1 Department of Cardiac Surgery, Centre Hospitalier de l’université de Montréal (CHUM), Montreal, QC H2X 0C1, Canada
2 Department of Surgery, Faculty of Medicine, Université de Montréal, Montreal, QC H3T 1J4, Canada
†These authors contributed equally.
Academic Editor: Giuseppe Santarpino
Abstract
Aortic interventions remain the most effective treatment for severe aortic stenosis. In the recent years, advances in bioprosthetics and newer data have reduced the cut-off age for the use of bioprosthetic valves in younger patients, but the debate on whether to favor mechanical valves in younger patients remains a constant, especially with the undesired effects and considerations of anticoagulation therapy with vitamin K antagonists in this age group. Other options like the Ross procedure are gaining traction, despite still being undervalued and necessitating expertise centers. Hemodynamic considerations and durability of these options are important to consider, especially in this age group. Regardless of the choice of the prosthesis, patient informed consent is paramount since the decision affects the lifetime management of their initial condition, and expectations given must remain realistic.
Keywords
- aortic stenosis
- young adults
- aortic valve replacement
- bioprosthetic valve
- mechanical valve
- ross procedure
Aortic stenosis (AS) remains the most common type of valvular heart disease in
Western countries and can affect patients of any age. Data of prevalence of AS in
the general population is lacking but it is estimated at 2–4% in patients
The previous guidelines for the management of valvular heart disease from the
American College of Cardiology (ACC)/American Heart Association (AHA) (2014) [5]
as well as that from the European Society of Cardiology (ESC) and the European
Association for Cardio-Thoracic Surgery (EACTS) (2017) [6] recommended, in terms
of the choice of the prosthesis, the use of mechanical valves in patients
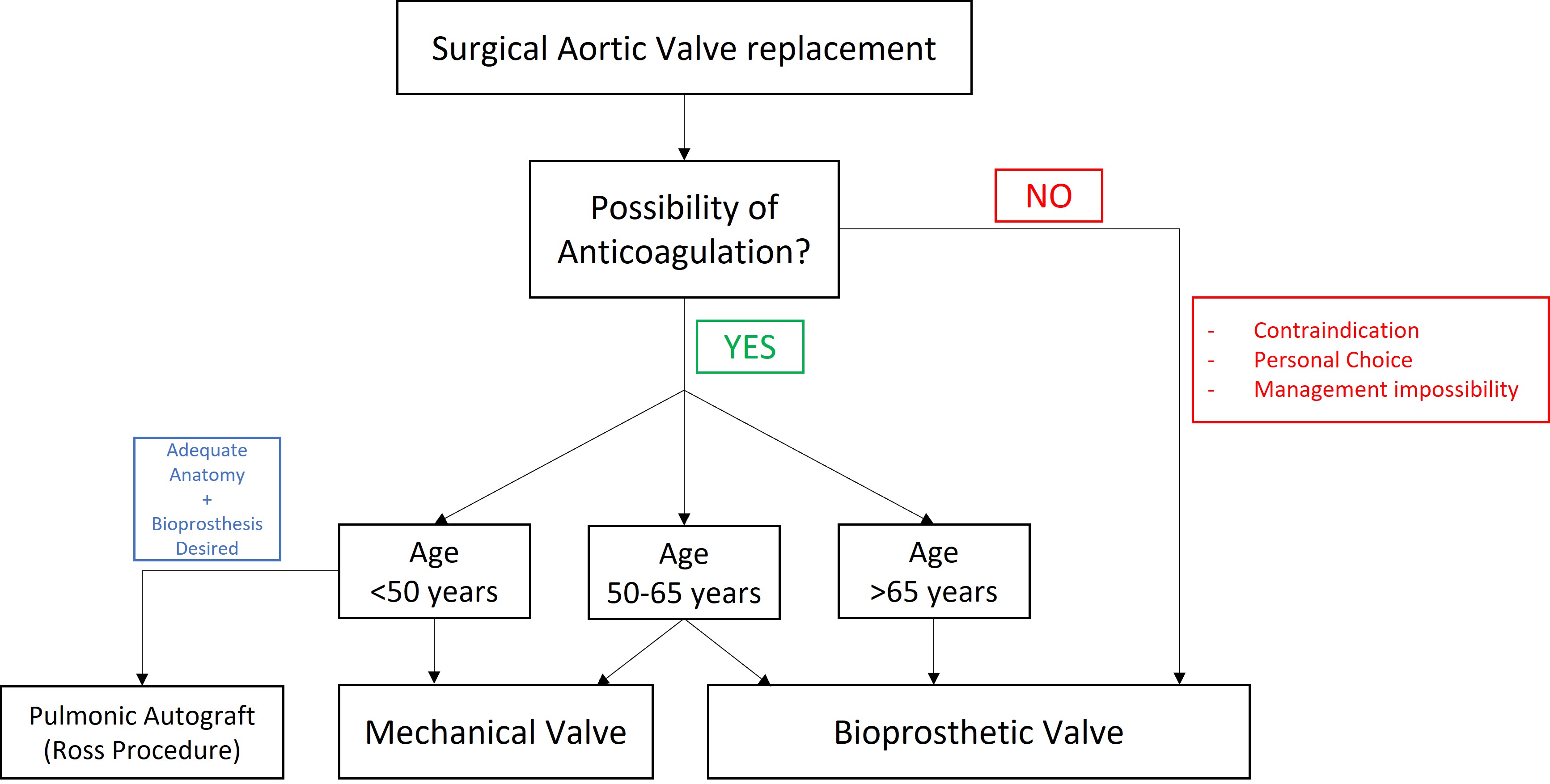 Fig. 1.
Fig. 1.Choice process of prosthetic valve adapted from the 2020 ACC/AHA Guideline for the Management of Patients With valvular Heart Disease [8].
Throughout the recent years, the advancements in valve designs and durability
allowed for a dramatic increase in the number of bioprosthetic aortic valve
implants in comparison to mechanical one. In fact, from 1997 to 2012, in the
state of New York alone, the use of bioprosthetic valves went from 15 to 74% in
young adults (age 50–69 years old) [10]. In the German Heart Surgery Report
of 2020, 88% of patients had a bioprosthetic valve implanted [11]. The
schematic representation of a bioprosthetic surgical aortic valve replacement is
shown in Fig. 2. Guidelines have also further decreased the age cut-off for the
use of biological prosthesis during aortic valve replacement throughout the
recent years [8, 9]. This can be explained by the advancements in design and the
long-term data on durability and survival of patients who had those prostheses
implanted, which are encouraging [12]. Other factors that contributed to the
potential use of bioprosthetics in younger patients are the advents of new
technologies that changed our field such as TAVR, and the advancements in that
regard that allowed for more reliable implants and the possibility for
Valve-in-Valve procedure in the future. In addition, regardless of durability and
advancements, bioprosthetics have been used in young patients who refuse (or are
contraindicated) to take long-term anticoagulation [10, 11]. According to Dr.
Bourguignon and his colleagues, the expected valve durability of the
Carpentier-Edwards Perimount aortic valve was 17.6 years for the younger patients
(
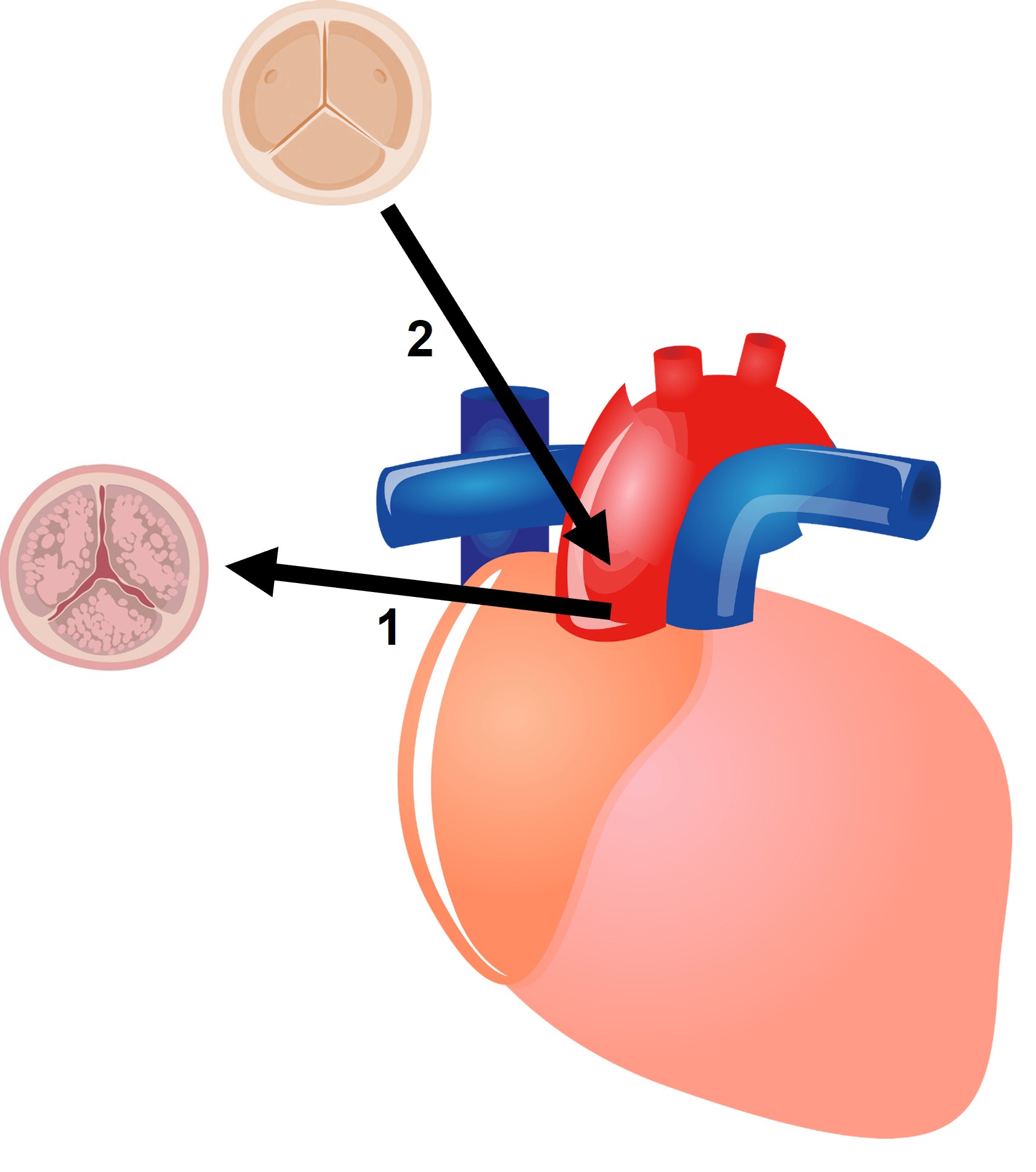 Fig. 2.
Fig. 2.Schematic representation of a biological surgical aortic valve replacement. (1) The diseased aortic valve is removed. (2) A bioprosthesis is inserted in its place.
Despite the advancements in bioprosthetic valves, the debate regarding whether mechanical or bioprosthetic valves should be used in patients aged 50–70 years remains a constant. Leviner and colleagues published in 2022 a meta-analysis comparing mechanical vs bioprosthetic aortic valve replacement in patients younger than 70 years old. They showed an overall survival benefit for patients who received a mechanical valve [17]. Also, in 2017, Goldstone et al. [18] published a comparative study comparing both types of valves. They showed that among patients who underwent aortic valve replacement, receipt of a biologic prosthesis was associated with significantly higher 15-year mortality than receipt of a mechanical prosthesis among patients 45 to 54 years of age (30.6% vs 26.4% at 15 years; p = 0.03) but not among patients 55 to 64 years of age.
On the counter part, Joanna Chikwe and her group compared mortality and
morbidity in young adults (18–50 years of age) in the states California and New
York who received mechanical versus tissue valve between 1997 and 2006. They
observed that the use of bioprosthetic valves increased from 14% to 47% from
1997 to 2014. There was no survival difference with bioprosthetic versus
mechanical aortic valves in the propensity score-matched cohort: actuarial
15-year survival was 79.0% vs 81.5 respectively; p = 0.20). There was
more stroke and bleeding in the mechanical valve group and more reoperation in
the bioprosthesis valve group. They suggested that in patients 18–50 years,
bioprosthesis are a reasonable alternative to mechanical valves for aortic valve
replacement [19]. Also, two other propensity-matched analyses found that
survival was comparable between the types of valves. McClure et al. [20]
reported a single-center analysis of 722 propensity-matched patients younger than
65 years and a mean age of 53 who were followed for a median of 6–7 years.
Survival after bioprosthetic and mechanical implantation was 78% vs 79% at 10
years, respectively, and 65% vs 75% at 15 years, respectively (p =
0.75). Chiang et al. [10] analyzed 2002 patients aged 50–69 years
from the New York State registry and followed these patients for a median of 10.8
years. At 15 years, survival was 60.6% in the bioprosthetic valve group and
62.1% in the mechanical group (p = 0.74). A group from Germany
published in 2021 a propensity-adjusted analysis in patients of two subgroups
(
Anticoagulation therapy with vitamin K antagonist (VKA) in the context of a mechanical prosthetic remains necessary to prevent thrombo-embolic and valve thrombosis events as newer anticoagulants have noy yet been proven to be safe or effective in these patients. This corresponds to a level I recommendation in the current guidelines. These same guidelines only find it reasonable to give aspirin 70 to 100 mg daily post-bioprosthetic AVR in all patients and VKA for 3 to 6 months in patients who are at low risk of bleeding. However, these correspond to a level IIa recommendation [8]. The prospective randomized On-X valve anticoagulation clinical trial (PROACT) showed that a lower INR target of 1.5 to 2 post-operatively, with the On-X mechanical prosthesis, decreases the incidence of both major and minor bleeding events when compared to the control group with an INR of 2 to 3 (1.48%/pt-yr versus 3.31%/pt-yr, and 1.18%/pt-yr versus 3.31%/pt-yr respectively) without increasing the risk of thrombo-embolic events (2.96%/pt-yr versus 1.85%/pt-yr, p = 0.178) [22, 23]. The LOWERING-IT trial evaluated the impact of lower anticoagulation targets (INR = 1.5–2.5 vs 2–3) with various mechanical valves and showed similar results to the PROACT trial with a significant decrease in bleeding (OR = 0.36, CI: 0.11–0.99, p = 0.04) and no difference in thrombo-embolic events (OR = 0.33, CI: 0.006–4.20, p = 0.6) [23, 24]. Despite the lower dosages, undesired effects and restrictions related to long-term VKA treatment sometimes push patients to avoid mechanical prosthesis because of medication interactions, dietary restrictions, the inconvenience of monitoring, and the need to restrict participation in certain activities, especially in young patients [8]. The management of VKA during pregnancy is also a concern in women of childbearing age undergoing an AVR [25, 26, 27]. Is the jury still out for the use of mechanical or biological valves in those younger patients?
Other options do exist, like the pulmonary autograft (Ross procedure). The
schematic representation of a Ross procedure is shown in Fig. 3. The current
guideline mentions the possibility of choosing the pulmonary autografts in young
patients
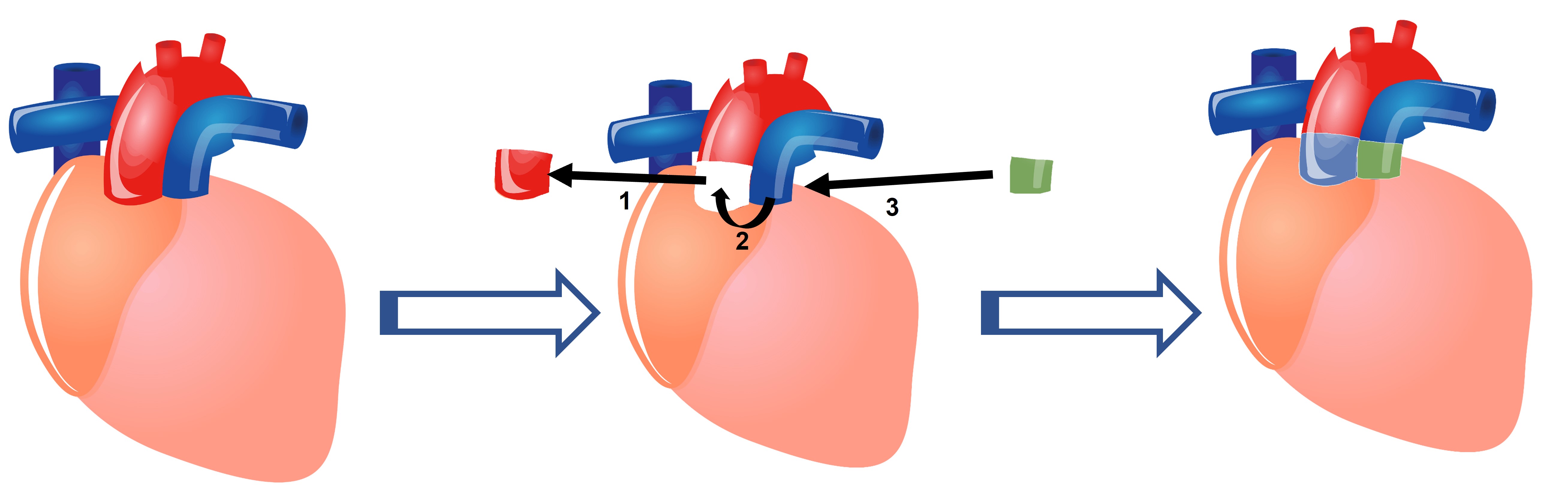 Fig. 3.
Fig. 3.Schematic representation of a Ross procedure (Pulmonary Autograft). (1) The diseased aortic valve is removed with a portion of the aorta. (2) The patient’s own pulmonic valve and a portion of the pulmonary artery are excised and placed in the aortic position. (3) A homograft (Allograft) consisting of the pulmonary valve and a portion of the pulmonary artery are placed in the pulmonary position.
Despite the abundance of evidence, including randomized trial [19], a systematic
review and meta-analysis [30] and several cohort studies, the use of the Ross
procedure remains low, representing
Aortic homografts could also be an option for young patients, but it was shown
that the survival of patients who received a homograft is decreased compared to
patients who had the Ross procedure (at 13 years, survival was 78%
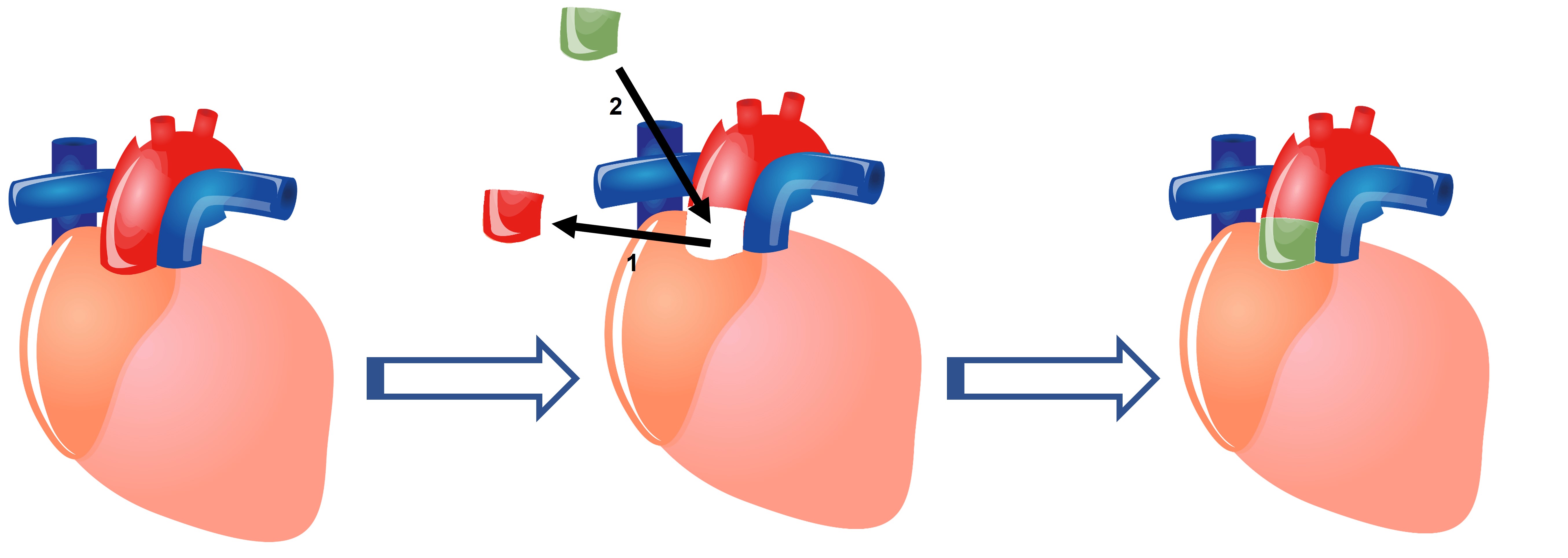 Fig. 4.
Fig. 4.Schematic representation of an aortic homograft procedure. (1) The diseased aortic valve is removed with a portion of the aorta. (2) A homograft (Allograft) consisting of the aortic valve and a portion of the aorta are placed in its place.
Many young adults wish to maintain an active lifestyle and pursue higher levels
of physical activities post-op. Therefore, the choice of procedure in young
patient adds an extra factor that should be taken into consideration in addition
to minimizing the risk of valve-related complication and restoring normal
survival; it should also provide durable hemodynamic properties [41]. Both
biological and mechanical prosthetics fix the annulus but are inherently
obstructive. The pulmonary autograft, on the other hand, preserves aortic root
mobility. This could be explained by the viability of the autograft and its
capacity to remodel in the new hemodynamic environment. This allows for a better
hemodynamic profile in patients who underwent the Ross procedure when compared to
a prosthetic, whether mechanical or biological. The aortic gradient is a good
indicator of hemodynamic performance. Lower gradients, closer to that of a
normally functioning valve, are important at rest for any patient for congestive
heart failure risk reduction [42]. A systematic review and meta-analysis by Um
and colleagues reported that in observational studies, the mean aortic gradients
were significantly lower at both discharge (–9 mmHg, CI: –13 to –5, p
In conclusion, the use of a bioprosthetic valve implanted in the aortic position is increasing, but the choice of an aortic valve prosthesis is still a complicated matter, especially in young patients. Regardless of the decision, informed consent remains paramount since patients need to be carefully informed of the next steps, because this procedure becomes a lifetime management of their initial condition. This is why patient preference, in terms of valve type and willingness/ability to take anticoagulant therapy, is an important decisive factor that is integral to the decision process and that is clearly accounted for in the guidelines nowadays. With newer data coming every day, guidelines can change, and recommendations can be updated. Would the use of the novel anticoagulants with mechanical valve change the trends of implantation in those younger patients? And what about the Ross procedure? It seems to be a great operation in terms of survival, freedom from valve-related complications, and hemodynamic profile for those younger patients in dedicated centers of expertise. Will the number of cases increase in light of the most recent data, and will the Ross procedure finally penetrate the modern surgical practice? Other innovative methods like the AV-Neo, consisting of constructing leaflets from the patient’s own pericardium, are gaining traction worldwide with satisfactory results and are also potential areas of interest for future treatment options [49]. Those are the contemporary options that we have in our armamentarium to treat aortic valve stenosis and no matter the technique used, younger patients must be given very realistic expectations of the need of re-interventions, their survival benefit, and implications of having this or that type of procedure, and at what time point during their lifetime.
KK and JF contributed equally to original ideas, text writing and editing. All authors have read and agreed to the published version of the manuscript.
Not applicable.
We would like to express our gratitude to the peer reviewers for their opinions and suggestions and to all those who helped us during the writing of this manuscript.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.




