1 Division of Cardiology, West Vicenza General Hospitals, 36071 Arzignano-Vicenza, Italy
2 Department of Clinical and Molecular Medicine, University La Sapienza, 00185 Rome, Italy
3 Department of Health Promotion Sciences Maternal and Infantile Care, Internal Medicine and Medical Specialties, University of Palermo, 90127 Palermo, Italy
Academic Editors: Manuel Martínez-Sellés and John Elefteriades
Abstract
Nowdays a small proportion of patients with high/very high/extreme atherosclerotic cardiovascular disease risk achieves the optimal target of LDL-cholesterol, because of drug intolerance, poor adherence to the therapy, or inapplicability of the stepwise strategy in lipid lowering therapy, recommended by the current guidelines. The new oral agent bempedoic acid lowers plasma LDL-cholesterol by inhibiting adenosine triphosphate-citrate lyase, an enzyme involved in the synthesis of cholesterol, and, ultimately, by up-regulating the LDL receptors. Several clinical trials in patients with atherosclerotic cardiovascular disease or familial heterozygous hypercholesterolemia demonstrated that bempedoic acid alone or combined with statins and/or ezetimibe significantly reduced LDL-cholesterol and high-sensitivity C-reactive protein. Bempedoic acid is well tolerated with no significant increase in muscle-related symptoms, since it can be activated only in the liver but not in the skeletal muscles. Bempedoic acid provides an effective tool to further reduce LDL-cholesterol as add on therapy in patients unable to reach the target despite maximally tolerated lipid lowering therapy.
Keywords
- bempedoic acid
- ATP citrate lyase
- LDL-cholesterol
- lipid-lowering treatment
- novel LDL-C treatment
According to the current European Society of Cardiology/European Atherosclerosis
Society guidelines on managment of dyslipidemia, LDL-cholesterol (LDL-C) below 55
mg/dL and a 50% reduction of the baseline LDL-C value are recommended in
patients with very high cardiovascular (CV) risk [1]. Indeed, the currently
available drug treatments are able to reduce LDL-C up to 85% of the basal
levels. However, despite the efficacy of the lipid lowering therapy, a very small
proportion of coronary artery disease (CAD) patients reaches the recommended
lipid targets. In the DA VINCI study [2] which enrolled 2888 secondary prevention
patients from 18 European countries, the target of LDL-C
BA is a small molecule, which can be administered orally, as prodrug, once daily
as a single dose of 180 mg. It is rapidly absorbed in the small intestine and has
a half-life of 15–24 hours. BA oral bioavailability is not affected by food, nor
its pharmacokinetic properties by age, sex, race, or weight. BA inhibits
adenosine triphosphate-citrate lyase (ACL). ACL is an enzyme involved in the
cholesterol synthesis pathway, acting upstream of the hydroxy-methylglutaryl
coenzyme A reductase (HMGCR). Results from Mendelian randomization studies
suggest that inhibiting ACL lowers plasma LDL-C levels in the same way that
inhibiting HMGCR by a statin does—that is, through up-regulation of the LDL
receptors. Moreover, genetic variants that mimic the effect of ACL inhibitors
lower the risk of CV events [4]. Furthermore, BA lowers plasma glucose by
activating adenosine 5
As prodrug, BA needs to be converted to its active form, bempedoyl-CoA, by the enzyme very-long-chain acyl-CoA synthetase-1 (ACSVL1). Because ACSVL1 is expressed in the hepatocytes but not in adipose tissue, intestine, or skeletal muscle [6], BA should cause considerably less muscle-related adverse effects compared to statin therapy [7]. The fact that bempedoyl-CoA is not found in the plasma of individuals treated with bempedoic acid and likely it does not escape the hepatocyte suggests that its activity is limited to the liver and may contribute to the reduction of the muscle-related adverse effects [8].
The glucuronides of BA and bempedoyl-CoA are the major metabolites found in the plasma. BA is eliminated mainly by the kidneys, with 70% recovered in urine and 30% in feces [8].
In vitro metabolic interaction studies suggest that BA is not
metabolized by and does not inhibit or induce cytochrome P450 enzymes. As a
result, drug-drug interactions with drugs metabolized by this path, including
warfarin, are not anticipated. BA inhibits organic anion transporter OAT2
in vitro, which plays a role in uric acid and creatinine uptake from
blood to proximal tubular cells, and may explain the minor elevations in serum
creatinine and uric acid [5]. The most significant drug-drug interactions involve
BA and simvastatin and pravastatin. Co-administration of simvastatin 40 mg with
BA 180 mg in healthy participants, for example, caused an approximately 2-fold
and 1.5-fold increase in simvastatin AUC and Cmax, respectively. Considering that
many patients under BA treatment concomitantly take statins, a certain grade of
caution should be kept and doses of simvastatin
Based on the above characteristics, BA represents a novel therapeutical option to effectively reduce LDL-C, as demonstrated by the CLEAR program, which encompasses four clinical trials on the safety and the efficacy of BA in a wide range of patients.
So far, five phase III randomized clinical trials have been published (Table 1,
Ref. [9, 10, 11, 12, 13]). The CLEAR program includes four studies. The CLEAR Harmony [9] and
the CLEAR Wisdom [10] trials enrolled 3009 individuals with previous
atherosclerotic cardiovascular disease (ASCVD) and/or heterozygous familial
hypercholesterolemia (HeFH) and elevated LDL-C despite the treatment with
maximally tolerated statin therapy. The follow-up period was 52 weeks but the
Harmony trial will also provide long-term safety results in its open-label
extension (OLE) up to an extra 82 weeks of follow-up [14]. By contrast, the CLEAR
Tranquility [11] and the CLEAR Serenity [12] trials enrolled 614 patients with
hyperlipidemia and statin intolerance. In these studies the follow-up was 24
weeks maximum. All the studies were conducted between 2016 and 2018 at European,
US and Canadian sites. The primary end point of the CLEAR Harmony trial was the
overall safety, based on the occurrence of adverse events and/or clinical safety
laboratory findings. The average percent reduction in LDL-C at week 12 was the
major secondary end point in the CLEAR Harmony study and the primary end point in
the CLEAR Wisdom, Tranquillity and Serenity trials. In all studies, additional
secondary/tertiary end points included percent reduction of LDL-C at week 24
and/or absolute or percentage changes in total cholesterol, non-HDL-C,
apolipoprotein B, and high-sensitivity C-reactive protein at different time
points. In general, adverse events of particular attention embraced hepatic and
renal events, muscle-related symptoms, hyperuricemia, gout, metabolic acidosis,
hypoglycemia or new-onset or worsening diabetes, and neurocognitive disorders.
Fasting triglycerides
| Study | Duration | Population | Treatment groups (n) | LDL-C reduction at 12 weeks (BA versus placebo) | Muscle symptoms (BA versus placebo) |
| CLEAR Harmony [9] | 52 weeks | ASCVD and/or HeFH patients with LDL-C |
BA (n = 1488) | –18.1% 95% CI, –20.0, –16.1 | 13.1% vs 10.1% |
| placebo (n = 742) | p | ||||
| CLEAR Wisdom [10] | 52 weeks | BA (n = 522) | –17.4% 95% CI, –21.0, –13.9 | Not available | |
| placebo (n = 257) | p | ||||
| CLEAR Tranquility [11] | 12 weeks | Hypercholesterolemic patients with statin intolerance requiring additional LDL-C lowering | BA (n = 181) | –28.5% 95% CI, –34.4, –22.5 | 1.7% vs 2.3% |
| placebo (n = 88) | p | ||||
| CLEAR Serenity [12] | 24 weeks | BA (n = 234) | –21.4% 95% CI, –25.1, –17.7 | 12.8% vs 16.2% | |
| placebo (n = 111) | p | ||||
| Ballantyne [13] | 12 weeks | ASCVD and/or HeFH or multiple CVD risk factors | BA (n = 110) | BA: –19.0% | BA: 8.0% |
| Ezetimibe (n = 109) | Ezetimibe: –25.0% | Ezetimibe: 8.1% | |||
| BA + Ezetimibe (n =108) | BA + Ezetimibe: –38.0% 95% CI, –46.5, –29.6 | BA + Ezetimibe: 7.1% | |||
| Placebo (n = 55) | p |
Placebo: 7.3% | |||
| Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BA, Bempedoic Acid; CI, confidence interval; HeFH, heterozygous familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; and LLT, lipid lowering therapy. | |||||
| ASCVD/HeFH on statin | statin intolerant | |||||
| % reduction (placebo-corrected) | 95% CI | p | % reduction (placebo-corrected) | 95% CI | p | |
| total cholesterol | –11.1 | –12.2, –9.9 | –16.2 | –18.4, –13.9 | ||
| LDL cholesterol | –17.8 | –19.5, –16.0 | –24.5 | –27.8, –21.1 | ||
| non-HDL cholesterol | –13.1 | –14.7, –11.6 | –20.4 | –20.4, –17.5 | ||
| apolipoprotein B | –12.1 | –13.6, –10.7 | –16.9 | –19.6, –14.2 | ||
| hs-CRP | –18.1 | –22.7, –13.5 | –27.4 | –36.1, –18.5 | ||
| ASCVD indicates atherosclerotic cardiovascular disease; HeFH, heterozygous familial hypercholesterolemia. | ||||||
As said before, the Clear Harmony trial was extended in an open-label follow-up to 82 weeks. The definitive results are not yet available in the literature. However, some preliminary observations [14] have shown that BA therapy guaranteed a enduring LDL-C lowering in the long period with an elevate (86.2%) patient adherence. Moreover, during the open-label follow-up no different safety issues emerged compared to the original Harmony study and the overall BA phase 3 CLEAR clinical program.
The efficacy of BA in association with ezetimibe has been studied in a
fixed-dose combination (FDC) trial, which included patients at high CVD risk
because of the presence of ASCVD, HeFH or multiple CVD risk factors. In this
study, the combination of BA plus ezetimibe reduced LDL-C by –38.0% at 12 weeks
(placebo-corrected, p
BA is effective also with other lipid lowering agents, such as proprotein
convertase subtilisin/kexin type 9 inhibitors (PCSK9i): in a small phase 2,
randomized, double-blind, placebo-controlled study on 57 patients, BA added to
background PCSK9i therapy significantly lowered LDL-C by 30.3% (p
RCTs have undoubtedly shown that lowering LDL-C with statins reduces cardiovascular events in both the primary and the secondary prevention setting with a linear relationship between degree of LDL-C lowering and clinical benefit [16]. Preliminary data from phase III trials on BA suggest a reduction of cardiovascular events according to the achieved LDL-C reduction: so far, the pooled data from these phase III trials are encouraging with a risk reduction of composite cardiovascular outcome (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, and coronary revascularization) of 25% (RR 0.75, 95% CI 0.56 to 0.99) [17]. However, definite insights will come from the CLEAR Outcomes trial that will determine whether BA added to standard medical therapy reduces the incidence of major cardiovascular events in high risk patients with statin intolerance during an expected median duration of 3.5 years [18].
As far as safety is concerned, there is evidence from pooled analysis,
encompassing 3623 patients, that the most common treatment-emergent adverse
events (TEAE) did not differ between treatment groups. Other TEAEs of special
interest were quantitatively low with a
The incidence of decreased glomerular filtration rate (GFR) was 0.7% among
patients treated with BA and 0.1% (p = 0.02) in the placebo arm [7]. BA
increased the plasma levels of hepatic enzymes (2.8% versus 1.3%, p =
0.004) [7]. In all the cases, these elevations were asymptomatic, and
aminotransferase levels returned to
New-onset or worsening diabetes resulted significantly lower in BA treated patients (4.0% versus 5.6%; p = 0.03) [7], with an OR of 0.66 (95% CI 0.48 to 0.90), according to a meta-analysis that included 3629 patients [21].
A rare but potentially serious TEAE is the tendon rupture or injury which
occurred in 10 (0.5%) out of 2009 BA patients compared to none of the 999
placebo individuals [19]. All of the tendon ruptures or injuries occurred in
patients taking statins or with other risk factors such as fluoroquinolone or
systemic corticosteroid use, diabetes, gout, rheumatoid arthritis, renal failure,
age
According to the characteristics described above, BA results in a very “easy-to-use” and “handy” molecule. Indeed, the single dose, once-daily, neutral to food intake administration, the theoretical absence of muscular effects (i.e., hepatic selectivity), the efficacy independent of the background therapy, the potentiality as add-on treatment, the favorable effect on hs-CRP and the not negative interaction with glucose metabolism give the physicians a wide spectrum of opportunities.
BA is available alone or in fixed-dose combination with ezetimibe. The choice between the two should be guided by the patient risk category, the percent of LDL-C reduction to achieve, and the background of other lipid lowering therapies. From a general point of view, taking BA and ezetimibe in a fixed dose combination than separately improves adherence and compliance and allows to modulate the statin types and doses reducing the risk of statin-associated muscle symptoms.
BA represents a valid new therapeutical option in controlling LDL-C in the
elderly (
As mentioned before, renal and hepatic impairment are of little or no concern
with regard to BA utilization. Although no studies in end-stage renal disease and
in patients with severe liver disease (Child-Pugh class C) are available, in most
of the cases no dosage adjustment is necessary. Indeed, mean difference in
creatinine levels at week 12 was 0.048 mg/dL for BA treatment versus –0.002
mg/dL for placebo [19]. These changes were observed within the first 4 weeks of
treatment, were stable over time, and were reversible after treatment
discontinuation. On the other hand, pooled data from RCTs have reported that
treatment with BA was associated with slight elevations in the liver enzymes. The
rate of repeated and confirmed (2 consecutive incidences) elevations in
aminotransferase levels
A careful monitoring is required when BA is prescribed in individulas with a
prior history of gout. In these cases a increase vigilance for hyperuricemia and
gout is mandatory, but it should be not a reason to interrupt a priori the
treatment, considering that (1) the clinical benefits of BA treatment might
balance the potential risk of gout and (2) the fully reversibility of the
elevation in acid uric after discontinuation of BA treatment. However, subjects
with asymptomatic hyperuricemia (serum urate
In patients aged 60 and older, who are taking corticosteroids or
fluoroquinolones, or with renal failure or history of tendon rupture,
discontinuation of BA is mandatory if tendon rupture occurs. Withdrawal of BA is
recommended in all patients with joint pain, swelling or inflammation [8].
Similarly, BA should be avoided in patients taking simvastatin
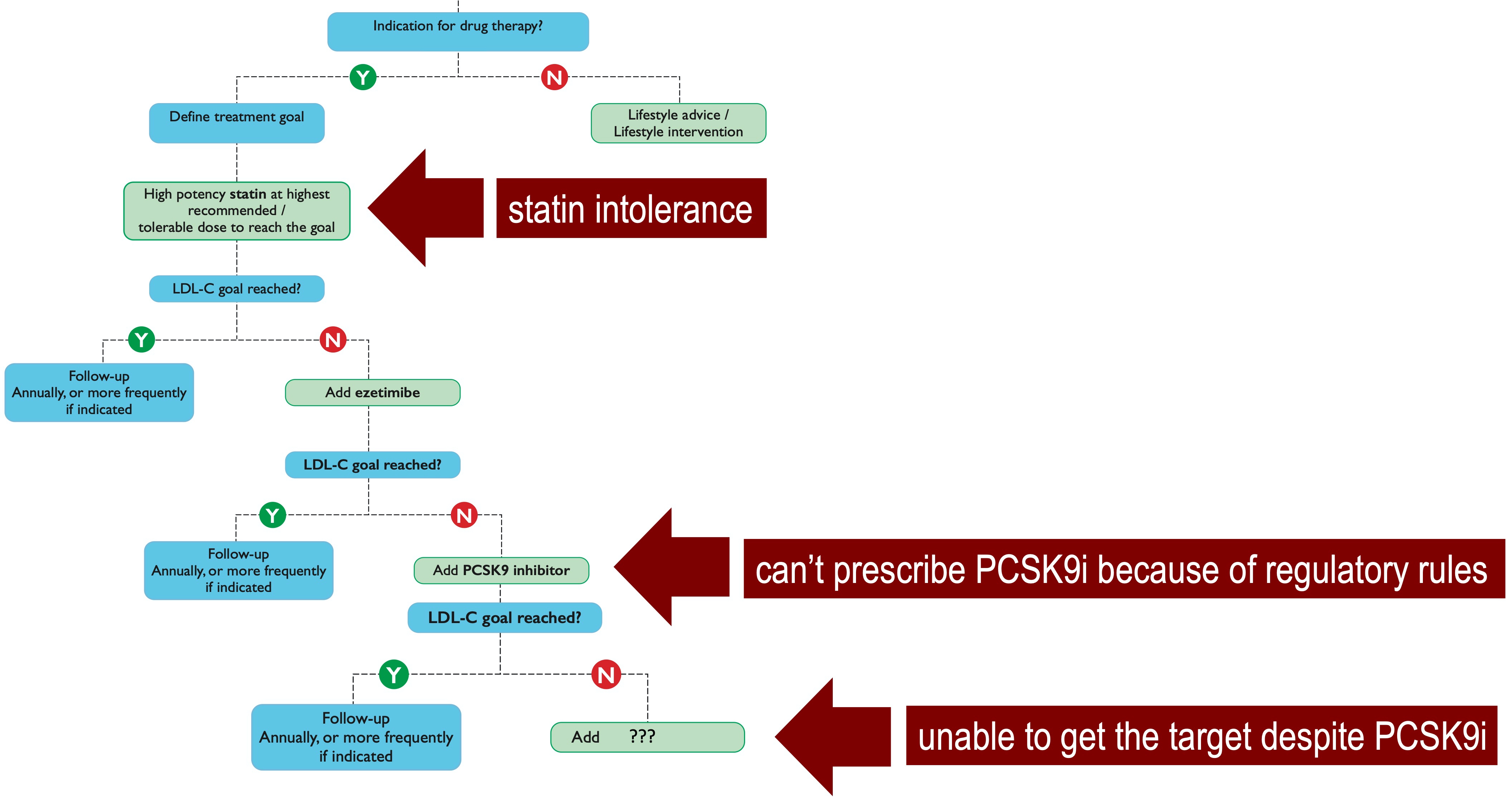
In patients with high/very high cardiovascular risk, current European guidelines for the management of dyslipidemias recommend a stepwise strategy starting with maximum tolerated dose of high intensity statin, followed by a combination with ezetimibe if the goals are not achieved after 4 weeks. If the further subsequent 4-week treatment of statin/ezetimibe dual therapy fails to reach the target, the addition of PCSK9 inhibitor is then recommended [1]. From a theoretical point of view, this step-by-step approach in lipid lowering treatment should guarantee the achievement of the therapeutical goals in all the patients, considering that the triple therapy (statin/ezetimibe/PCSK9i) is able to reduce the baseline LDL-C levels by 85%. This strategy, however, is affected by, at least, three potential barriers (Fig. 1, Ref. [1]), without considering other practical obstacles such as the cost and/or the route of administration.
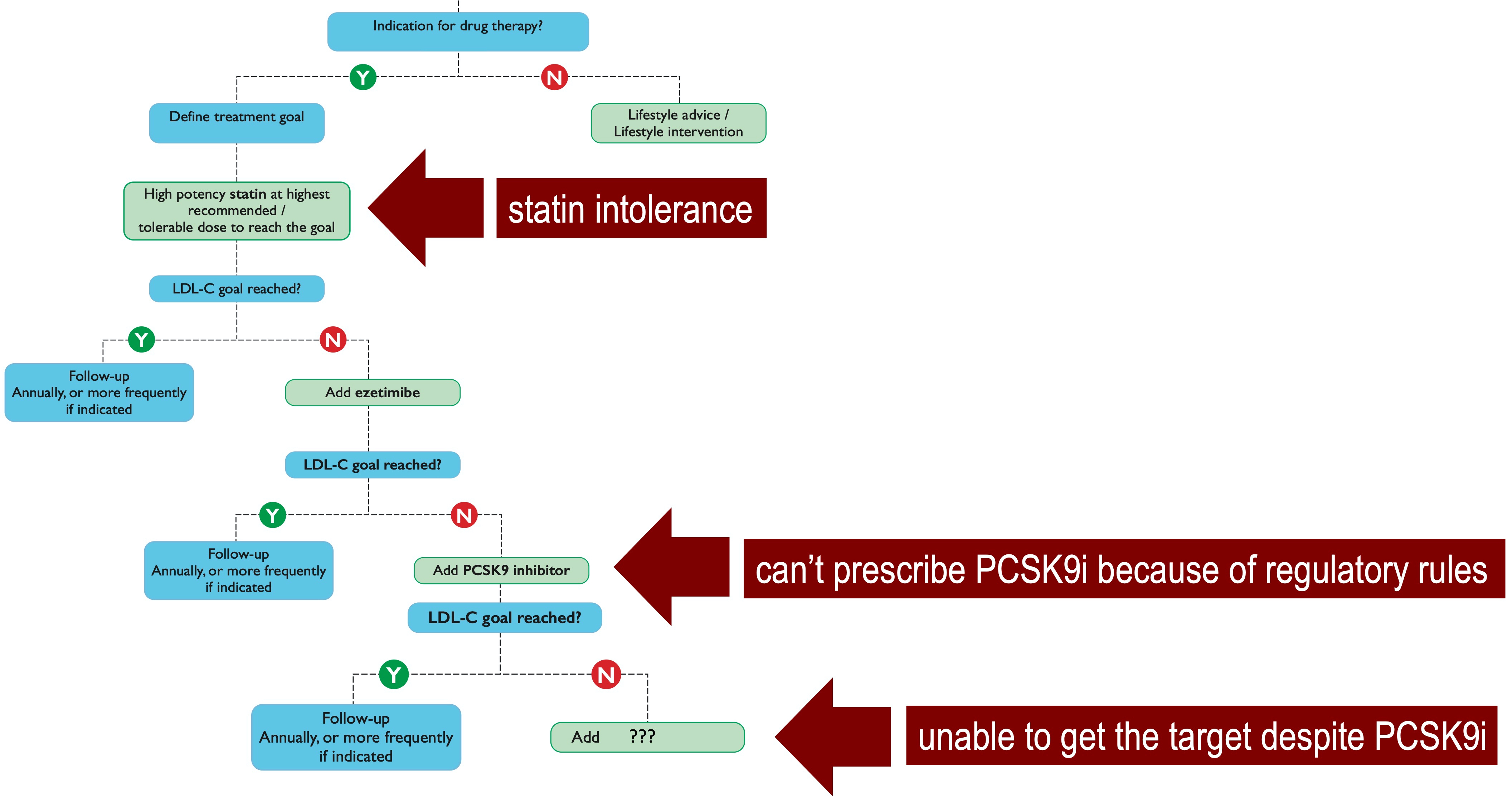 Fig. 1.
Fig. 1.The stepwise therapeutical strategy according to the recommendations of the 2019 ESC/EAS guidelines for the management of dyslipidemias [1], in which single statin, dual statin/ezetimibe and triple statin/ezetimibe/PCSK9 inhibitor therapies are gradually introduced every 4 weeks until the achievement of LDL-C target.
First, the high proportion of patients with intolerance to statins, and, in
particular, statin-associated muscle symptoms who likely contribute significantly
to the very high discontinuation rates of statin therapy (up to 75%) within 2
years of initiation [25]. Second, when the patients are not eligible for PCSK9i
because national regulatory agencies do not allow to prescribe them and/or do not
recognize their reimbursement, if specific criteria are not fully satisfied. As
result, it has recently been reported that, in Europe, patients initiated on
PCSK9i had baseline LDL-C levels almost 3 times higher than the reccomended
threshold for PCSK9i use [26]. Third, when despite the use of a multiple lipid
lowering therapy (i.e., triple therapy: statin/ezetimibe/PCSK9i), the patient is
unable to reach the recommended goal, especially if the subject is at very high
(LDL-C should be
BA is not a competitor of PCSK9i, but in some circumstances it might be used before PCSK9i, being more cost-effective compared to more expensive therapies. In particular, in high and very high ASCVD risk patients not at LDL-C goal, BA alone might be utilized, on top of high intensity statin plus ezetimibe, when the distance from LDL-C goal is less than 20%. BA in FDC with ezetimibe, instead, might be used, on top of high intensity statins, when the distance from LDL-C goal is less than 40%.
Finally it should be recognize that, although clinical studies enrolled mainly patients at high and very high risk, BA or BA/ezetimibe in FDC might be used in moderate ASCVD risk patients, to contribute to the LDL-C reduction and might have a role in primary prevention, especially in patients with insulin resistance, metabolic syndrome and non-alcoholic fatty liver disease.
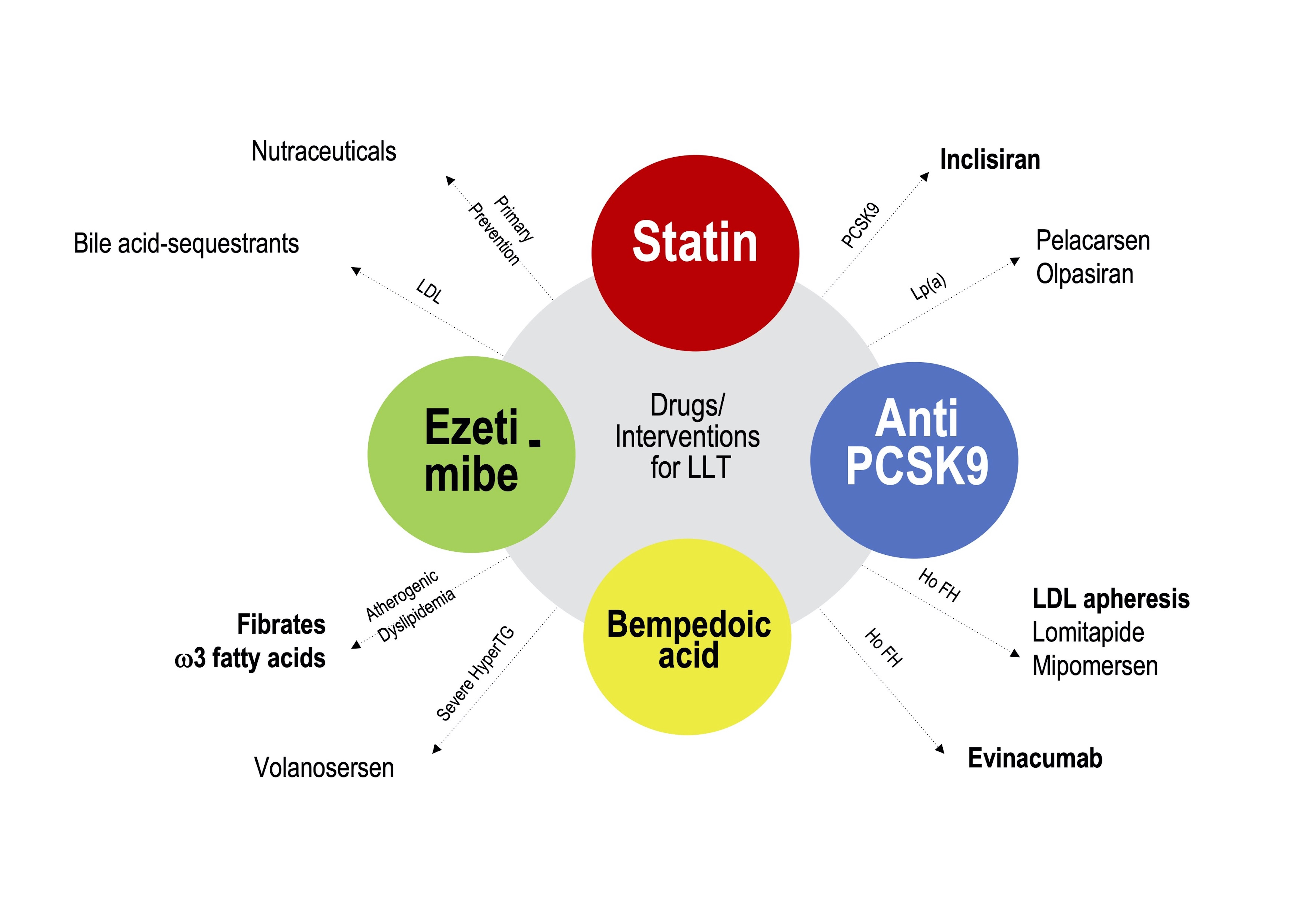
In conclusion, the pharmacokinetic and pharmacodynamic characteristics of BA make this drug a very useful tool in control LDL-C especially in high/very high/extreme ASCVD risk patients which, in a framework of personalized medicine, will play, along with other lipid lowering therapies, an important role in Preventive Cardiology. Indeed, in the next years, several molecules are expected to be introduced in the clinical practice. Many of them will permit an individual tailoring of the therapies directed non only toward LDL-C control but also to non LDL-C targets such as anti-apoCIII or anti-ANGPTL3 agents or anti-Lp (a) RNA therapeutics. For now, however, as illustrated in Fig. 2 (Ref. [27]), BA rapresents along with statin, ezetimibe and PCSK9i one of the 4 pillars of the modern lipid lowering treatment.
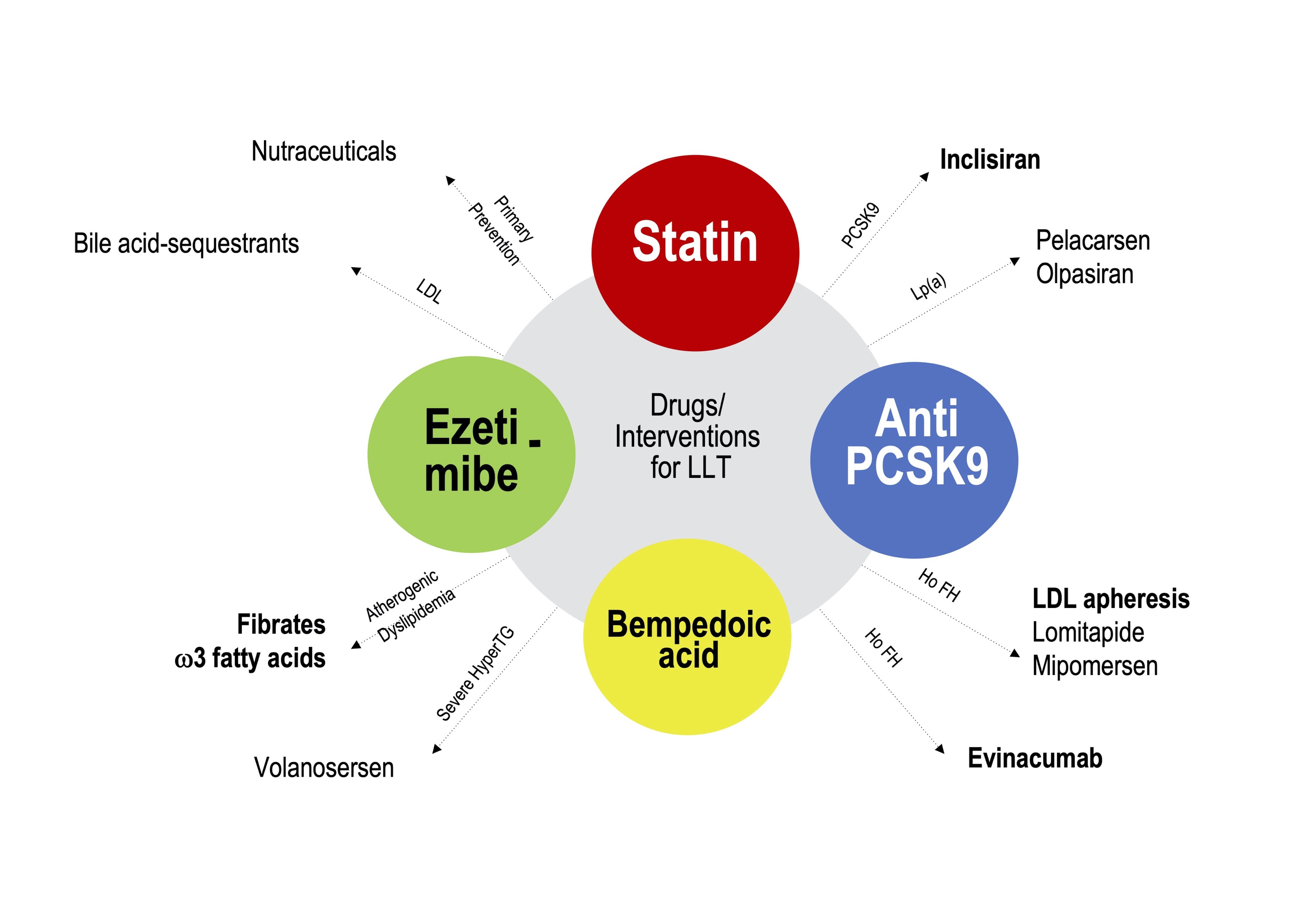 Fig. 2.
Fig. 2.The current lipid lowering available drugs. Modified from: Johann Bauersachs, Heart failure drug treatment: the fantastic four, Eur Heart J (2021) 42, 681–683. (Ref. [27])
ASCVD, atherosclerotic cardiovascular diseas; BA, Bempedoic acid; CV, cardiovascular; CAD, Coronary Artery Disease; FDC, fixed-dose combination; GFR, glomerular filtration rate; HeFH, heterozygous familial hypercholesterolemia; hs-CRP, high sensitive C-reactive protein; HMGCR, Hydroxy-methylglutaryl coenzyme A reductase; LDL-C, LDL-cholesterol; TEAE, Treatment-emergent adverse events; ULN, upper limit of normal; ACSVL1, Very-long-chain acyl-CoA synthetase-1.
Conceptualization, CB, GS and MA; methodology, CB, GS and MA; validation, GS and MA; formal analysis, CB, MA; investigation, CB, GS and MA; resources, CB; data curation, CB, GS and MA; writing—original draft preparation, CB; writing—review and editing, CB, GS and MA; supervision, GS, MA. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.