risk factors, unique sex-specific risk factors contribute to increased CVD morbidity and mortality in women. Complications of pregnancy, autoimmune/inflammatory conditions, premature menopause, and stress/depression are some of the nontraditional risk factors that may help identify women at increased CVD risk. Women have a high burden of comorbid conditions that impact their health-related quality of life. Early identification and treatment of modifiable CVD risk factors may alleviate CVD risk in women. A multi-disciplinary approach to not only provide comprehensive care, but also targeted public health education regarding CVD risk, will be beneficial to all women.
Writing (original draft preparation, review, and editing)—PKM, SG, AS, JEM; Supervision—JEM. All authors have read and agreed to the published version of the manuscript.
Not applicable.
We thank Esha Dave, MS for her assistance with figures and tables.
This work was supported in part by a developmental grant on Specialized Center of Research Excellence in Sex Differences (SCORE) from the NIH (1U54AG062334-01), 1R01HL157311, Mrs. Marcia Taylor, and Mr. Jim Hills.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.