Bleeding Complications in Patients Undergoing Percutaneous Coronary Intervention
1 Department of Cardiovascular and Pulmonary Sciences, Catholic University of the Sacred Heart, 00168 Rome, Italy
2 Department of Cardiology, Maria Cecilia Hospital, GVM Care & Research, 48033 Cotignola, Italy
3 Department of Cardiovascular Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS , 00168 Rome, Italy
Academic Editor: Julio Núñez Villota
Abstract
Percutaneous coronary intervention (PCI) is considered a relatively safe procedure associated with low rates of complications, but is inevitably associated with short and mid-to-long term increased bleeding risk. Besides the short term risk associated with the arterial access to perform PCI, enhanced bleeding risk persists for several months, given the need for antithrombotic therapy to prevent procedure-related thrombotic complications as well as ischemic recurrences. Bleeding is a powerful harbinger of adverse outcomes. This awareness has fuelled intense research on bleeding reduction strategies, including new PCI devices and techniques as well as new medications and antithrombotic regimens. We here review the mechanisms and prevalence of bleeding in PCI patients, discuss the available evidence from a practical point of view, and explore future perspectives on how to treat and prevent bleeding complications in these patients.
Keywords
- percutaneous coronary interventions
- bleeding
- complications
- antithrombotic therapy
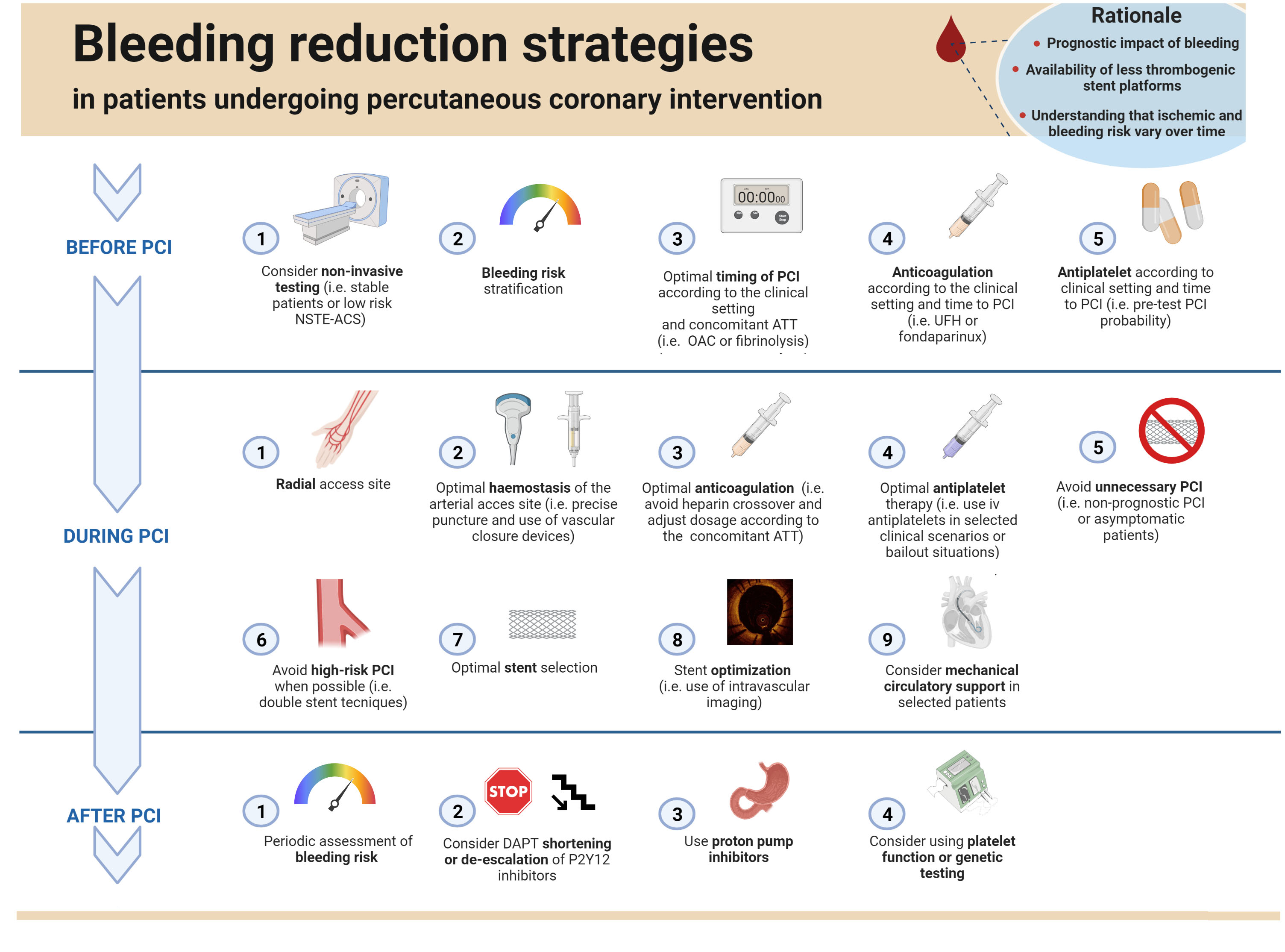 Graphical abstract.
Graphical abstract.Bleeding reduction strategies in patients undergoing percutaneous coronary intervention. Abbreviations. NSTE-ACS, Non-ST Elevation- Acute Coronary Syndrome; PCI, percutaneous coronary intervention; OAC, oral anticoagulation; ATT, anti-thrombotic therapy; UFH, unfractioned heparin; CTO, chronic total occlusion; DAPT, double antiplatelet therapy.
Since the first coronary angioplasty performed by Andreas Grüntzig in 1977,
there has been significant progress in the field of percutaneous coronary
intervention (PCI), which currently represents a cornerstone in the management of
ischemic heart disease (IHD) [1]. PCI, however, requires an arterial vascular
access and adjunctive antithrombotic therapy, such as intraprocedural parenteral
anticoagulation, as well as mid-to-long-term dual antiplatelet therapy (DAPT),
consisting in the association of aspirin plus a P2Y
The first challenge when dealing with bleeding among PCI patients stems from the fact that bleeding is a complex clinical phenomenon, which is hard to enclose under an univocal classification, given its broad range of severities, sites, and hemodynamic consequences. Several bleeding definitions have been proposed over time, generating some confusion and hindering comparisons of incidence and prognostic relevance of bleeds across different studies [7, 8] (Fig. 1).
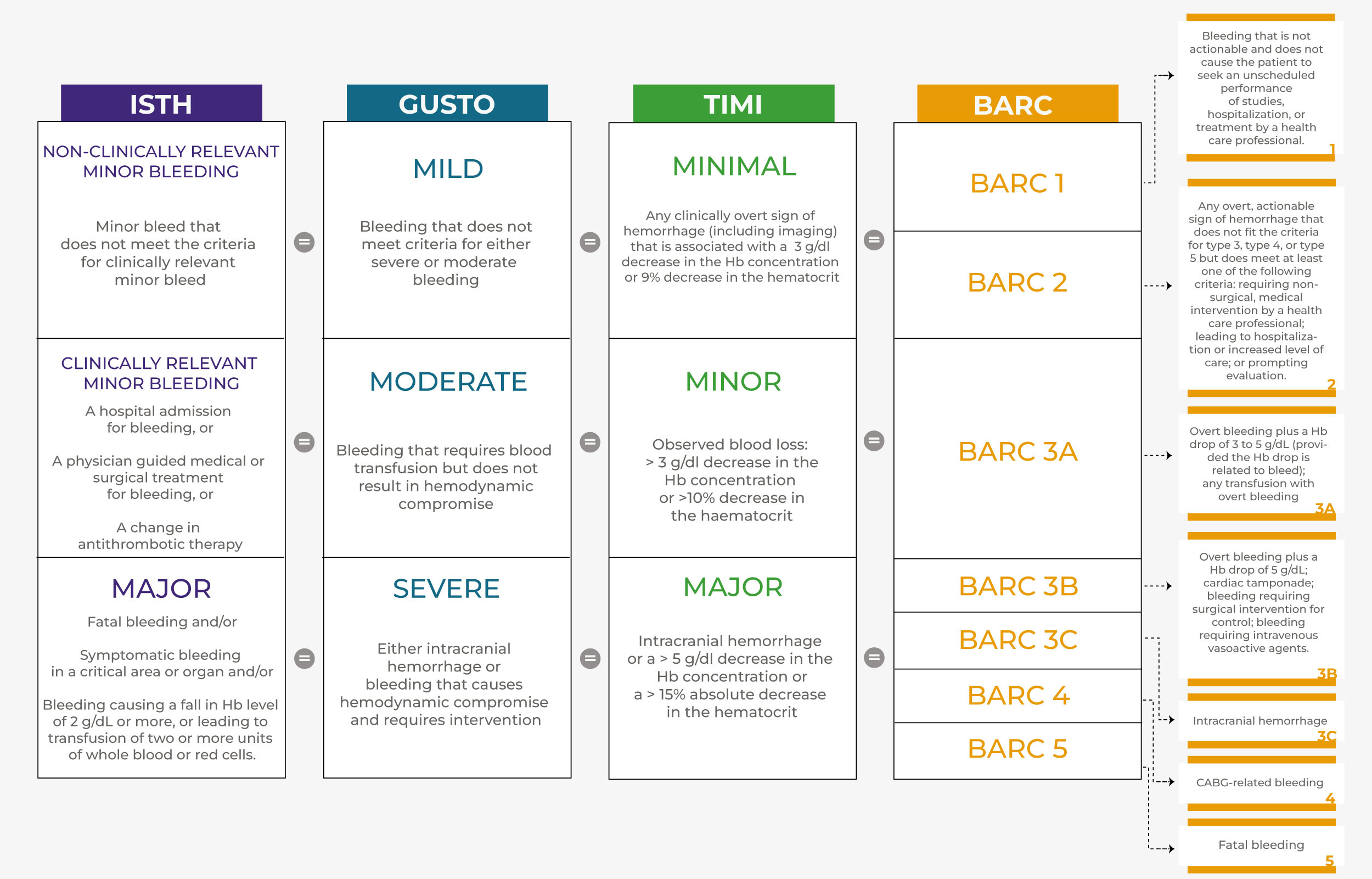 Fig. 1.
Fig. 1.Comparison of the most used bleeding classifications. ISTH classifies bleeding according to clinical relevance. TIMI, GUSTO and BARC represent the most used bleeding classifications. ISTH, TIMI and GUSTO are divided into three levels of severity, while BARC is divided into five, numerically graded, levels. Prognostically, a bleed classified as “mild” by GUSTO corresponds to a “minimal” bleed by TIMI and to a “1” or “2” bleed by BARC. A bleed classified as “moderate” according to GUSTO, corresponds to a “minor” bleed by TIMI and to a “3a” bleed according to BARC. Finally, a bleed classified as “severe” according to GUSTO corresponds to a “major” bleed according to TIMI and to a “3b”, “3c”, “4” or “5” bleed according to BARC. Abbreviations. ISTH, International Society on Thrombosis and Haemostasis; BARC, Bleeding Academic Research Consortium; GUSTO, Global Use of Strategies to Open Occluded Coronary Arteries; TIMI, The Thrombolysis in Myocardial Infarction.
The first widely used definitions were the GUSTO (Global Use of Strategies to Open Occluded Arteries) and the TIMI (Thrombolysis in Myocardial Infarction), developed in patients with ST-elevation myocardial infarction (STEMI) receiving thrombolytic therapy [9, 10]. These definitions were designed to classify relatively severe bleeds, and are suboptimal in capturing less severe events typical of the post-fibrinolytic era [8]. The TIMI classification stratifies events according to coronary artery bypass graft (CABG) or not. It is mainly based on laboratory parameters (fall in hematocrit or hemoglobin), with the limitation of not specifying their timing, which may lead to changeable peaks and nadirs [8, 9].
Converseley, the GUSTO classification is mainly driven by life-threatening (major) bleeds, such as intracranial or hemodynamically unstable, and those requiring transfusion in the absence of hemodynamic instability (moderate bleeds) [8, 10]. Because transfusion criteria may vary according to local clinical practice, adjudication may not be consistent across geographic regions [8].
In 2005, the International Society on Thrombosis and Haemostasis (ISTH) defined bleeding as major, stratified by surgery or not, or minor, stratified by clinical relevance or not [11]. This definition groups heterogeneous events of variable severity, presentation and course, and has been less implemented in recent years [12].
In the attempt to overcome the limitations of previous classifications, several trials have created their own definitions, combining elements from both TIMI and GUSTO definitions and adding new parameters [8]. Notable examples include the CURE, ACUITY, OASIS, STEEPLE and PLATO definitions [8]. The PLATO definition, for example, classifies bleeds as major (life-threatening or not), minor or minimal [8]. PLATO major bleeds include a broader range of events compared to TIMI or GUSTO criteria. Thus, bleeding with a drop in hemoglobin of 3 to 5 g/dL would be defined as major by PLATO and minor by TIMI criteria, while a clinically stable event requiring transfusion would be considered PLATO major and GUSTO moderate. Trial specific definitions present the inherent limitation of hindering the comparison of incidence and of the prognostic impact of bleeding across studies.
More recently, the Bleeding Academic Research Consortium (BARC), uniting academia, professional societies and federal agencies, has focused on clinical (such as health care intervention or harmfulness of bleeding site) and laboratory criteria (such as hematocrit and hemoglobin) to classify bleeds, using ordinal numbers rather than qualitative terms, and showing increasing bleeding severity and mortality with increasing BARC grades [8].
Because the BARC classification captures a larger proportion of clinically significant bleeding than the GUSTO or TIMI scales and provides more precise sub-classifications, it has been extensively adopted over the years, becoming the most used and reliable bleeding definition.
Bleeding is one of the most common complications in patients treated with DAPT or with the combination of antiplatelet agents and oral anticoagulation (OAC), typical of patients with atrial fibrillation (AF) undergoing PCI [5]. The risk of bleeding is proportional to the intensity and duration of antithrombotic treatment [4, 13] (Fig. 2, Ref [14]).
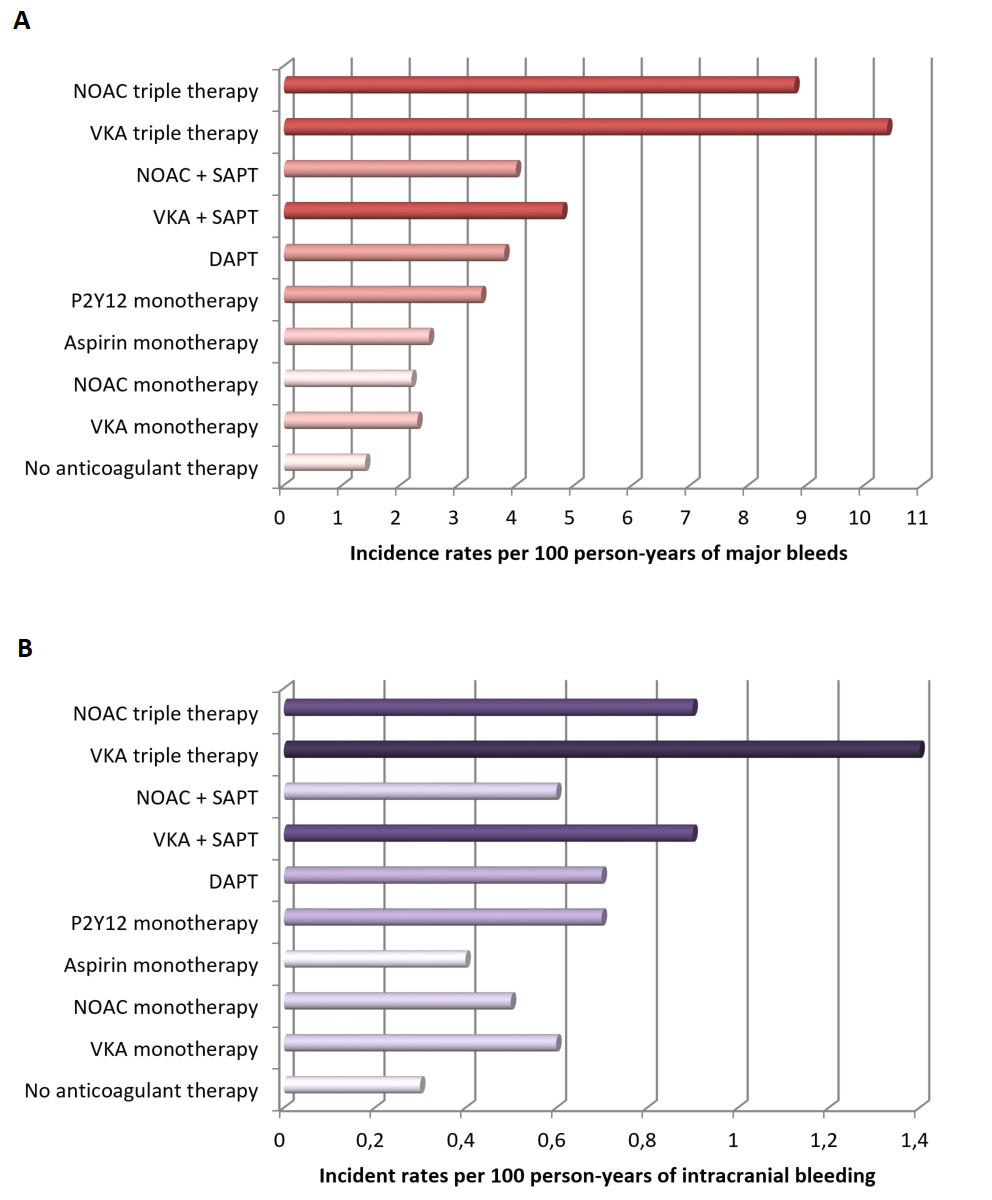 Fig. 2.
Fig. 2.Bleeding incidence associated with single, dual, and triple therapy (modified from Nienke van Rein et al. [14]). (A) Incidence rates of major bleeding. (B) Incidence rates of intracranial hemorrhage. Abbreviations. DAPT, dual antiplatelet therapy; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist; SAPT, single antiplatelet therapy.
In fact, patients with AF on triple antithrombotic therapy (TAT) experience
higher rates of major bleeding compared to patients on dual antithrombotic
therapy (DAT, i.e., single antiplatelet therapy plus OAC) or DAPT [14]. In a
national Danish cohort of 272,315 patients with AF aged 50 years or older, during
a one year follow-up, major bleeding was lowest in patients not treated with any
antithrombotic agent and increased with the number of anticoagulants or
antiplatelet drugs, with incidence rates between 1.3 and 10.4 per 100
patient-years (Fig. 2) [14]. Importantly, the type of P2Y
As expected, bleeding rates are significantly increased in patients defined as being at high-bleeding risk (HBR). In fact, these patients have a 38% higher risk of major or clinically relevant non-major bleeding and a 71% higher risk of major bleeding compared to their non-HBR counterparts [19]. Therefore, stratification aimed at identifying HBR patients is crucial as discussed below [5].
Bleeding complications can occur in-hospital or post-discharge. In-hospital, the
Cath-PCI registry of
Out-of-hospital, data from 8582 patients enrolled in the ADAPT-DES study showed a strong association of post-discharge bleeding vs. none with all-cause mortality (13.0% vs. 3.0%) [22]. Importantly, as compared to post-discharge MI, post-discharge bleeding had an even greater effect on subsequent mortality (hazard ratio HR, 1.92; 95% confidence interval, CI 1.18 to 3.12; p = 0.009) [22].
The association between MI, post-discharge bleeding and all-cause mortality was
also elegantly assessed by a post-hoc analysis of the TRACER trial that included
12,944 patients with non-ST Elevation MI (NSTEMI) [4]. In this study, MI was
associated with a greater risk of mortality compared with BARC 2 (relative risk,
RR, 3.5; 95% CI 2.08–4.77; p
Multiple mechanisms may be responsible for the adverse outcomes associated with bleeding, apart from the direct consequences of massive bleeding on hemodynamic status or tissue injury in case of intracranial bleeding (Fig. 3).
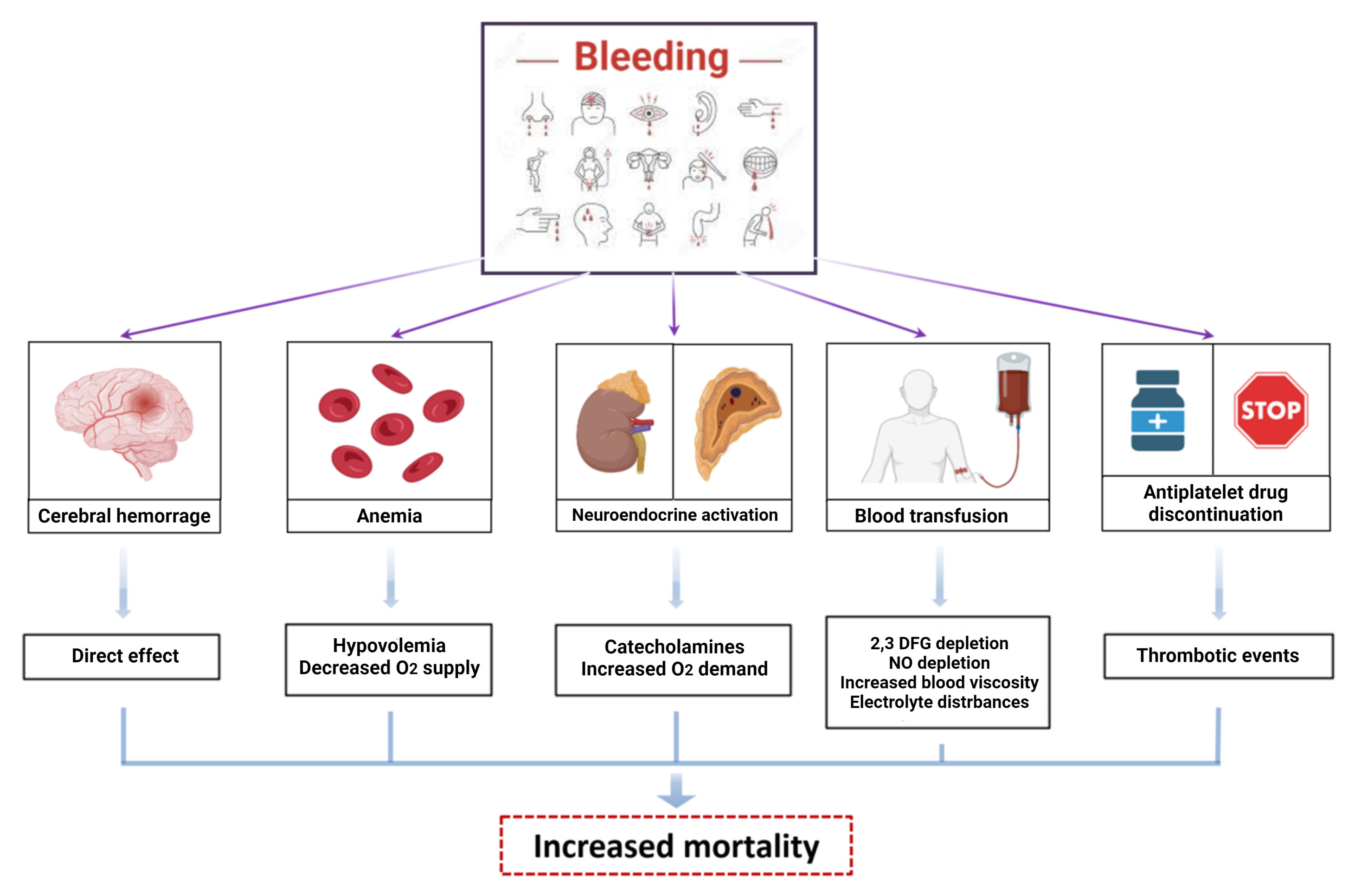 Fig. 3.
Fig. 3.Mechanisms of damage of bleeding. Bleeding increases mortality
and morbidity in patients treated with antithrombotic therapies after PCI.
Multiple mechanisms can explain the adverse outcomes related to bleeding: direct
cerebral injury in case of intracranial hemorrhage, anemia and hypovolemia in
case of huge losses leading to decreased O
Bleeding, including minor, is associated with a higher rate of ischemic events, mainly related to the unplanned interruption of antiplatelet treatment in response to bleeding [24]. Furthermore, activation of coagulation and inflammatory pathways are a physiological counter-response to blood loss, increasing the risk of thrombotic events and atherosclerotic plaque destabilization [25]. Moreover, blood transfusion used to correct severe and acute anemia is associated to a three-fold increase of 30-day mortality, caused by depletion of 2–3 diphosphoglycerate (DPG) and nitric oxide, which reduce tissue oxygen delivery and lead to vascular constriction and platelet aggregation, respectively [26].
In conclusion, bleeding complications after PCI are associated with a rise in the rates of short- and long-term death, non-fatal spontaneous MI, stroke, blood transfusions, longer hospital stay and re-hospitalisation.
Identifying clinical and procedural features associated with HBR is essential to define patients with HBR, allowing prompt application of targeted bleeding avoidance strategies and standardized bleeding risk across trials [5]. Several scores have been developed to predict major bleeding. They can be divided according to the setting (in-hospital vs. out-of-hospital), validation cohort, type of events (bleeding events only vs. ischemic and bleeding events) and the approach (semi-quantitative vs. quantitative) (Table 1).
| PRECISE DAPT score | DAPT score | CRUSADE score | ARC-HBR criteria | |
| Validation | Pooled analysis of 8 randomized trials (n = 14,936) | DAPT randomized trial (n = 11,648) | Registry of high-risk patients with NSTEMI (n = 71,277) | Consensus of experts |
| (subsequent validations) | ||||
| Bleeding outcome | Out-of-hospital bleeding | Major bleeding between 12 and 30 months after PCI | In-hospital major bleeding | Out-of-hospital major bleeding |
| Bleeding definition used | TIMI major and minor | GUSTO moderate and severe | Crusade major bleeding | BARC major bleeding |
| Score threshold | Score |
Score –2 to 0 | Score |
1 major criterion or 2 minor criteria |
| Ischemic risk evaluation | No | Yes | No | No |
| Score range | 0 to 100 | –2 to 10 | 0 to 100 | Qualitative |
| Clinical setting | Stable and unstable patients undergoing PCI | Stable and event-free patients 12 months after PCI | NSTEMI | Stable and unstable patients undergoing PCI |
| Several risk stratification scores have been developed to predict major bleeding
events in the past years. Among these, guidelines recognize the PRECISE-DAPT,
DAPT and CRUSADE scores and the ACR-HBR criteria. Scores can be divided according
to in-hospital vs. out-of-hospital setting, validation cohort, type of predicted
events (bleeding only vs. both ischemic and bleeding events), bleeding definition
used, approach (semi-quantitative vs. quantitative) and the clinical setting (i.e.,
CCS and/or ACS). Abbreviations. PCI, percutaneous coronary intervention; DAPT, dual antiplatelet therapy; NSTEMI, non-ST elevation myocardial infarction; CCS, chronic coronary syndrome; ACS, acute coronary syndrome. | ||||
For in-hospital bleeding, the “Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/American Heart Association (AHA) guidelines” (CRUSADE) score is considered the most discriminatory among NSTEMI patients [27, 28]. It takes into account hemodynamic parameters at presentation (heart rate, systolic blood pressure, heart failure), laboratory findings (hematocrit, creatinine clearance) and clinical features (sex, history of diabetes mellitus or vascular disease). The sum of the weighted integers ranges from 1 to 100 points with a threshold of 50 points, above which the risk of in-hospital major bleeding is considered high [27].
The “PREdicting bleeding Complications In patients undergoing Stent
implantation and subsEquent Dual Anti Platelet Therapy” (PRECISE-DAPT) score was
introduced to predict the risk of out-of-hospital major bleeding at 1 year [29].
It is applicable at discharge to CCS or ACS treated with PCI and treated with
DAPT and no OAC and includes both clinical and laboratory features [30]. HBR
patients are defined as having a PRECISE-DAPT score
In 2019, The Academic Research Consortium for High Bleeding Risk (ARC-HBR)
proposed a definition encompassing patients having a BARC type 3 or 5 bleeding
risk
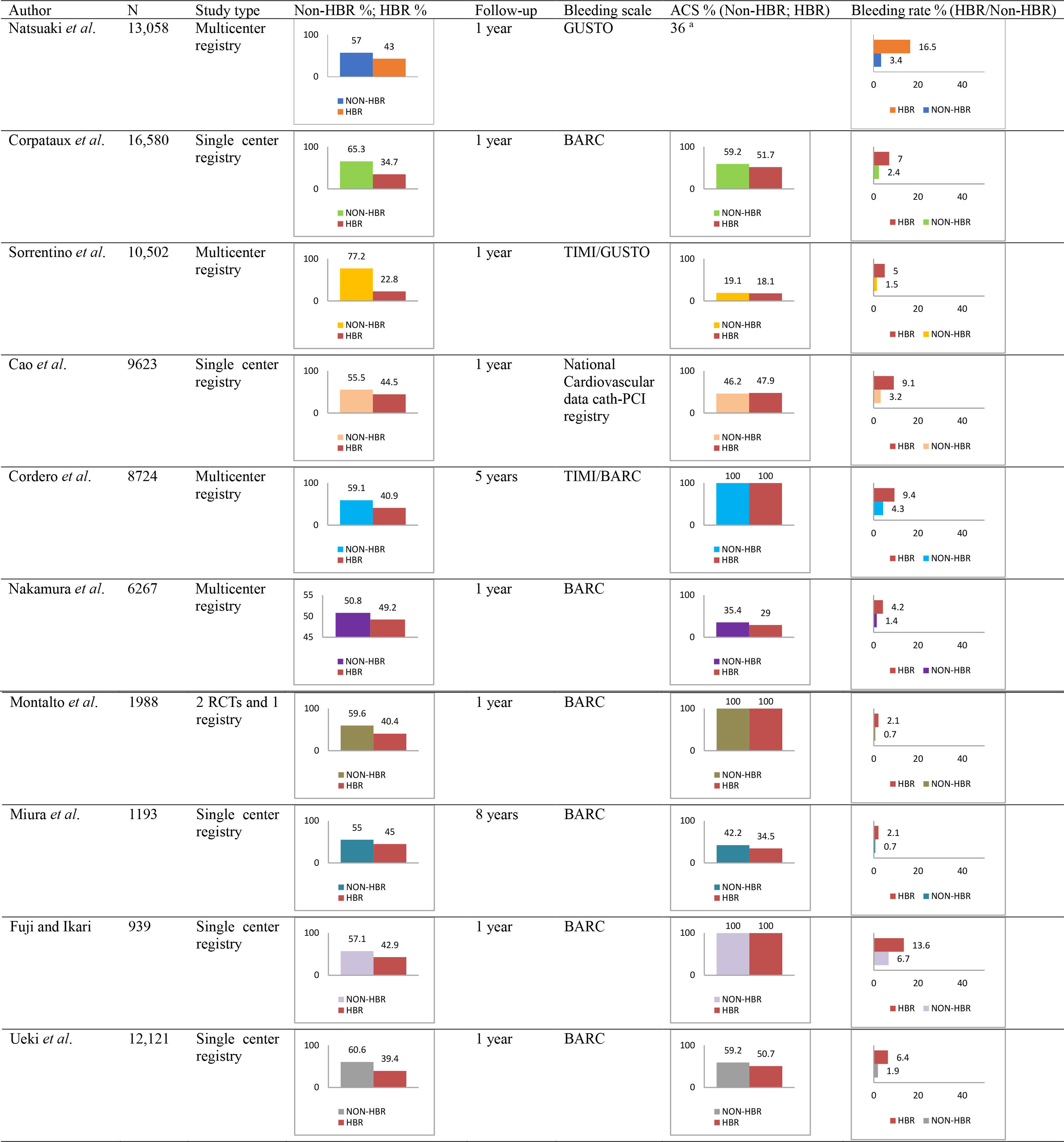
|
| List of all the studies reporting the occurrence of major
bleeding according to ARC-HBR status in patients undergoing PCI. Abbreviations: ACS, Acute coronary syndrome; BARC, Bleeding Academic Research Consortium; GUSTO, Global Use of Strategies to Open Occluded Arteries; ARC, Academic Research Consortium; HBR, High Bleeding Risk; RCT, Randomized Clinical Trial; TIMI, Thrombolysis in Myocardial Infarction. |
Among risk scores predicting out-of-hospital thrombotic and bleeding events, the
DAPT score aims at identifying patients that would benefit from a DAPT prolongation beyond 1 year after PCI [32]. A high-risk score (
Besides scores, a number of demographic (i.e., Asian ethnicity or elderly), clinical (i.e., cardiogenic shock or cardiac arrest, previous bleeding, anemia, reduced platelet count, prior stroke, malignancy, severe liver disease, fragility) and procedural (i.e., non-radial access, periprocedural antithrombotic therapy, use of mechanical support) features are associated with increased bleeding risk and should be considered for a correct stratification of individual patient’ bleeding risk (Graphical abstract) [37, 38, 39].
5.2.1.1 Periprocedural Anticoagulant Therapy
Periprocedural anticoagulation in patients undergoing PCI includes low molecular
weight heparin (LMWH), fondaparinux, unfractionated heparin (UFH) or bivalirudin.
The latter is recommended only for specific clinical scenarios because of high
costs and higher rates of stent thrombosis compared with heparin [27, 32, 33, 40].
With regards to intraprocedural anticoagulation, UFH represents the standard of
care regardless of the clinical setting, despite no trial has ever shown its
efficacy versus placebo. The only exception to this general rule is the use of
bivalirudin in patients with heparin-induced thrombocytopenia [33].
Anticoagulation before and after PCI is typical of the ACS setting and the
regimens vary between STEMI and NSTEMI. Before PCI, the anticoagulant associated
with the best performance in terms of safety and efficacy is fondaparinux in
NSTEMI, while in STEMI it is debated whether LMWH or UFH performs better [40].
Given that UFH is the anticoagulant of choice during PCI and that crossovers
between heparins is discouraged, the anticoagulant should be selected also
according to practical considerations related with the timing of PCI. In fact,
there is no reason for administering an anticoagulant different from UFH in a
patient scheduled to undergo PCI in few hours, regardless of clinical
presentation [40]. The benefit of UFH pre-treatment in the STEMI setting has been
shown in a sub-analysis of the TASTE trial including 7144 patients [41]. In this
study, patients pre-treated with UFH less often presented with intracoronary
thrombus (61.3% vs. 66.0%, p
With respect to the post-PCI setting, anticoagulation is generally not recommended after PCI, with the exception of ACS patients at high thrombotic burden or those not eligible for coronary revascularization, in whom anticoagulation in-hospital may be considered up to 7 days [44, 45]. In this scenario, the use of fondaparinux may represent the safest option, also in light of the fact that patients are already treated with concomitant DAPT [44].
5.2.1.2 Periprocedural Antiplatelet Therapy
Aspirin is considered the backbone of antiplatelet therapy in patients
undergoing PCI, regardless of clinical setting (acute or chronic), in association
with a P2Y
In STEMI, the recommendation towards P2Y
With regards to NSTEMI, a substantial proportion of these patients (up to 30%)
do not undergo revascularization or undergo CABG after CA, making routine
P2Y
Finally, in stable patients in whom the risk of thrombotic events is lower
compared to ACS, the rationale as well as the evidence in support of P2Y
5.2.1.3 Vascular Access Site
Among in-hospital bleeding avoidance strategies, the optimal choice of vascular access site is well known to play a key role. Three large RCTs, have strongly demonstrated a reduction of access site-related bleeding, access site-vascular complications and cardiac mortality with radial compared to femoral access site [62, 63, 64]. Therefore, radial access is now considered the standard of care [1, 27, 32, 33]. Nevertheless, in those situations in which femoral artery may be the only available access, a variety of methods have been developed to reduce access site-vascular complications. These include the optimization of femoral artery puncture by using fluoroscopy and ultrasounds or the micro-puncture technique [65]. The optimization of haemostatic process is also fundamental for reducing bleeding risk and vascular complications at puncture site. Femoral artery haemostasis can be obtained with either manual compression or vascular closure devices, with recent studies suggesting improved outcomes with the use of active closure systems [66]. Therefore, the use of vascular closure devices is highly recommended to reduce bleeding, especially for large bore access.
5.2.1.4 Stent Choice and Optimization
After stent implantation, DAPT is required to avoid local ischemic events such as ST until endothelial coverage occurs [2]. Because different rates of ST have been associated with different stent platforms, the stent choice may impact bleeding risk and DAPT durations after implantation [67, 68]. An emblematic example is the recommended DAPT duration after bare metal stent (BMS) implantation in stable patients is one month' while it was 12 months after first-generation drug eluting stent (DES) implantation given the increased risk of ST with these earlier DES [29].
The limitation of first-generation DES has led to the development of second-generation DES characterized by smaller strut thickness and reduced thrombogenicity [68]. RCTs and single-group studies using historical cohorts as controls have compared the performance of different stent platforms in the setting of short DAPT durations (1 to 6 months versus 12 months) [69, 70, 71, 72, 73, 74]. RCTs represent the highest level of evidence while the latter should be interpreted in light of their methodological limitations (non-randomized design). Among the RCTs, LEADERS FREE compared a drug-coated-stent (BioFreedom, Biolimus A9) versus BMS among 2466 patients at high risk of bleeding (57% CCS, 43% ACS) undergoing 1 month of DAPT and found the former to reduce the primary composite endpoint of cardiac death, MI, or ST (HR 0.71; 95% CI 0.56–0.91; p = 0.005) [69]. Similarly, SENIOR showed a 29% reduction of the primary composite endpoint of all-cause mortality, MI, stroke, or ischaemia-driven target lesion revascularization with an everolimus-eluting, biodegradable polymer stent (Synergy, Boston Scientific) compared with BMS in 1200 older patients (55% CCS, 45% ACS) receiving a short duration of DAPT (1 month for CCS and 6 months for ACS) [70]. Finally, Onyx ONE found that the current-generation zotarolimus-eluting, durable polymer stent (Resolute Onyx, Medtronic) was non-inferior to BioFreedom DES in 1996 patients (48% CCS, 51% ACS) at high risk of bleeding treated with 1 month-DAPT-regimen, with regards to the primary composite endpoint including death from cardiac causes, MI, or definite or probable ST at 1 year [71].
Furthermore, non-randomized studies using historical cohorts as controls of patients treated with DES have suggested the safety of the following stent platforms in the setting of short DAPT (1–3 months): Ultimaster (Terumo), Xience (Abbott Vascular) and Synergy (Boston Scientific) [72, 73, 74]. Based on the available data, regulatory agencies have approved the use of Resolute Onyx, Synergy, Xience in the United States and of these platforms plus BioFreedom and Ultimaster in Europe, for patients at high risk of bleeding requiring short DAPT duration.
Regardless of stent platform, there are technical aspects in the setting of PCI that can deeply influence not only stent-related adverse events, including ST and restenosis, but also bleeding. Indeed, the use of double stents for the treatment of bifurcation lesions, the occurrence of edge dissections after stent implantation and stent malapposition and underexpansion, are associated with increased risk of ischemic events, that can be at least partially outweighed by a more intense antiplatelet therapy, which, in turn, increases the risk of bleeding [75, 76]. To this extent, intravascular imaging (intravascular ultrasound, IVUS, and optical coherence tomography, OCT) to guide PCI can reduce the risk of cardiovascular death and major adverse events (MACE) compared with angiography guided PCI [77, 78, 79]. A pioneering study by Colombo et al. [80], published in 1995, showed that among patients in which IVUS-guided stent optimization after PCI was performed, the rate of ischemic events was very low despite the implementation of low-intensity antithrombotic regimens (ticlopidine plus aspirin for 5 days or aspirin alone, both without periprocedural anticoagulation). Therefore, refraining from PCI in the absence of prognostic or symptomatic benefits, using last generation stent platforms with best stent optimization, reduced number of implanted stents and reduced technical complexity when possible (i.e., use of provisional rather than double stenting for bifurcation lesions) may reduce the risk of thrombotic events and allow for less intense and shorter antithrombotic regimens, reducing bleeding (Graphical abstract).
5.2.1.5 Special Clinical Settings
5.2.1.5.1 Mechanical Circulatory Support
Mechanical circulatory supports, such as intra-aortic balloon pump, IMPELLA®, and venoarterial extracorporeal membrane oxygenation are increasingly adopted for PCI patients requiring urgent hemodynamic support during ACS or for elective patients undergoing high-risk PCI [81].
Despite providing important hemodynamic benefits (i.e., left ventricular unloading, increased cardiac output, reduced afterload, and increased blood pressure), there is growing evidence supporting the risk of associated complications, particularly systemic and access-related bleeding [82]. Therefore, an appropriate patient selection is needed to reduce the risk of adverse events related to the use of these devices [83].
5.2.1.5.2 PCI after Fibrinolysis
Despite primary PCI is considered the standard of care for STEMI patients,
fibrinolysis is recommended when PCI is not feasible within 120 minutes from
diagnosis [33]. PCI may be performed after fibrinolysis in three different
scenarios: (i) rescue-PCI (r-PCI), that is performed immediately after
unsuccesful fibrinolysis; (ii) facilitated PCI (f-PCI), that is performed
immediately after successful fibrinolysis (a strategy not recommended by
guidelines); and (iii) early (
Among patients undergoing fibrinolysis, the recommended antithrombotic therapy is represented by aspirin, clopidogrel and parenteral anticoagulation, given until revascularization if performed, or for the duration of hospital stay, up to 8 days [33]. The anticoagulant of choice is represented by enoxaparin, followed by UFH [31]. Fondaparinux may be considered only in STEMI patients treated with streptokinase [33]. The administration of a GPI is not recommended in this setting because it may increase bleeding without improving clinical outcomes. Ticagrelor, prasugrel and bivalirudin have not been extensively studied in STEMI patients treated with fibrinolysis, therefore they do not represent a safe option [33]. The addition of oral antiplatelet and parenteral anticoagulant drugs on top of fibrinolysis may come at the expenses of increased bleeding, which may be exponentially increased in patients undergoing PCI soon after fibrinolysis, because of the need for intraprocedural heparin on top of the antithrombotic cocktail already administered [84].
The REACT trial randomized 427 STEMI patients undergoing thrombolysis to either repeated thrombolysis, conservative management or r-PCI, the latter was associated with reduced MACE (death, recurrent MI, or severe heart failure) and cerebrovascular events, but at the cost of increased minor bleeding (28% versus 3% in the repeated thrombolysis and 0%, in the conservative therapy arm), without, however, any significant difference among groups in major bleeding events [85].
Notably, studies testing f-PCI found that PCI early after fibrinolysis is associated with higher rates of major bleeding, including hemorrhagic stroke, and higher rates of death, indicating that PCI early after fibrinolysis may significantly increase bleeding [86, 87, 88].
In conclusion, PCI early (
5.2.1.5.3 Concomitant Anticoagulation
Between 10% and 15% of patients undergoing PCI is on treatment with oral
anticoagulation, raising concerns over the optimal timing of interruption of
this therapy or additional intraprocedural anticoagulation [89]. This dilemma is
particularly true among patients requiring an urgent invasive strategy [27, 32].
Indeed, in case of elective PCI, the procedure can be delayed until the effects
of OAC have waned off, but such delay is not possible for ACS patients undergoing
urgent or emergent CA. For elective PCI, the recommendation is to wait for an INR
The availability of stent platforms with less thrombogenic profiles, together
with the increasing understanding of the prognostic relevance of bleeding events
and the fact that ischemic and bleeding risks may vary over time, with the former
being highest during the first months after PCI and the latter remaining
relatively stable over time, has fuelled the interest towards antithrombotic
strategies aiming at reducing the incidence of bleeding without any trade-off in
ischemic events among patients who have undergone PCI [2] (Fig. 4). Furthermore,
there is increasing evidence supporting differences in individual responsiveness
to P2Y
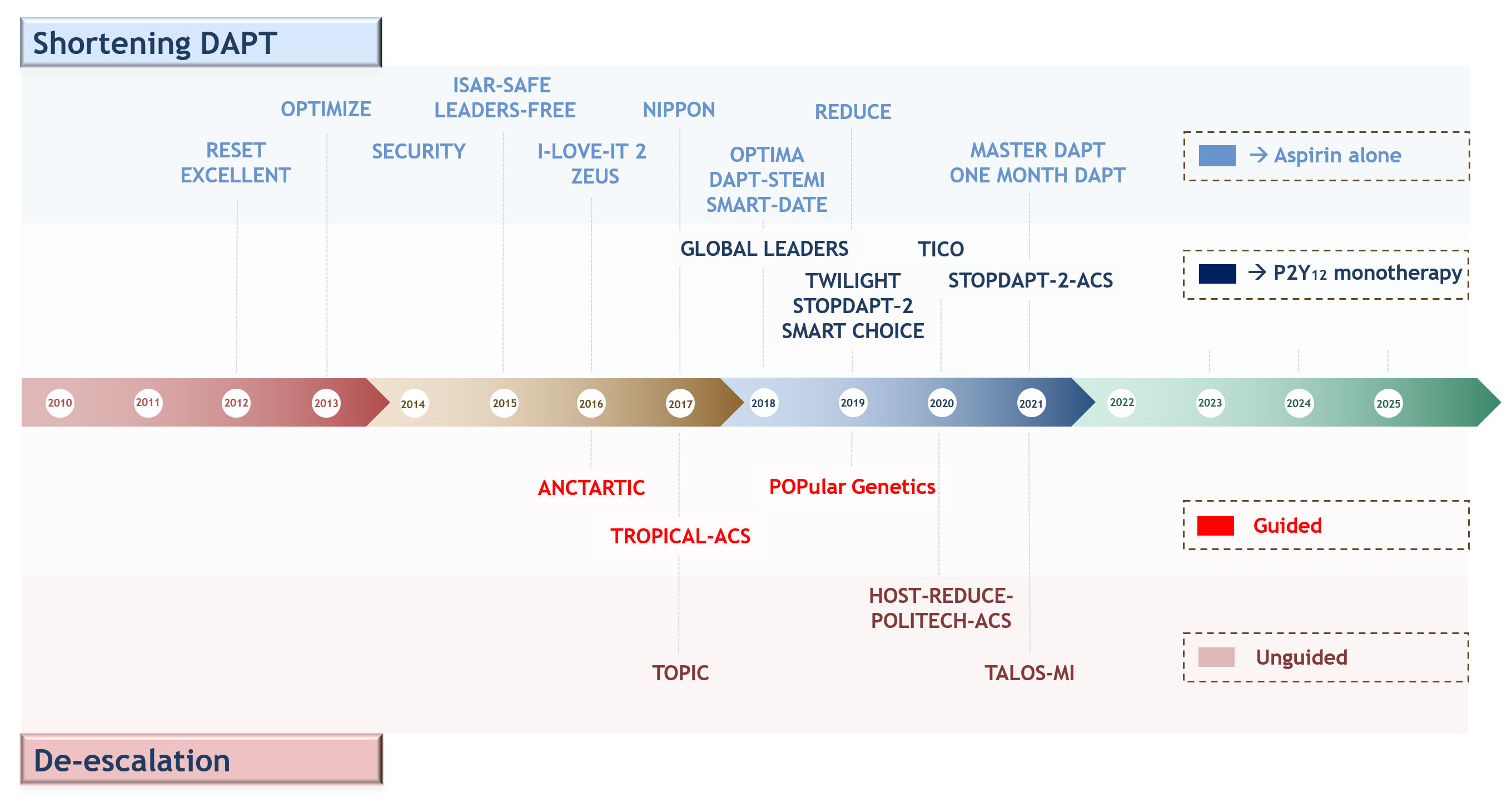 Fig. 4.
Fig. 4.Timeline of randomized controlled trials testing antiplatelet regimens aiming at reducing bleeding events after percutaneous coronary interventions or acute coronary syndrome. Upper: shortening DAPT and aspirin-free strategies; lower: guided and un-guided DAPT de-escalation. Abbreviations. DAPT, dual antiplatelet therapy.
5.2.2.1 Shortening DAPT Duration
The first and most largely adopted strategy in this setting consists in
shortening DAPT duration (1 to 6 months), followed by the use of aspirin alone.
This strategy has been evaluated in thirteen RCTs, of which seven compared
6-month versus 12-month DAPT, four trials included 3-month versus 12-month DAPT
and two a one-month versus 6- and 12-months DAPT [2]. Overall, individual RCTs
and pooled analyses have shown shortening DAPT duration may reduce bleeding
without any trade-off in ischemic events in patients with CCS, while the abrupt
shortening of DAPT duration in ACS patients may be associated with an increase in
ischemic events. The, SMART-DATE trial, randomized 2717 ACS patients (38% STEMI,
31% NSTEMI and 31% unstable angina) to either 6-month or 12-month DAPT (mainly
using clopidogrel as P2Y
5.2.2.2 P2Y
Pharmacodynamic (PD) investigations showing that aspirin provides limited
antithrombotic effects in addition to potent P2Y
The fact clopidogrel but not ticagrelor monotherapy after a short course of DAPT has been associated with increased ischemic events in ACS is consistent with the well-known higher risk of ischemic events of ACS patients compared to CCS patients and by the fact that about 30% of patients treated with clopidogrel, but less than 5% of those treated with ticagrelor (or prasugrel), result in inadequate platelet inhibition leading to high platelet reactivity (HPR), a modifiable marker of thrombotic risk [91, 92]. This difference in the PD response to clopidogrel is related to the fact that clopidogrel is a pro-drug that requires a 2-step biotransformation oxidative process by the hepatic cytochrome (CYP) P450 system to be activated. The CYP2C19 enzyme is involved in both metabolic steps of clopidogrel biotransformation and the gene responsible for its transcription is highly polymorphic, with carriers of loss-of-function (LoF) alleles *2 and *3 being associated with reduced generation of clopidogrel’s active metabolite leading to high HPR rates, and increased thrombotic complications [92, 104].
5.2.2.3 De-Escalation of P2Y
Prasugrel and ticagrelor are characterized by more potent and predictable
pharmacodynamic effects compared with clopidogrel, which however lead to an
increased risk of bleeding [105, 106]. Switching from a more potent (prasugrel or
ticagrelor) to a less potent (clopidogrel) or lower dose P2Y
5.2.2.3.1 Guided De-Escalation
The rationale for the use of a guided de-escalation is to selectively administer
a potent P2Y
Three RCTs have tested a guided de-escalation strategy, two using PFT and one
using genetic test [109, 110, 111]. ANTARCTIC failed to show reduced NACE with
PFT-guided de-escalation versus standard therapy in 877 elderly patients with ACS
undergoing PCI. Nevertheless, reduced dose (5 mg daily) of prasugrel rather the
recommended 10 mg daily was used in this trial, potentially blunting the superior
safety of a de-escalation strategy [109]. On the contrary, TROPICAL-ACS met the
composite primary endpoint for non-inferiority of NACE in 2610 patients with ACS
[110]. Furthermore, POPular Genetics, which randomized to either genotype-guided
de-escalation or standard therapy (mainly ticagrelor) 2488 STEMI patients, showed
the non-inferiority in the primary endpoint of NACE and a significant 22%
reduction in the co-primary endpoint of PLATO major and minor bleeding at 12
months [111]. Nevertheless, the use of a primary endpoint including both ischemic
and bleeding outcomes and the non-inferiority design of these two latter trials
represent important limitations contributing to the relatively weak recommendations
on the use of PFT or genetic guidance in clinical practice (Class IIb, level of
evidence A) [27]. Indeed, such trials were not powered for hard, individual,
ischemic or hemorrhagic endpoints such as CV death, MI, ST, major bleeding and
intracranial hemorrhage. To this extent, meta-analysis are useful to overcome
the limited statistical power for rare endpoints. A recent comprehensive
meta-analysis overcoming this limitation showed that a guided de-escalation is
associated with a 19% reduction of bleeding without any trade-off in ischemic
events [112]. Moreover, a network meta-analysis comparing guided de-escalation
versus prasugrel or ticagrelor among more than 60,000 ACS patients from 15 RCTs
showed guided de-escalation to be associated with the most favorable balance
between safety and efficacy [113]. Collectively, PFT or genetic testing
represents a promising strategy for reducing bleeding without any trade-off in
ischemic events among ACS patients and future guidelines are likely to provide
stronger recommendations on the use of a guided selection of P2Y
5.2.2.3.2 Unguided De-Escalation
The rationale for the use of an unguided de-escalation strategy stems from the
fact that while ischemic risk decreases after 1 to 3 months post-PCI, bleeding
risk, although being higher in the periprocedural phase, tends to be stable over
time [2]. Therefore, potent P2Y
Three RCTs, for a total of 5681 patients have tested an unguided de-escalation 1
month after ACS versus standard 12-month DAPT. In two of them, de-escalation
consisted in switching from a potent P2Y
Limitations of the unguided de-escalation of antiplatelet therapy are the
following: (1) 5035 of the 5681 patients in which this strategy was tested were
East Asian patients, a population in which bleeding events are higher and
ischemic events are lower compared to other populations, therefore, further
studies are needed before generalization of their results to different
populations; (2) further evidence are needed to show how a de-escalation
consisting in a dose reduction of potent P2Y
In summary, unguided de-escalation is a very effective and promising strategy in reducing bleeding among ACS patients undergoing PCI, but whether this strategy may be broadly adopted regardless of individual response to clopidogrel requires further investigation.
5.2.2.3.3 Special Clinical Scenarios
Patients Requiring Long Term Anticoagulation
Up to 15% of patients undergoing PCI are affected by a concomitant medical
condition requiring OAC, among which AF is the most frequent [117]. Because the
addition of DAPT to OAC (the so called triple antithrombotic therapy, TAT)
increases the risk of bleeding two- to three-fold compared to OAC alone,
strategies to reduce bleeding are particularly important in this clinical setting
[14]. To this extent, recent guidelines propose the use of NOAC over VKA and the
shortening of TAT to one week followed by clopidogrel plus OAC for the majority
of patients [118, 119, 120]. These recommendations are based on the evidence of 4
RCTs comparing each of the 4 available NOACs plus a P2Y
Moreover, it may be argued that these trials present important limitations, such as: (1) none of them focused on ACS patients; (2) procedural complexity was not reported; (3) low ischemic patients were included; and (4) the strategy tested does not reflect the clinical question of whether TAT with NOAC lasting one month would be beneficial compared to TAT lasting 7 days [128]. Furthermore, in light of the fact that approximately 30% of patients treated with clopidogrel are non-responders, such an early drop of aspirin could be particularly detrimental. In summary, these data suggest that shortening TAT duration to 7 days may be a very effective strategy in reducing bleeding but caution should be paid with patients at high ischemic risk or those with ACS, in which prolonging TAT with a NOAC for 1 month may represent a more balanced strategy [128].
Gastrointestinal (GI) bleeding is the most frequent source of out-of-hospital bleeding after PCI [129]. Several trials have shown that PPIs (proton pump inhibitors) and histamine H2-receptor antagonist reduce the rate of recurrent gastrointestinal bleeding in patients receiving aspirin at high-risk of GI bleeding [130, 131]. On the basis of these results, international guidelines recommend the routine use of PPI in combination with DAPT, regardless of GI bleeding risk [29]. Among PPIs, pantoprazole or rabeprazole should be preferred over others due to their potential interaction with the CYP2C19 which is also implied in clopidogrel metabolism [132].
Additional strategies to reduce the risk of bleeding in patients treated with anti-thrombotic drugs include optimal control of blood pressure and avoidance of non-steroidal anti-inflammatory drugs [133, 134].
Acute management of bleeding complications in patients treated with antithrombotic therapy is very challenging and scarce evidence is available from RCTs. Therefore, recommendations on acute bleeding management are based on expert opinion or observational studies [90, 135]. Fig. 5 provides a flow chart for the management of bleeding in patients treated with OAC +/ DAPT or SAPT (single antiplatelet therapy) [29].
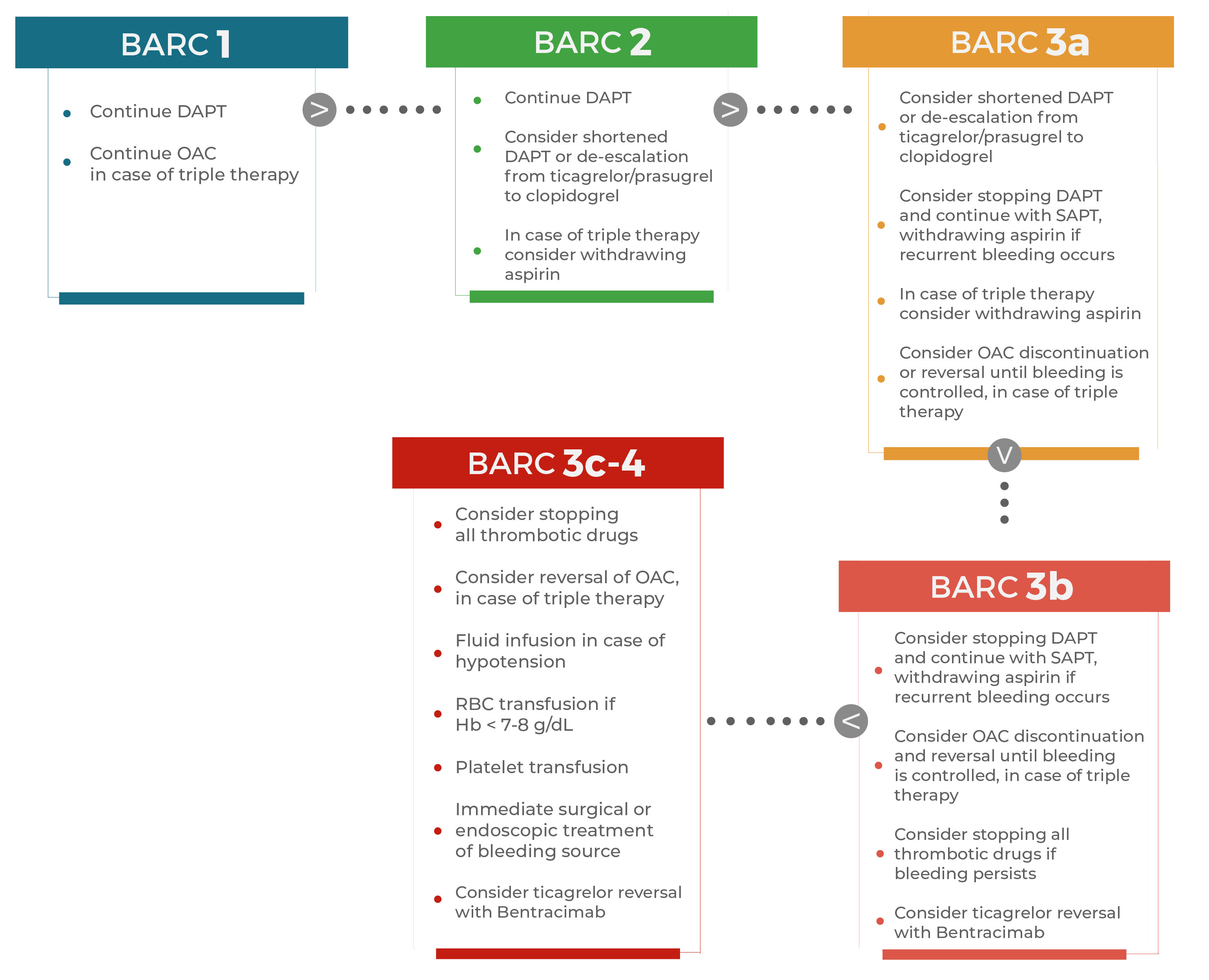 Fig. 5.
Fig. 5.Practical considerations for the management of bleeding
(classified by BARC) in patients treated with dual antiplatelet therapy with or
without concomitant oral anticoagulation. Several strategies can be implemented
in case of bleeding, depending on bleeding severity according to BARC classification. BARC 1, BARC 2 and BARC 3a
bleeds do not require integrative treatments apart from redefinition of duration
and type of antithrombotic treatment (i.e., withdrawal of aspirin or
de-escalation of P2Y
With respect to non-specific reversal agents for antiplatelet therapies,
platelet transfusion or desmopressin have been proposed over years, but none of
these is strongly recommended by guidelines [135]. Platelet transfusion has been
tested in two RCTs reporting clinical endpoints that have included 970 patients
with intracranial hemorrhage taking antiplatelet therapy [136, 137]. These trials
showed that platelet transfusion increases the risk of death compared with
standard care in patients who do not need a neurosurgical intervention, while it
reduces mortality in patients with intracranial hemorrhage undergoing
neurosurgery [136, 137]. Desmopressin, has been mainly studied in the setting of
elective or emergent cardiac surgery in patients on antiplatelet therapy or
affected by platelet dysfunction [138]; it was associated with a 25% reduction
in total volume of red blood cells transfused and a 23% reduction in blood loss,
as well as with lower risk of re-operation due to bleeding [138]. Nevertheless,
there was no decrease in mortality nor increase in thrombotic events with its use
[138]. Current guidelines recommend a single dose of desmopressin for
intracranial hemorrhage associated with aspirin or P2Y
Because ticagrelor has reversible binding kinetics and a relatively long half-life (9–12 hours) as opposed to the irreversible binding of aspirin, clopidogrel and prasugrel, platelet transfusion is ineffective in reversing platelet function within 24 hours from ticagrelor withdrawal [139]. To this extent, bentracimab, a recombinant monoclonal antibody fragment that reverses the antiplatelet effects of ticagrelor within 5 minutes has been produced and has been recently tested in the REVERSE-IT trial, among patients undergoing urgent surgery/procedure or with major bleeding. At the interim analysis, bentracimab successfully met the primary reversal endpoint consisting in minimum % inhibition of VerifyNow PRU within 4 hours, with onset of action after 5 minutes of drug initiation [140]. No safety concern emerged from the interim analysis. The trial is still ongoing and completion is expected in 2023.
In the presence of a major or life-threatening bleeding on a VKA, reversal agents are represented by prothrombin complex concentrate, fresh frozen plasma or recombinant activated factor VII [141]. Prothrombin complex is the first choice reversal agent, followed by plasma and factor VII, because it seems to be more effective than plasma in correcting INR, does not require crossmatching, is virally inactivated, does not pose a risk of volume overload and is associated with a lower risk of thrombosis than factor VII, whose use is restricted to cases in which prothrombin complex and plasma are not available [27, 142].
For NOAC-treated patients with intracranial hemorrhage or bleeding involving a critical organ, in case of treatment with dabigatran, first-line treatment is represented by its specific antidote idarucizumab, followed by prothrombin complex concentrates in case of its unavailability [27, 143].
For patients treated with apixaban, edoxaban or rivaroxaban, prothrombin complex concentrate should be first-line treatment [27]. A specific antidote, andexanet, has been developed for factor X inhibitors and evaluated in a single trial, involving 67 patients with acute major bleeding [144]. It reached effective hemostasis in 79% of patients, with no serious side effects [144]. Further studies, with a larger sample size and a control arm, are needed to assess efficacy and safety of this antidote.
A number of strategies may be implemented to reduce bleeding in patients undergoing PCI (Graphical abstract). Among these, a careful selection of patients undergoing PCI, the increasing adoption of more advanced stent platforms and more refined techniques and technologies to optimize stent implantation and the use of more balanced antithrombotic regimens will be key in reducing the risk of bleeding after PCI. It is becoming increasingly clear that a “one-size-fits-all” approach is not successful when selecting antithrombotic therapy in these patients, given to the broad individual response to treatments. Personalization of antithrombotic therapy, taking into account individual ischemic and hemorrhagic risks but also individual responses to antiplatelet agents such as clopidogrel represents the most promising strategy for an optimal balance between bleeding and ischemic prevention at the individual patient’s level [145].
Novel antithrombotic regimens as well as their combinations are currently being
tested and may play a key role not only in reducing bleeding but also in reducing
the still high rate of ischemic recurrences by promoting plaque stabilization
reducing systemic inflammation [146]. Indeed, inflammatory and thrombotic
pathways have been shown to be strictly connected and play a key role in the
pathogenesis of atherosclerotic disease. To this extent, targeting inflammation
on top of antithrombotic drugs (i.e., anti-IL-1
Another promising line of research is represented by FXIa inhibitors. FXIa has been considered to contribute to thrombosis while playing a relatively minor role in haemostasis. Therefore, its inhibition may potentially lead to reduced ischemic events without increased bleeding [150]. Three compounds are in clinical development: (1) asundexian, a small molecule FXI(a) inhibitor; (2) osocimab, anti-FXI(a) antibody, and (3) fesomersen, a FXI-ligand-conjugated antisense oligonucleotide [151, 152]. Asundexian has recently shown to reduce bleeding without any trade-off in efficacy in a phase II trial comparing different doses versus apixaban among AF patients [153]. Other phase II studies, including over 4000 patients, are ongoing in patients with recent ischemic stroke or recent MI [153].
New formulations of aspirin have been proposed in the attempt to make aspirin more tolerable and reduce bleeding in the GI tract, such as designing enteric-coated aspirin with cellulose or silicon which resists disintegration in the stomach, permitting aspirin to dissolve specifically in the duodenum, avoiding topic epithelial injury [154]. A liquid formulation of a novel pharmaceutical lipid–aspirin complex (PL-ASA) was designed to prevent disruption of protective gastric phospholipid barrier, avoiding direct acid injury and has provided promising results in pharmacokinetic and pharmacodynamic studies [155].
The development of new reversal agents is under way and may be of particular interest for the prompt treatment of bleeding complications among patients treated with antithrombotic agents. Among these, ciraparantag is a small molecule that has been reported to bind all NOACs as well as LMWH and UFH and fondaparinux [156]. Therefore, ciraparantag may potentially function as a universal reversal agent for several classes of anticoagulants, enhancing their safety profile. A phase II RCT is ongoing to evaluate the efficacy and safety of ciraparantag for reversal of anticoagulation induced by different anticoagulant drugs (edoxaban, apixaban or rivaroxaban) in generally healthy adults, whose results are expected in December 2022 [157]. Finally, UHRA-7 is a multivalent polymer designed to be a universal heparin reversal agent (both UFH and LMWH) that is currently being studied in preclinical trials [158].
For many years, the main concern in patients undergoing PCI has been the reduction of ischemic complications. The increasing awareness that bleeding complications are relatively common and carry important prognostic implications has recently shifted the interest towards the implementation of bleeding reduction strategies. To this extent, prevention represents the most effective and cost-effective strategy. Bleeding prevention strategies include patient bleeding risk stratification, careful assessment of the eligibility for invasive and high-risk procedures, personalized antithrombotic therapy and implementation of advanced stent platforms and procedural techniques. Finally, when a bleeding occurs, prompt and effective treatment is essential and may be achieved by new reversal agents and technologies.
ACCOAST, Comparison of Prasugrel at the Time of Percutaneous Coronary
Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST
Elevation Myocardial Infarction; ACS, Acute Coronary Syndrome; ADAPT-DES,
Assessment of Dual AntiPlatelet Therapy With Drug Eluting Stents; AF, Atrial
Fibrillation; ANTARCTIC, Platelet function monitoring in elderly patients on
prasugrel after stenting for an acute coronary syndrome; ARC-HBR, Academic
Research Consortium for High Bleeding Risk; ASA, AcetylSalicylic Acid; ATLANTIC,
Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST
Elevation Myocardial Infarction to Open the Coronary Artery; AUGUSTUS, An
Open-Label, 2
MG—conceived, structured, and organized this review. Writing—original draft preparation—MG and RL; writing—review and editing—FA, DD, RV, RAM, AI, CT, FB, FC; supervision—FA, DD, RV, RAM, AI, CT, FB, FC; visualization—MG and RL. All authors have read and agreed to the published version of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
M.G. declares that he has received consulting fees or honoraria from Terumo, outside the present work. F.A. declares that she has received consulting fees or honoraria from AstraZeneca, Amgen, Bayer, BMS/Pfizer and Daiichi-Sankyo, outside the present work. F.B. declares that he has received consulting fees or honoraria from Abbott, Abiomed, Medtronic and Terumo, outside the present work. C.T. declares that he has received consulting fees or honoraria from Abbott, Abiomed, Medtronic and Terumo, outside the present work. F.C. declares to be member of the advisory board of GlyCardial Diagnostics. The remaining authors report no disclosures. Mattia Galli is serving as one of the Guest editors of this journal. We declare that Mattia Galli had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Julio Núñez Villota.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






