1 Department of Cardiology, The First Affiliated Hospital of Sun Yat-sen University, 510080 Guangzhou, Guangdong, China
2 Department of Medicine College, Sun Yat-sen University, 510080 Guangzhou, Guangdong, China
†These authors contributed equally.
Academic Editors: Matteo Bertini, Bernard Belhassen and Fabian Sanchis-Gomar
Abstract
Background: Alcohol septal ablation (ASA) has been more commonly
applied in medical refractory hypertrophic obstructive cardiomyopathy (HOCM)
compared with septal myectomy (SM), however its potential to create a
proarrhythmic substrate is increased. Methods: A systematic search was
performed in PubMed, EMBASE, Web of Science, and the Cochrane Library from
inception to October 2020. Fixed or random effects models were used to estimate
the risk ratios (RR) for ventricular arrhythmia events or other outcomes between
the SM and ASA cohorts. Results: Twenty studies with 8025 patients were
included. Pool analysis showed that the incidence of ventricular tachycardia
(VT)/ventricular fibrillation (VF), which included appropriate implantable
cardioverter defibrillator (ICD) intervention, was significantly higher in the
ASA cohort than that in the SM cohort (ASA vs SM: 10% (345/3312) vs 5%
(161/3227) (RR = 1.98, 95% CI (confidence interval), 1.65–2.37; p
Keywords
- hypertrophic cardiomyopathy
- septal reduction therapy
- alcohol septal ablation
- septal myectomy
- ventricular arrhythmias
- meta-analysis
Hypertrophic cardiomyopathy (HOCM) is the most common inherited cardiovascular disease. The majority of patients have an abnormally thickened ventricular septum, which could lead to systolic anterior motion of the mitral valve and obstruction of the left ventricular outflow tract (LVOT) [1]. Initial pharmacological therapy including beta-blockers and verapamil produces a negative inotropic effect to relieve the obstruction [2, 3]. In patients who are refractory to medical treatments, septal reduction therapy (SRT) is indicated. Surgical septal myectomy (SM) and alcohol septal ablation (ASA) are the two most common SRTs [4]. Although there is no randomized controlled study (RCT) comparing these two treatments, an increasing number of ASAs are being performed due to its reduced invasiveness [5].
Unlike the direct resection of the hypertrophic cardiac muscle, ASA works by inducing an iatrogenic myocardial infarction. In theory, it could be a potential substrate for ventricular arrhythmias and sudden cardiac deaths (SCD) [6, 7]. Although the possible proarrhythmic properties of ASA has been proposed since its emergence [8], this has not been substantiated in more recent studies [9, 10]. Furthermore, previous meta-analyses or systematic reviews comparing SM and ASA, such as the latest two from Mohammed et al. [11] and Ibadete et al. [12] in 2019 and 2020, respectively, rarely compared ventricular arrhythmic events. Therefore, in the current study, we focused on the incidence of post-procedure ventricular arrhythmic events between these two therapies. The aim was to analyze whether ASA would increase the risk of ventricular arrythmias.
The preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement was followed in this meta-analysis. Due to the study design, neither institutional review board (IRB) approval nor informed patient consent was needed.
A systematic search was performed in PubMed-Medline, EMBASE, the Cochrane
Library and Web of Science databases using the terms “hypertrophic obstructive
cardiomyopathy”, “idiopathic hypertrophic sub-aortic stenosis”, “asymmetric
septal hypertrophy”, “septal reduction therapy”, “septal myectomy”, “Morrow
septal myectomy”, “modified morrow septal myectomy”, “alcohol septal
ablation” and “percutaneous transluminal myocardial ablation”. No time limit
to the start date was applied, and the search was conducted up to October 2020.
The detailed search strategies are presented in Supplementary Table 1.
The inclusion criteria included (a) studies comparing the outcomes of ASA and SM;
(b) enrolled patients
Two examiners (WT, ML) independently screened the titles and abstracts (if
available) of the entries identified in different databases. Next, the full text
of all studies that met the eligibility criteria or those with insufficient
information from the titles or abstracts to make a decision, were obtained for
the next screening phase. All studies that did not meet the criteria were
excluded, and the reasons for exclusion were noted. Case reports and series,
review articles, editorials and duplicate reports were excluded. For those
studies that reported the same study or utilized the repeated data at different
follow-up intervals, we pooled all the relevant details together and used the
most comprehensive data for further analysis. A third member (JL) would further
check the data whenever there was disagreement until a consensus was reached.
Data from the included studies were then extracted by two independent reviewers
(WT, ML) based on a predesigned outline. The extracted information included the
design of the study, study population (number of participants, age, and sex),
length of follow-up, clinical characteristics and outcomes such as the pre- and
post-procedure left ventricular outflow tract pressure gradient, sustained
ventricular tachycardia or ventricular fibrillation (VT/VF) events (including
appropriate implantable cardioverter defibrillator (ICD) intervention) during and
post-procedure, SCD and resuscitated sudden cardiac arrest (SCA) events
post-procedure, the ICD implantation rate and permanent pacemaker (PPM)
implantation rate post procedure, the reintervention rate and all-cause or
cardiac mortality post procedure. Additional details are presented in Table 1
(Ref. [10, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31]). VF/VT events were divided into an early-phase (which occurred
during the procedure, during hospitalization or within 30 days after the
procedure) and a late-phase (which occurred
| Study, year | No. of patients (n) | Age (mean |
Male (%) | Resting LVOT PG (mmHg) | Post-procedure LVOT PG (mmHg) | Prior ICD (n) | Follow-up (years) | Reported outcomes | ||||||
| ASA | SM | ASA | SM | ASA | SM | ASA | SM | ASA | SM | ASA | SM | |||
| Nagueh SF, 2001 [31] | 41 | 41 | 49 |
49 |
NA | NA | 76 |
78 |
8 |
4 |
NA | NA | 1 | Mortality, PPM/ICD, VA |
| Qin JX, 2001 [30] | 25 | 26 | 63 |
48 |
28 | 62 | 64 |
62 |
28 |
7 |
NA | NA | 0.25 | Mortality, NYHA class, Reintervention, PPM |
| Firoozi S, 2002 [29] | 20 | 24 | 49 |
38 |
60 | 54 | 91 |
83 |
22 |
15 |
NA | NA | 2 | Mortality, PPM, NYHA class |
| Jiang TY, 2004 [28] | 43 | 11 | 45 (13–74) | 36 (11–69) | NA | NA | 76 |
95 |
20 |
12 |
NA | NA | 2 | Mortality, NYHA class, VA |
| Ralph-Edwards A, 2005 [27] | 54 | 48 | 59 |
46 |
48 | 62 | 74 |
64 |
15 (0, 96) | 5 (0, 17) | NA | NA | 2.2 | Mortality, PPM |
| Van der Lee C, 2005 [26] | 43 | 29 | 52 |
44 |
NA | NA | 101 |
100 |
23 |
17 |
NA | NA | 1 | Mortality, NYHA class, PPM, VA, reintervention |
| Valeti US, 2007 [25] | 24 | 24 | 62 |
50 |
50 | 62.5 | 76 |
75 |
7 |
3 |
NA | NA | 1.2 | CMR outcomes, VA, PPM |
| Ten Cate FJ, 2010 [24] | 91 | 40 | 54 |
49 |
55 | 53 | 92 |
86 |
NA | NA | 0 | 4 | 5.4 | Mortality, SCD, VA, PPM |
| Sorajja P, 2012 [23] | 177 | 177 | 63 |
62 |
32 | 32 | 70 |
67 |
NA | NA | 8 | 12 | 5 | Mortality, SCD, VA, PPM |
| Steggerda RC, 2014 [21] | 161 | 102 | 59 |
56 |
53 | 46 | 32 (18–75) | 50 (25–75) | 10 (7–19) | 9 (4–10) | 4 | 3 | 5.1 | Mortality, NYHA class, VA, reintervention |
| Samardhi H, 2014 [22] | 47 | 23 | 57 |
47 |
55 | 43.5 | 74.0 |
75.5 |
27.2 |
12.9 |
3 | 3 | 2 | Mortality, NYHA class, VA, reintervention, PPM |
| Vriesendorp PA, 2014 [20] | 321 | 253 | 58 |
52 |
55 | 54 | 102 |
92 |
10 |
9 |
NA | NA | 7.5 | Mortality, SCD, VA |
| Sedehi D, 2015 [19] | 52 | 171 | 57.3 |
48.0 |
56 | 49 | 67.1 |
67.4 |
23.9 |
11.2 |
6 | 0 | 3.2 | NYHA class, survival, PPM |
| Yang YJ, 2016 [18] | 22 | 37 | 45.5 |
44.6 |
80 | 67 | 79.7 |
69.0 |
43.7 |
15.0 |
0 | 0 | 1 | NYHA class, CMR outcomes, VA |
| Cavigli L, 2018 [17] | 55 | 71 | 49 |
42 |
42 | 62 | 70 |
52 |
22 |
11 |
1 | 6 | 5 | Mortality, SCD, ICD/PPM, reintervention |
| Guo HC, 2018 [16] | 68 | 158 | 42 |
37 |
63 | 49 | 70.30 |
74.58 |
39.78 |
13.95 |
NA | NA | 2 | Mortality, VA, ICD/PPM, reintervention |
| Nguyen A, 2019 [10] | 167 | 334 | 65 |
64 |
44.3 | 45.8 | 65 (29–100) | 60 (32–85) | 5 (0–15) | 0.0 (0.0–3.0) | NA | NA | 2.6 | NYHA class, Survival, ICD/PPM |
| Kimmelstiel C, 2019 [15] | 99 | 378 | 66.3 |
52.7 |
37 | 58 | 65.7 |
58.0 |
NA | NA | NA | NA | 4.0 | Mortality, NYHA class, ICD/PPM, VA, reintervention |
| Lemor A, 2020 [13] | 2245 | 2113 | 62.0 |
53.5 |
41.3 | 46.5 | NA | NA | NA | NA | 245 | 285 | 5 | Mortality, VA, ICD/PPM |
| Afanasyev AV, 2020 [14] | 105 | 105 | 52.2 |
51.9 |
52.4 | 54.3 | 72 (48–90) | 78 (63–90) | 10 (0–20) | 12 (8–20) | NA | NA | 4 | Mortality, SCD, PPM, Reintervention |
Abbreviations: LVOT, left ventricular outflow tract; PG, pressure gradient; ICD, implantable cardioverter defibrillator; ASA, alcohol septal ablation; SM, septal myectomy; NYHA, New York heart association; VA, ventricular arrhythmias; SCD, sudden cardiac death; PPM, permanent peacemaker; CMR, cardiac magnetic resonance. | ||||||||||||||
Since all of the included studies were observational, the assessment of the risk of bias was evaluated by a modified version of the Newcastle-Ottawa scale (NOS), which is a quality assessment tool for nonrandomized studies in three domains: the selection of participants, comparability of study groups, and the outcome of interest. The risk of bias in each study was evaluated by calculating the aggregate score on the 9 items. The detailed assessment of each study is shown in Supplementary Table 2.
The data analysis was conducted by Review Manager 5.4 (The Cochrane
Collaboration, Oxford, England) and Stata (version 15.1, StataCorp, College
Station, TX, USA). Continuous variables were reported as the means
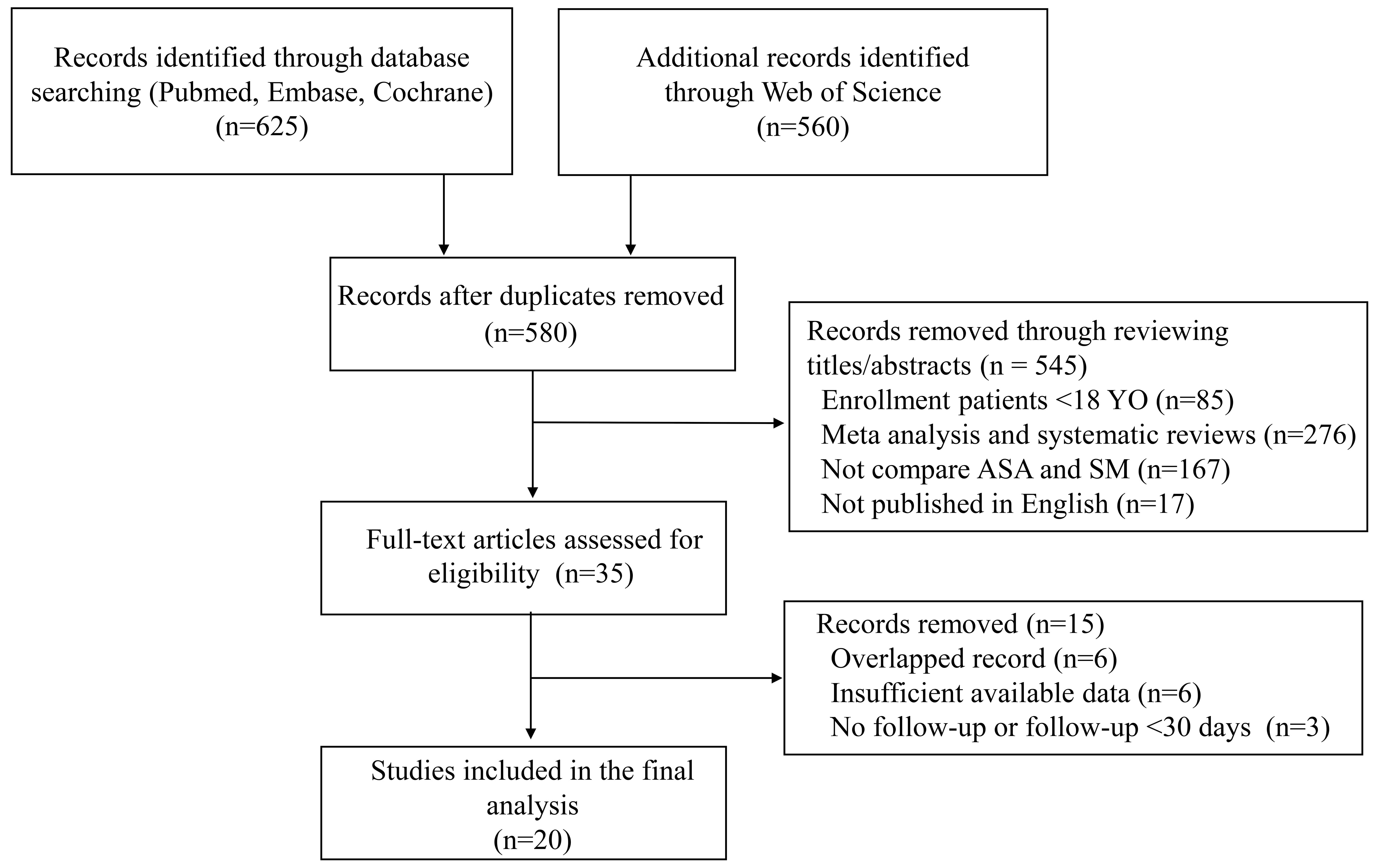
A total of 1185 articles were initially retrieved from PubMed, Embase, the Web of Science, and the Cochrane Library. After removing duplicates, 580 articles were left for title and abstract review. Thirty-five full-text articles were further assessed for eligibility, and 20 studies were ultimately included for data extraction and analysis [10, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31] (Fig. 1). All studies were observational studies. The risk of bias was evaluated by the Newcastle Ottawa Scale (Supplementary Table 2). Among them, 5 studies acquired 6/9 points, 12 studies acquired 7/9 points, and the remaining 3 studies acquired 8/9 points.
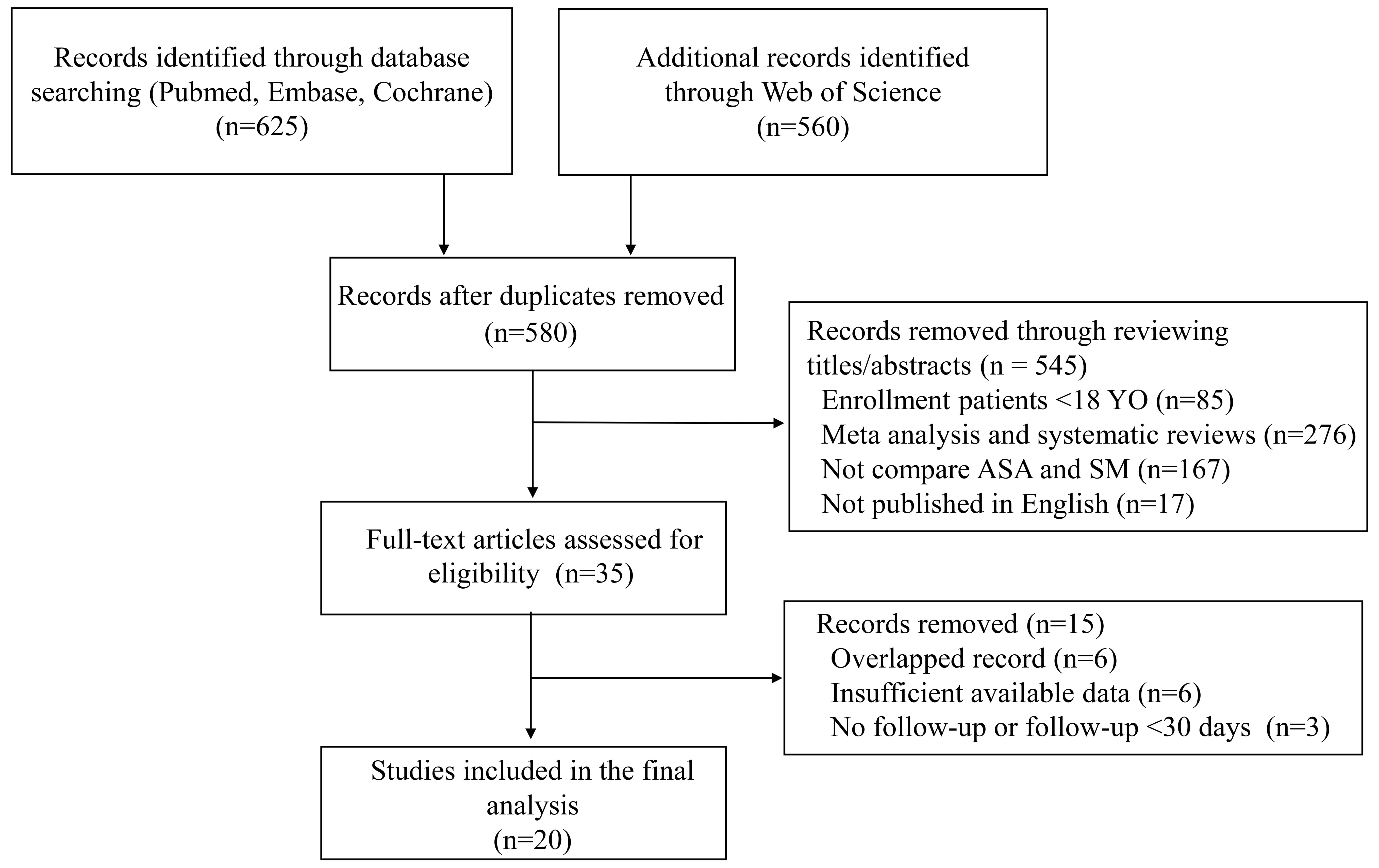 Fig. 1.
Fig. 1.Flow chart of literature search.
The study characteristics are presented in Table 1. A total of 8025 patients
were included in the 20 studies. Among them, 3860 patients received ASA
treatment, and the remaining 4165 patients underwent SM. The mean follow-up
periods varied from 3 months to 120 months. Of the 20 enrolled studies, twelve
studies had a specific description of VT/VF events (including appropriate ICD
intervention), eight studies contained descriptions about SCD and resuscitated
SCA, and ten studies collected data about ICD implantation. The baseline LVOT
pressure gradient was higher in the ASA groups (WMD = 5.89; 95% CI: 3.03–8.75;
p
Among the 20 enrolled studies, 12 studies contained descriptions of VT/VF events
(including ICD intervention). The pooled analysis showed that the incidence of
total VT/VF events was almost twice as high in the ASA group (345/3312, 10.42%)
than in the SM group (61/3227 patients, 4.99%) (RR = 1.98; 95% CI: 1.65–2.37;
p
 Fig. 2.
Fig. 2.Comparisons of ventricular fibrillation (VT)/ventricular tachycardia (VF)/appropriate ICD intervention events between ASA groups and SM groups. (a) Total VF/VT events. (b) Early-phase VF/VT events. (c) Late-phase VF/VT events.
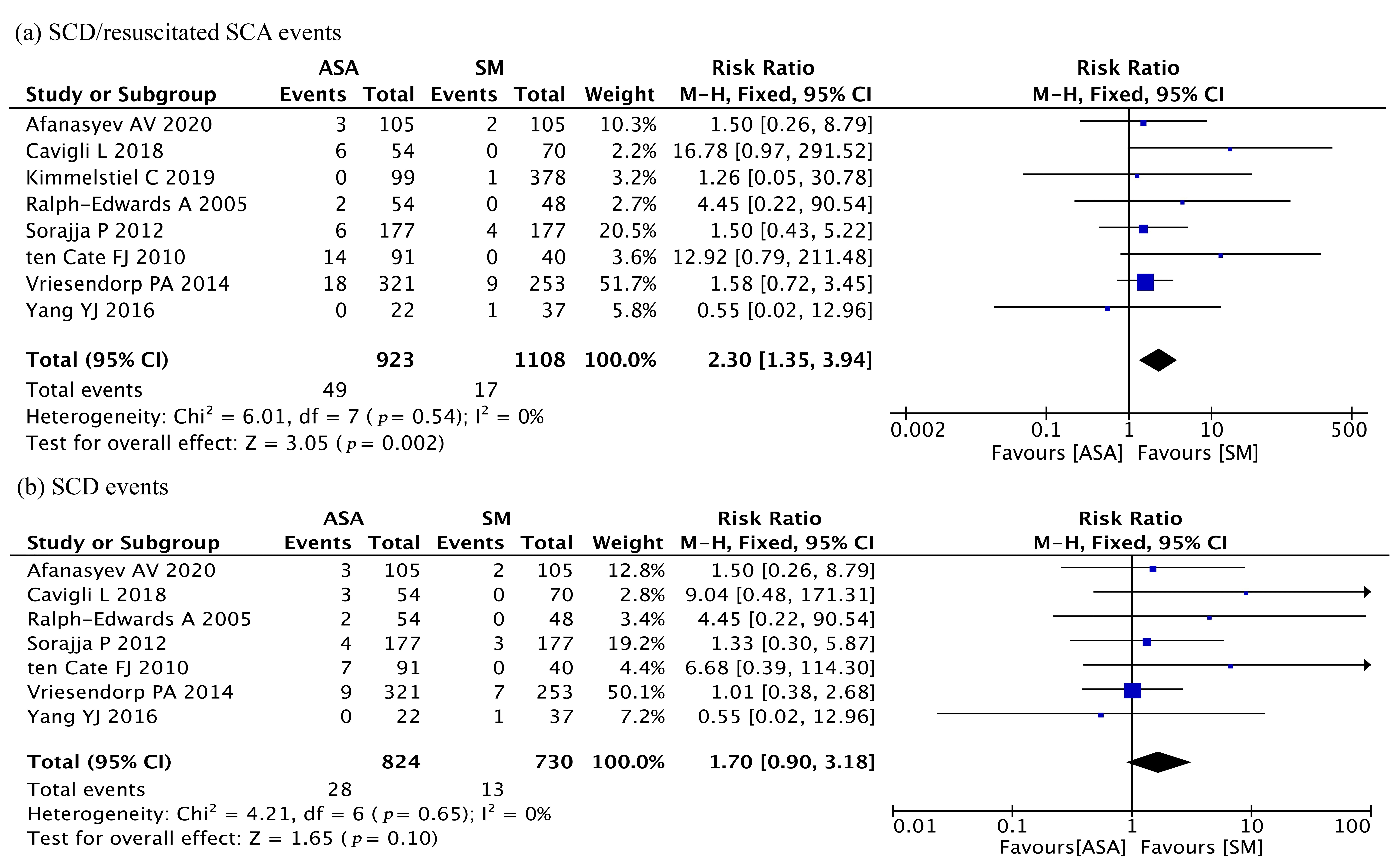
The SCD and resuscitated SCA were combined as SCA to estimate the risk for SCD
together in this analysis. In the 8 studies that described SCD and/or
resuscitated SCA events, a total of 49 events were reported in the ASA group
(SCD: 26; resuscitated SCA: 23), and 17 events were reported in the SM group
(SCD: 13; resuscitated SCA: 4). The pooled analysis showed that the ASA group had
a higher incidence of SCA (RR = 2.30; 95% CI: 1.35–3.94; p = 0.002;
I
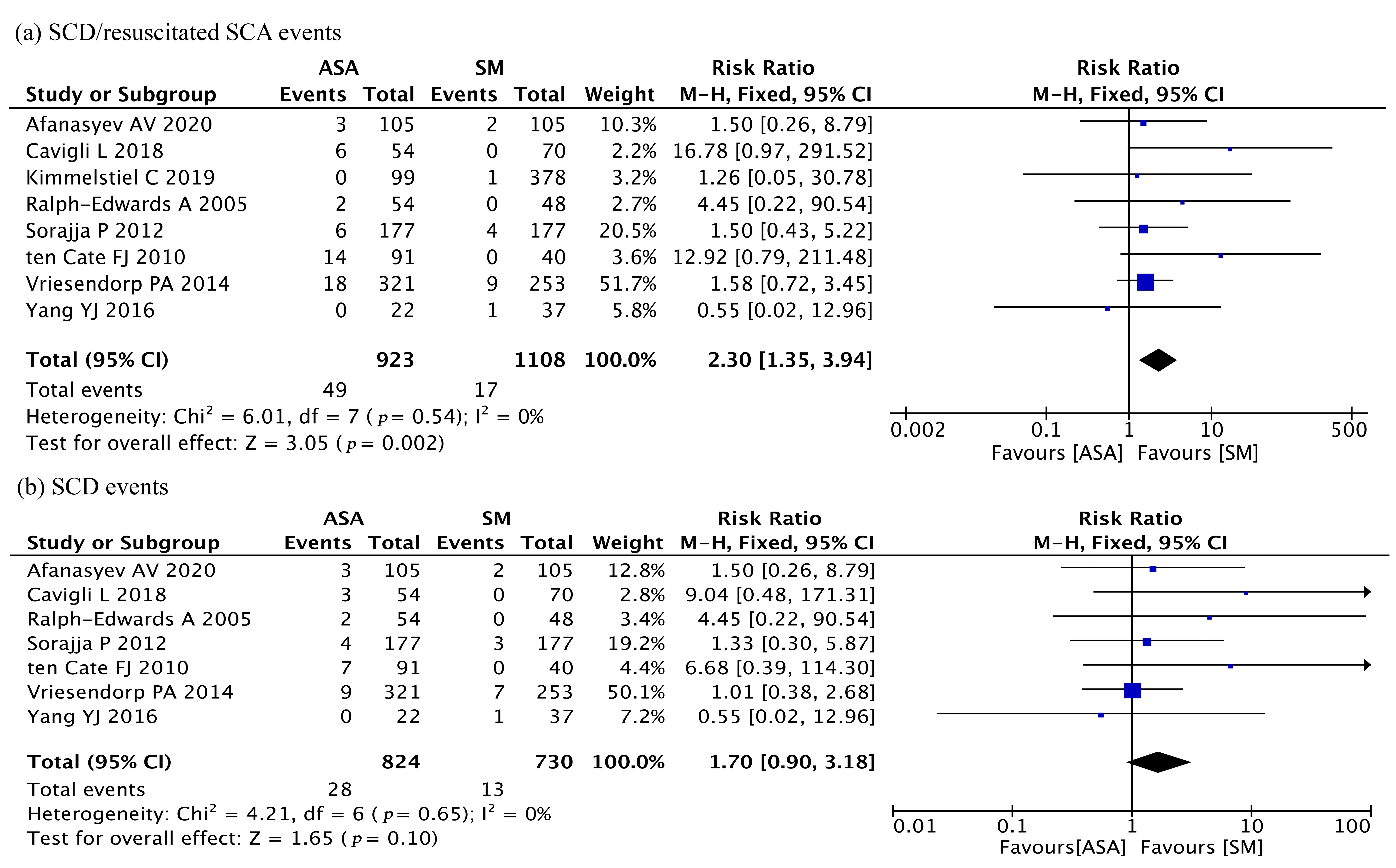 Fig. 3.
Fig. 3.Comparison of the incidence of sudden cardiac death (SCD)/resuscitated sudden cardiac arrest (SCA) after ASA and SM. (a) Combined SCD/resuscitated SCA events. (b) SCD events alone.
All-cause mortality within 30 days and above 30 days after receiving ASA or SM
was regarded as early-phase and late-phase mortality, respectively. For the ASA
cohorts, there were 38 early-phase deaths and 89 late-phase deaths among 3361
(1.13%) and 1055 patients (8.43%), respectively, while in the SM cohorts, there
were 75 early-phase deaths and 88 late-phase deaths among 3520 (2.13%) and 1304
patients (6.75%), respectively. The pooled analysis showed no significant
differences between ASA and SM in either early-phase mortality (RR = 0.72, 95%
CI: 0.37–1.41; p = 0.34; I
| Post-procedure outcomes | Number of studies | ASA group | SM group | RR | 95% CI | p value | I | ||
| Event | Total patients | Event | Total patients | ||||||
| Early-phase death | 11 | 38 | 3361 | 75 | 3520 | 0.72 | 0.37–1.41 | 0.34 | 31.00 |
| Late-phase death | 9 | 89 | 1055 | 88 | 1304 | 1.04 | 0.63–1.69 | 0.89 | 39.00 |
| PPM implantation | 17 | 404 | 3474 | 240 | 3864 | 1.99 | 1.39–2.83 | 50.00 | |
| ICD implantation | 10 | 123 | 1208 | 89 | 1589 | 1.67 | 0.98–2.86 | 0.06 | 64.00 |
| Reintervention | 8 | 68 | 603 | 5 | 892 | 10.50 | 5.10–21.64 | 0.00 | |
| Abbreviation: ASA, alcohol septal ablation; SM, septal myectomy; PPM, permanent peacemaker; ICD, implantable cardioverter defibrillator; RR, risk ratio; CI, confidence interval. | |||||||||
After ASA, 404 patients were implanted with a permanent pacemaker in the 3474
pooled patients (11.63%), which was significantly higher compared with
(240/3864, 6.21%) after SM (RR = 1.99; 95% CI: 1.39–2.83; p = 0.0002;
I
Our results showed that the risk of ventricular arrhythmias (VT/VF and appropriate ICD intervention) and SCA (SCD and resuscitated SCA) were higher in the ASA cohort than in the SM cohort. Additionally, pacemaker implantation and reintervention were required more often in the ASA group, but there was no significant difference in all-cause mortality between the two groups.
Due to several metabolic, autonomic, and electrophysiological changes,
myocardial infarction can be arrhythmogenic and is closely related to lethal
arrhythmias and SCD [32]. Hence the iatrogenic myocardial infarction resulting
from ASA increases the potential risk for life-threatening arrhythmias [1, 6, 7, 8].
However, in recent years, these concerns have not been substantiated [9, 33].
Therefore, the latest 2020 American Heart Association/American College of
Cardiology guideline still indicate that further studies are needed [34]. In this
analysis, we compared the incidence of VT/VF between the ASA and SM groups. The
results showed that the ASA cohort had a higher risk of VT/VF, even when it was
divided into the early phase (during the procedure, during hospitalization or
within 30 days after the procedure) and late phase (
SCD is one of the most devastating complications of HOCM. Most recent studies and meta-analyses showed no significant difference in SCD between ASA and SM [39]. In this analysis, we also did not find that the incidence of SCD was significantly different in the ASA and SM groups. However, when we regarded SCD and resuscitated sudden cardiac arrest (SCA) as a single event (SCA), the ASA group had more than twice the incidence than the SM group. This suggests that patients receiving ASA were more likely to be exposed to SCD and more dependent on effective resuscitation or ICD intervention. This was consistent with the higher incidence of malignant ventricular arrhythmias after ASA.
Our meta-analysis also found some results consistent with those of previous studies [11, 39]. ASA is as effective as SM in decreasing LVOT pressure and relieving obstructive symptoms. Patients receiving ASA are more likely to require permanent peacemakers and reinterventions. ASA and SM carry similar low risk for early- or late-phase mortality. Both therapies tend to be more effective in reducing symptoms than medical management alone. In those patients who are refractory to adequate medical therapy, the results of our meta-analysis may be helpful in determining the selection of ASA vs SM for an individual patient in order to achieve the best clinical outcomes.
There are several limitations in this study. First, the absence of an RCT to compare ASA and SM inevitably brings about the concern for selection bias since we conducted a sensitivity analysis regarding the incidence of VF/VT, which further supports our results. In addition, as the study from Lemor et al. [13] collected the data from the National Readmission Database, its study size was much larger than that of other studies. Thus, it was weighted as the absolute majority in the pooled analysis, which could lead to some bias; however, this concern could also be relieved by the sensitivity analysis, where we could obtain the same result even if this study were excluded from the cohorts. Another limitation is that we included some studies from 20 years ago. Since that time, there have been significant improvements in both but the development of ASA and SM techniques; however, Liebregts et al. [39] found no association between the study period and all-cause mortality.
Patients with HOCM who underwent ASA had nearly more than twice the risk of VF/VT events. Most events occurred during the procedure or during hospitalization or within 30 days post-procedure. In addition, patients who receive ASA were more likely to be exposed to SCD and resuscitated SCA.
ASA, Alcohol septal ablation; SM, Septal myectomy; SRT, Septal reduction therapy; HOCM, Hypertrophic obstructive cardiomyopathy; LVOT, Left ventricular outflow tract; VT, Ventricular tachycardia; VF, Ventricular fibrillation; SCD, Sudden cardiac death; SCA, Sudden cardiac arrest; ICD, Implantable cardioverter defibrillator; PPM, Permanent pacemaker; IVSd, Intraventricular septal diameter; RR, Risk ratio; WMD, Weighted mean difference; CI, Confidence interval; NYHA, New York Heart Association.
WT and ML contributed equally to this article. WT performed the article search and data extraction regarding the two treatments and was also a major contributor in writing the manuscript. ML analyzed and interpreted the final included data. JL and RC designed and prepared the tables and figures. CS and XZ were responsible for revising the manuscript. LW designed the work. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.