1 Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, University at Buffalo – SUNY, Buffalo, NY 14214, USA
Academic Editor: Lee Stoner
Abstract
Cardiovascular disease (CVD) is the leading cause of death among adults in the U.S. and elsewhere. Variation in the presence, severity, and control of major modifiable risk factors accounts for much of the variation in CVD rates worldwide. Cardiorespiratory fitness (CRF) reflects the integration of ventilation, circulation, and metabolism for the delivery and utilization of oxygen in support of dynamic aerobic physical activity. The gold standard measure of CRF is maximal oxygen uptake. Because the primary factor underlying differences in this measure between individuals is maximal cardiac output, it can serve as a clinical indicator of cardiac function. Higher CRF is associated with favorable levels of major CVD risk factors, lower prevalence and severity of subclinical atherosclerosis, and lower risks of developing both primary and secondary clinical CVD events. The beneficial associations between CRF and CVD are seen in women and men, older and younger adults, in those with multiple coexisting risk factors or prior diagnosis of CVD. Exercise training and regular physical activity of at least moderate intensities and volumes improves CRF in adults, and improvements in CRF are associated with lower risks of subsequent CVD and mortality. Routine assessment of CRF in primary care settings could enhance individual-level CVD risk assessment and thereby guide implementation of appropriate measures to prevent future clinical events.
Keywords
- heart disease
- exercise
- physical activity
- maximal oxygen uptake
- risk assessment
- exercise prescription
- prognosis
Cardiorespiratory fitness (CRF) is a strong, independent predictor of future cardiovascular clinical events and mortality [1, 2, 3]. When measured carefully in a clinical setting, CRF has been more strongly associated with cardiovascular outcomes than other exercise test responses including patient symptoms, electrocardiographic and hemodynamic factors [4, 5]. CRF reflects assimilation of anatomical, physiological, biochemical, and neuromuscular inputs that represent far more than an individual’s exercise habits. As such, CRF is considered a hallmark of aging resiliency [6]. While CRF is a recognized biomarker of physical function and cardiovascular health, it is not currently included with other established risk factors, such as blood pressure or cholesterol, in clinical practice guidelines for cardiovascular disease (CVD) risk assessment. Routine assessment of CRF in primary care settings not only will provide the physician with valuable clinical data on their patient’s health status, but could potentially foster health behavior changes to improve CRF knowing that it is part of the annual health record along with body weight, blood pressure, and other vital signs [5].
Beginning around 1950, numerous scientific publications have documented the relationship of physical activity (PA) and cardiorespiratory fitness (CRF) with cardiovascular health and disease [1, 2, 3]. Not surprising, these investigations have differed substantially with respect to study population, size and design, the cardiovascular outcome investigated, and the type of assessment used to measure PA or CRF. Nevertheless, the overwhelming finding among the studies of higher quality (e.g., adequate sample size and statistical power, well-documented quantification of PA or CRF) has been consistency in cardiovascular health benefits associated with higher levels of activity and fitness. Because of their relatively high prevalence at the population level, the population attributable risk (e.g., percentage of disease cases attributed to a risk factor) for all-cause and cardiovascular mortality associated with sedentary behavior and low CRF is comparable to that of other major modifiable cardiovascular risk factors such as hypercholesterolemia, hypertension, and smoking [7, 8]. Table 1 (Ref. [7]) illustrates this showing population attributable risks of CVD mortality for low CRF and other modifiable CVD risk factors in adults ages 18–98 years who were without known CVD or cancer and followed an average of 17 years [7]. Assuming the association between CRF and CVD mortality is causal, if all individuals with low CRF improved to even moderate levels of CRF then 1 in 4 CVD deaths among women and men each in this population might have been avoided. Only hypertension accounted for a high proportion of deaths therein. While population attributable risk is a theoretical estimate, it does bring into context the force an exposure exerts on population health which depends on the amount of exposure and the strength of its association with CVD [9]. Because of the relatively high prevalence of low CRF and its strong association with CVD mortality, the potential population effect for delaying CVD mortality through increases in CRF is considerable. Indeed, leading authorities assert that low CRF could be the biggest public health threat of the 21st century [10] and, as such, CRF should be considered a standard clinical vital sign assessed regularly and targeted for modification just like other conventional risk factors monitored for cardiovascular health [11]. Because measured CRF is less prone to misclassification resulting from response biases or behavioral reactivity as compared to self-reported or directly monitored PA habits, CRF may better reflect the adverse consequences of a sedentary lifestyle [12]. This might not only be because due to more reliable measurement than reported PA levels, but also because CRF may better reflect the combined effects of genetics and behavior in determining an individual’s health status.
| Men (N = 40,872) | Women (N = 12,943) | |||||
| Risk Factor | P |
HR (95% CI) | PAR% | P |
HR (95% CI) | PAR% |
| Low CRF | 42.9 | 2.78 (2.29, 2.89) | 29.9 | 41.2 | 3.32 (2.31, 4.78) | 28.8 |
| Self-reported sedentary | 52.7 | 1.27 (1.11, 1.42) | 11.2 | 51.9 | 1.36 (0.93, 1.99) | 13.7 |
| Obesity | 19.3 | 2.08 (1.81, 2.39) | 9.9 | 13.7 | 3.01 (1.82, 4.97) | 9.2 |
| Current smoker | 25.5 | 1.51 (1.33, 1.72) | 8.6 | 19.1 | 1.61 (1.03, 2.51) | 7.2 |
| Hypertension | 56.9 | 2.23 (1.99, 2.49) | 31.4 | 50.4 | 3.24 (2.29, 4.57) | 34.8 |
| Hypercholesterolemia | 43.2 | 1.68 (1.51, 1.88) | 17.4 | 38.2 | 1.68 (1.18, 2.39) | 15.5 |
| Diabetes | 15.8 | 2.26 (1.94, 2.62) | 8.8 | 9.2 | 3.55 (1.96, 6.44) | 6.6 |
| HR (95% CI) adjusted for age and examination year. P Adapted from LaMonte MJ. Epidemiology of Cardiovascular Disease. In: JL Durstine, GE Moore, MJ LaMonte, BA Franklin (eds.) Pollock’s Textbook of Cardiovascular Disease and Rehabilitation (pp. 9–22). Human Kinetics: Champaign, IL. 2008. [7]. | ||||||
The objective of this report is to overview the cardiovascular health benefits associated with greater levels of CRF in both primary and secondary CVD prevention. Key points will be illustrated using results from selected individual studies that are frequently cited in consensus statements and systematic reviews. Streams of evidence from both observational and experimental studies will be discussed when possible.
CRF is one of several physiological attributes collectively referred to as
physical fitness, the other attributes being body composition, muscular strength
and endurance, agility, balance, and reaction time [13]. CRF (also referred to as
cardiovascular, cardiopulmonary, aerobic, or endurance fitness) reflects the
ability of the cardiopulmonary system to supply oxygen to working skeletal
muscles, and of muscles to effectively utilize oxygen to support performance of
dynamic PA [13]. CRF, thus, reflects an integrated system that links
ventilation (O
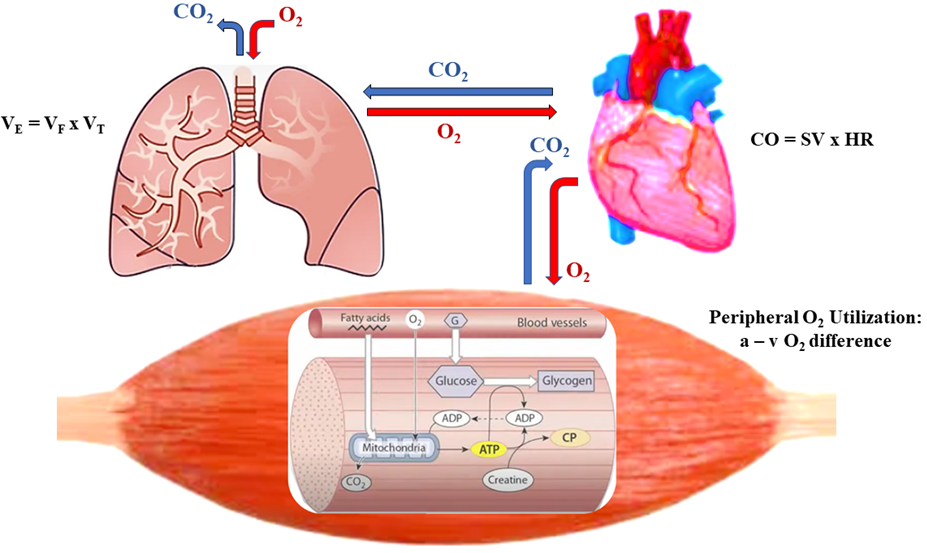 Fig. 1.
Fig. 1.Ventilation
In clinical settings CRF is often used as a measure of exercise tolerance or
physical functioning capacity expressed as metabolic equivalent (METs) or
multiples of resting oxygen uptake [4, 19]. One MET (resting oxygen
uptake) is assumed over a wide adult age range to be 3.5 mL O
| Age (years) | Percentile | Men | Women | ||
| mL O |
METs | mL O |
METs | ||
| 20–39 | |||||
| 20th–40th | 36.4–40.9 | 10.4–11.7 | 28.7–32.9 | 8.2–9.4 | |
| 40th–60th | 40.9–45.6 | 11.7–13.1 | 32.9–36.4 | 9.4–10.4 | |
| 60th–80th | 45.6–50.4 | 13.1–14.4 | 36.4–40.9 | 10.4–11.7 | |
| 40–49 | |||||
| 20th–40th | 34.7–37.8 | 9.9–10.8 | 26.6–29.8 | 7.6–8.5 | |
| 40th–60th | 37.8–42.7 | 10.8–12.2 | 29.8–32.9 | 8.5–9.4 | |
| 60th–80th | 42.7–47.3 | 12.2–13.5 | 32.9–37.8 | 9.4–10.8 | |
| 50–59 | |||||
| 20th–40th | 29.8–34.7 | 8.5–9.9 | 23.5–26.6 | 6.7–7.6 | |
| 40th–60th | 34.7–37.8 | 9.9–10.8 | 26.6–29.8 | 7.6–8.5 | |
| 60th–80th | 37.8–43.1 | 10.8–12.3 | 29.8–33.6 | 8.5–9.6 | |
| 20th–40th | 25.2–29.8 | 7.2–8.5 | 20.3–23.5 | 5.8–6.7 | |
| 40th–60th | 29.8–33.3 | 8.5–9.5 | 23.5–26.6 | 6.7–7.6 | |
| 60th–80th | 33.3–37.8 | 9.5–10.8 | 26.6–30.1 | 7.6–8.6 | |
| METs, metabolic equivalents; 1 MET = 3.5 mL O Adapted from Sui X, LaMonte MJ, Blair SN. American Journal of Epidemiology. 2007; 65: 1413–1423. [25]. | |||||
CRF can be measured using both submaximal and maximal exercise tests and a
variety of testing modalities in laboratory and field settings [19, 33, 34]. Direct
quantification of
Shorter timed walk tests, such as the 400 meter and 6-minute walk, are readily
used in clinical and epidemiological settings to assess physical function status
as well as to predict
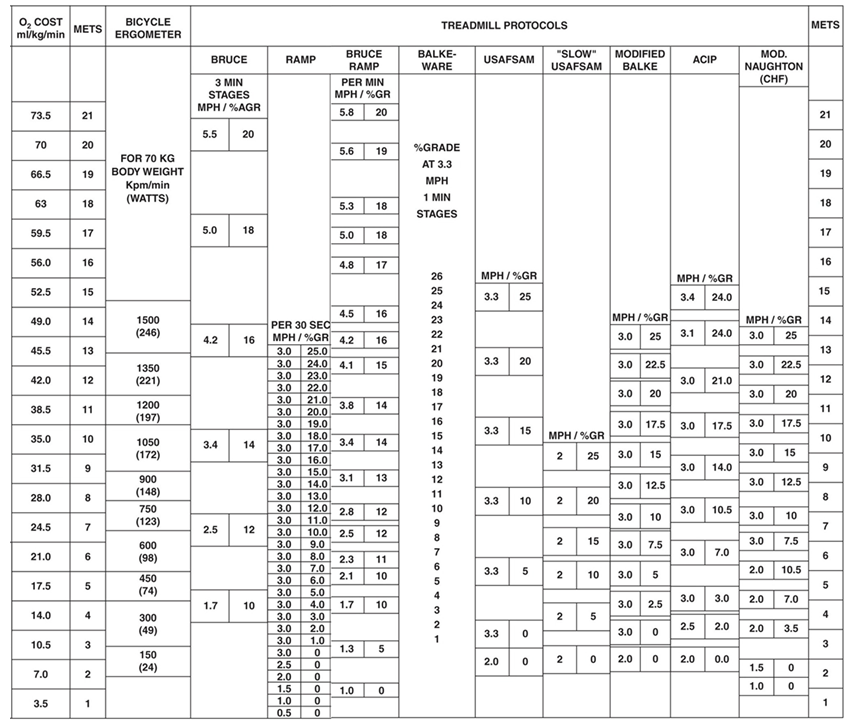 Fig. 2.
Fig. 2.Oxygen uptake according to various workloads and exercise
testing modality. O
Atherosclerosis, the underlying disease process of most CVD deaths in U.S. adults, is a complex process that starts early in life and progresses over decades in a subclinical state before manifestation of clinical CVD events in mid- to later life [7]. Interaction of environmental factors and individual-level susceptibility traits lead to development of major modifiable CVD risk factors which initiate formation of atherosclerotic lesions within the coronary arteries. If unchecked, the disease progresses and eventually presents clinically as angina, myocardial infarction, or sudden cardiac death. As illustrated in Fig. 3, there are several plausible pathways through which higher CRF impacts the initiation and progression of atherosclerotic CVD for both primary and secondary prevention of clinical CVD events. The following sections will briefly review evidence supporting this conceptual framework.
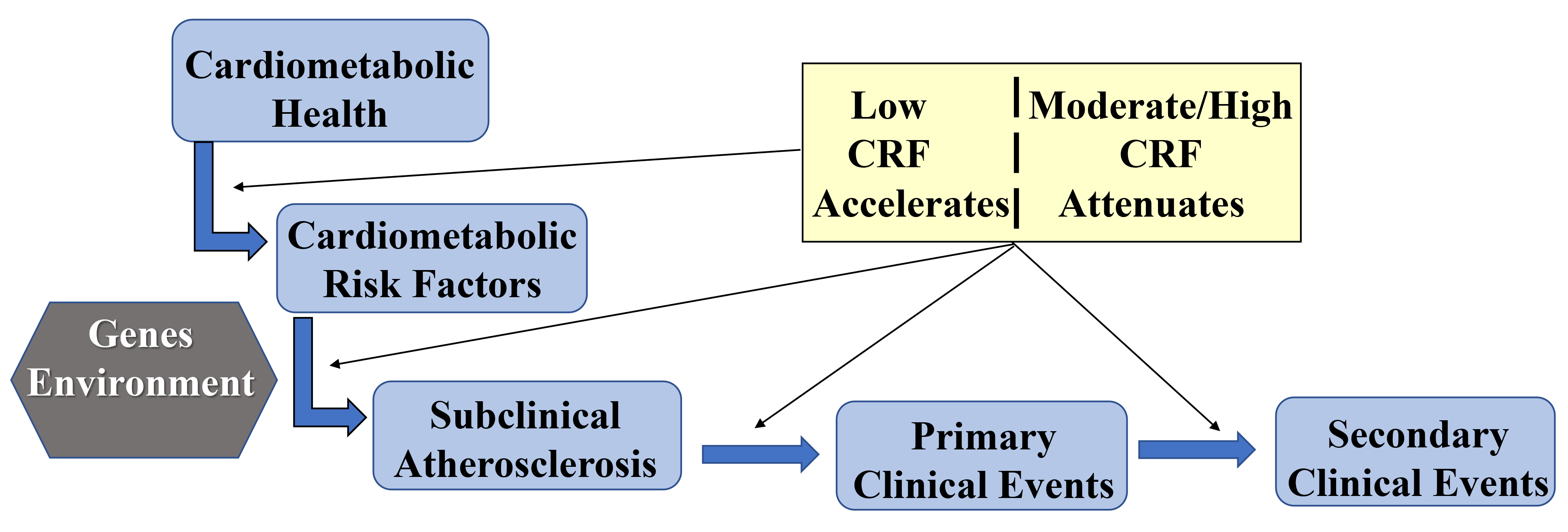 Fig. 3.
Fig. 3.Conceptual framework of CRF pathways to CVD prevention.
Variation in the presence, severity, and control of major modifiable CVD risk factors is a principal determinant of differences in CVD burden between populations [50, 51]. In the U.S. National Health and Nutrition Examination Survey between 2007 and 2018, trajectories for some modifiable risk factors (current smoking, leisure-time physical activity, serum total cholesterol) showed significant improvement whereas other risk factors (body mass index, dietary intake, blood pressure, serum glucose and hemoglobin A1c) worsened during the same time interval [52]. Variation in CVD risk factors was clearly evident according to subgroups defined by age (worse in older adults) and race and ethnicity (worse in black compared to white and Hispanic adults). While widespread use of pharmacotherapies to control major CVD risk factors is likely benefiting certain factors (e.g., lipids [53]), there remains a substantial burden of untoward risk factors in the population that will increase with an aging society and will translate into higher frequency of clinical CVD events if not brought into check [54]. Use of nonpharmacologic behavioral strategies to enhance risk factor control is, therefore, of high importance to preventive cardiology and public health.
Higher CRF is associated with favorable levels of traditional CVD risk factors
in cross-sectional studies of women and men with [55, 56] and without [56, 57, 58]
existing CVD. Fig. 4 (Ref. [56]) shows inverse associations for CRF, assessed by maximal
treadmill testing, with prevalence of clinically relevant CVD risk factors [56].
CRF is also associated with lower prevalence of coexisting cardiometabolic
factors, metabolic syndrome [59, 60], in cohorts of middle-aged adults
who were without known CVD at examination. The inverse association between CRF
and prevalent metabolic syndrome is quite steep. Among 7104 women whose mean age
was 44 years at the time of completing a symptom-limited maximal treadmill
fitness test, the age, smoking, and exam year-adjusted prevalence of metabolic
syndrome across incremental quintiles of CRF was 19%, 6.7%, 6.0%, 3.6%, and
2.3%, respectively (Trend, p
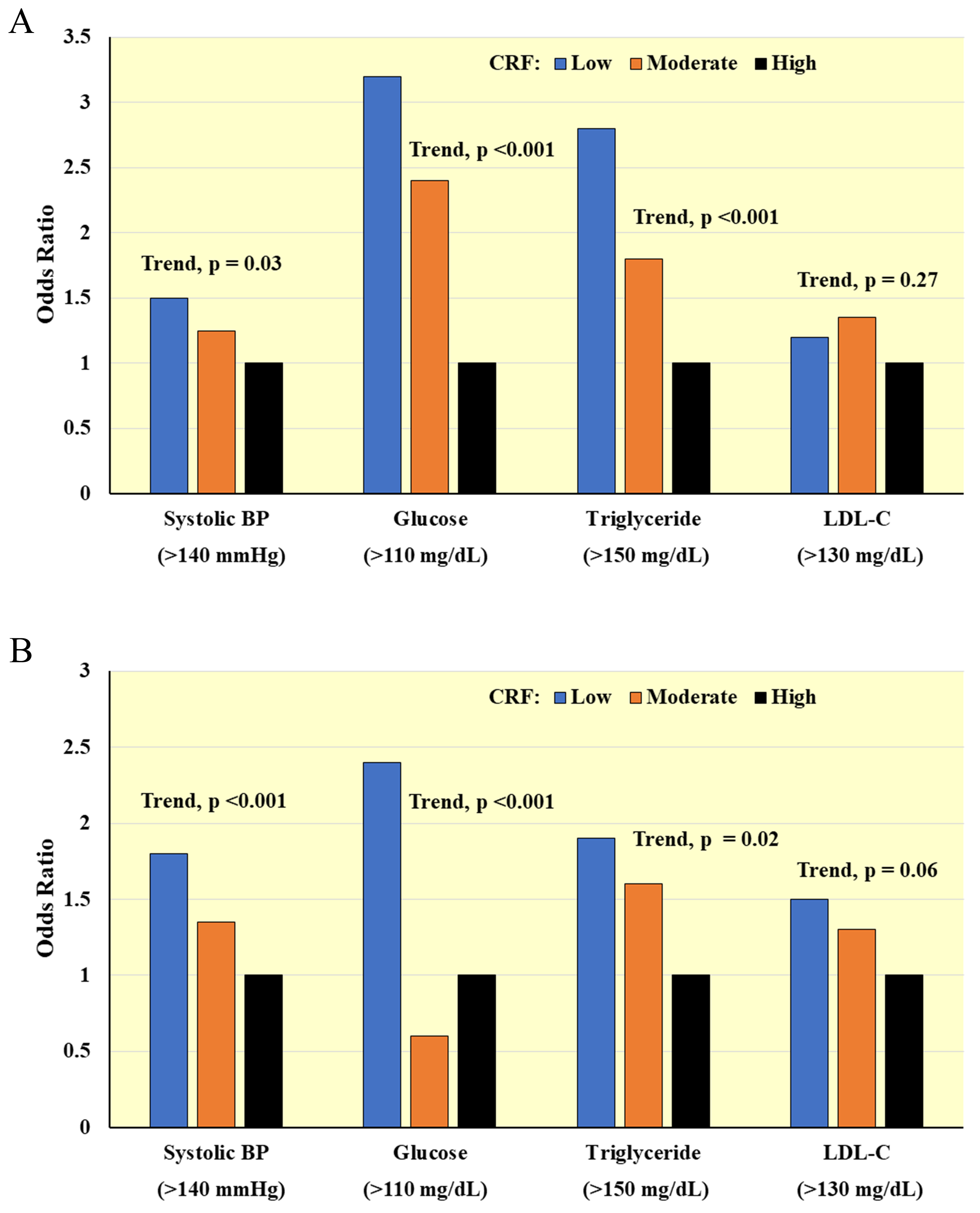 Fig. 4.
Fig. 4.Cross-sectional associations between CRF and clinically relevant CVD risk factors in (A) Men and (B) Women. Odds ratios adjusted for age, percent body fat, smoking, and family history of CVD. BP, blood pressure; LDL-C, low-density lipoprotein cholesterol. Adapted from LaMonte MJ et al., Circulation. 2000; 102(14): 1623–1628. [56].
CRF has also been associated with other biomarkers of cardiovascular health.
Higher CRF is favorably related to pulse wave velocity [62] and coronary arterial
diameter [63] and dilating capacity [64] (measures of arterial compliance), heart
rate variability [65, 66] (measure of cardiac autonomic function), pericardial
adipose deposition [67], and measures of cardiac size and function in adults
residing in the community setting [68, 69, 70, 71, 72]. In one study that evaluated
nitroglycerin-induced coronary vasodilation between runners and sedentary
controls, there was a 2-fold greater increase in arterial cross-sectional area
following nitroglycerin in runners that correlated (r = 0.68) strongly with
Evidence that CRF levels are predictive of future development of clinically
relevant risk factors would provide stronger inferences as compared to the
cross-sectional findings reviewed above. However, few studies have examined
prospective associations between a measure of CRF and incidence of
cardiometabolic risk factors. One of the most comprehensive studies to date was
reported in the CARDIA cohort where 2029 men and 2458 women, mean age 25 years at
the time of maximal treadmill fitness testing, were followed for 15 years [82].
In analysis adjusted for demographic, anthropometric, family history, and
self-reported PA information, the relative risk of incident hypertension,
diabetes, metabolic syndrome, and elevated low-density lipoprotein cholesterol
among non-obese participants was 1.21, 1.26, 1.28, and 1.08 (p
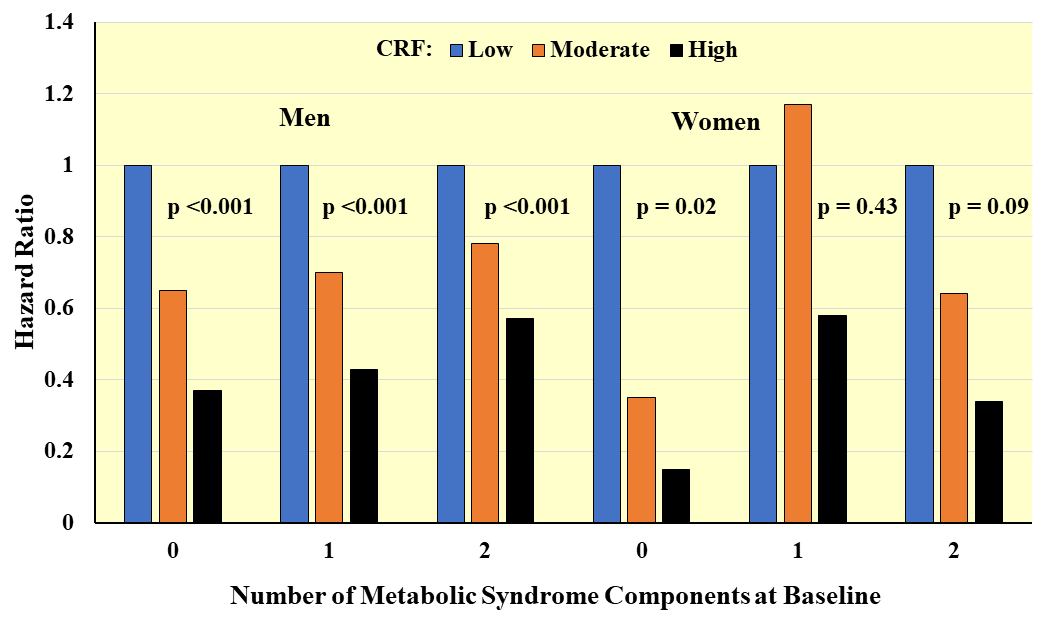 Fig. 5.
Fig. 5.Prospective associations between CRF and incident metabolic syndrome according to number of components at baseline. Hazard ratios adjusted for age, exam year, BMI, smoking, alcohol, family history of CVD and diabetes. Adapted from LaMonte MJ et al., Circulation. 2005; 112(4): 505–512. [83].
The ability to characterize atherosclerotic CVD while in its subclinical stage
provides new opportunities for arresting disease progression and preventing
clinical CVD events [88]. Several measures of subclinical disease have been used
in epidemiological and clinical investigations, some of which have been evaluated
against CRF levels. Higher CRF is associated with fewer resting and exercise
electrocardiographic indicators of obstructive coronary atherosclerosis in
asymptomatic adults [25, 47, 89]. An extensive analysis on 3722 Korean men, ages 40
and older without clinical CVD, included measurements of
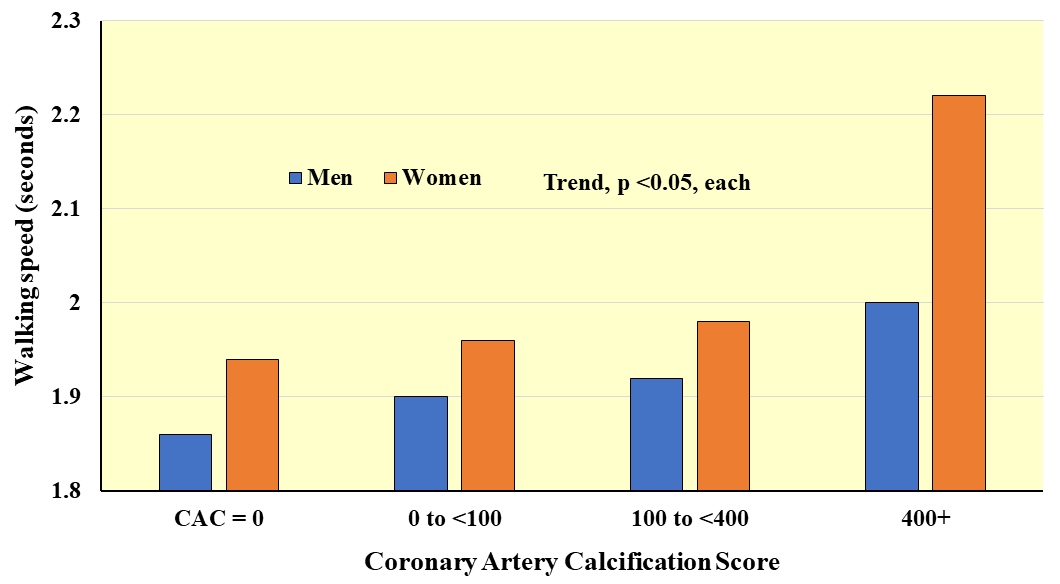 Fig. 6.
Fig. 6.Average walking speed over 8 feet according to coronary arterial calcification score. Adapted from Hamer M et al., Heart. 2010; 96(5): 380–384. [95].
A large body of epidemiological evidence supports inverse associations between CRF and the incidence of several primary clinical CVD outcomes [1, 2, 3]. Additionally, randomized controlled trials have demonstrated that aerobic exercise training in medically managed patients with existing CVD is safe and efficacious in the secondary prevention of recurrent events and mortality [99, 100, 101]. Guidelines have been published regarding the type, amount, and intensity of PA required to improve CRF and clinical cardiovascular status in both primary and secondary prevention settings [13, 34].
CVD Mortality. The seminal work was contributed by Blair and
coworkers who followed 13,344 adults ages 20–88 years for about 8 years after
completion of a maximal treadmill fitness test and showed steep inverse gradients
in age-adjusted rates of CVD mortality across incremental CRF tertiles in men
(24.6, 7.8, 3.1 per 10,000; Trend p
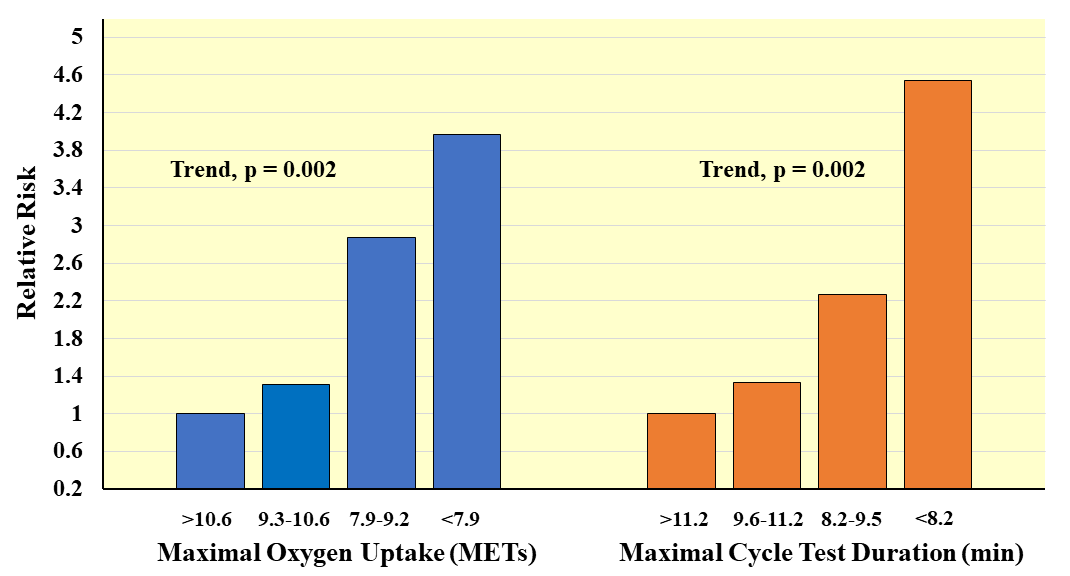 Fig. 7.
Fig. 7.Prospective association of measured maximal oxygen uptake and exercise test duration with CVD mortality in men. Relative risks adjusted for age and examination year. Adapted from Laukkanen JA, et al., Archives of Internal Medicine. 2001; 161: 825–831. [39].
Stroke Mortality. In an exceptionally large cohort of 1,166,035 Swedish men whose CRF was measured using maximal cycle ergometry at the time of entry into the military and who were followed 42 years for fatal stroke, the multivariable relative risks (95% CI) in the lowest and middle CRF tertile were 1.62 (1.35, 1.93) and 2.52 (1.82, 3.50), respectively, compared to high CRF [105]. Multivariable-adjusted relative risks for stroke mortality across incremental quartiles were 1.00, 0.47, 0.59, 0.50, Trend p = 0.004 in men and 1.00, 0.71, 0.62, 0.43, Trend p = 0.09 in women who completed maximal treadmill fitness testing in mid-life and were followed 17 years thereafter [106].
Non-fatal CVD. The vast majority of investigations on CRF and
CVD have evaluated fatal events as the study outcome. However, the role of CRF in
development on nonfatal clinical events is a critical piece of the primary
prevention framework. A 10-year follow-up subsequent to maximal treadmill fitness
testing showed significant inverse multivariable relative risks over tertiles of
CRF for nonfatal total CVD (1.00, 0.89, 0.75, p = 0.001), CHD (1.00,
0.89, 0.76, p = 0.001), MI (1.00, 0.87, 0.73, p = 0.02), and
stroke (1.00, 0.90, 0.71, p = 0.04) in 20,728 middle-aged men [25].
Among 5909 women in this study, inverse associations between CRF and each
nonfatal endpoint were observed but did not achieve statistical significance due
to the relatively small number of case counts. In Finnish men, each 1-MET
increment in measured
Population Subgroups. The protective association between CRF
and clinical CVD events also is apparent in higher risk clinical subgroups. In
40,718 men without CVD, significant inverse associations between CRF and CHD
mortality were observed in categories of
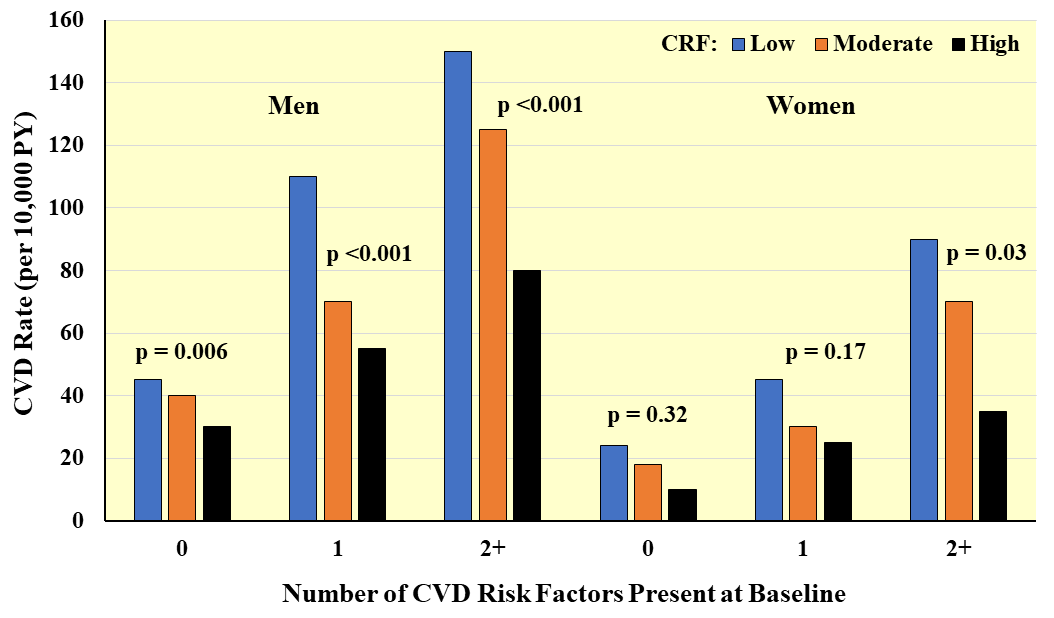 Fig. 8.
Fig. 8.Prospective association between CRF and CVD incidence according to number of major CVD risk factors present at baseline. Rates are adjusted for age and examination year. PY, person-years. Adapted from Sui X et al., American Journal of Epidemiology. 2007; 165: 1413–1423. [25].
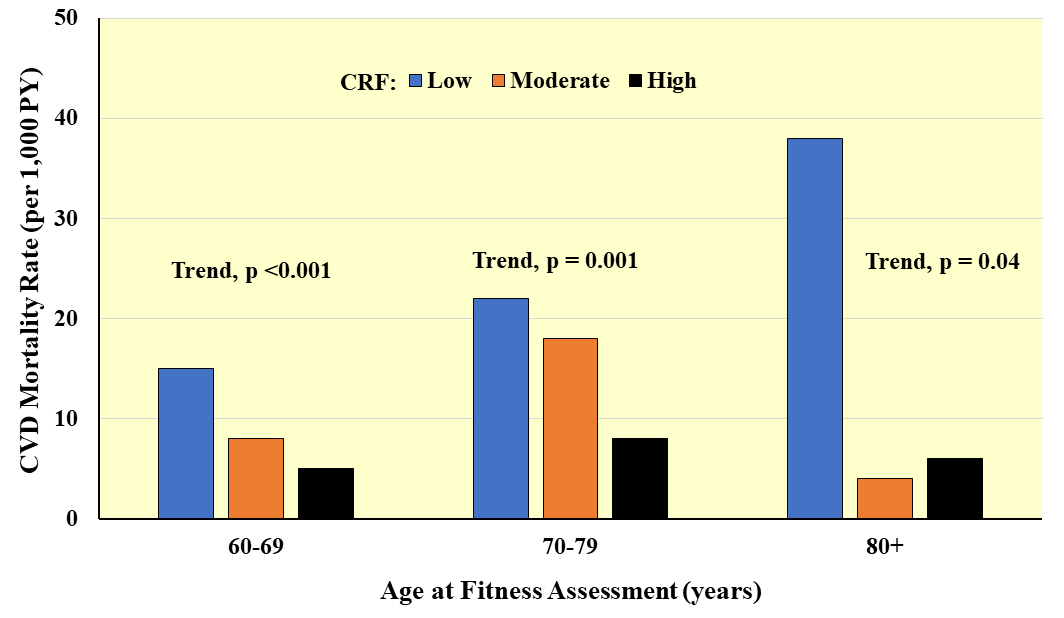 Fig. 9.
Fig. 9.Prospective association between CRF and CVD mortality according to age at baseline. Rates are adjusted for sex and examination year. PY, person-years. Adapted from Sui X et al., Journal of the American Geriatrics Society. 2007; 55: 1940–1947. [112].
Alternative CRF Indices. Several indicators of the hemodynamic
and autonomic response to exercise also have been associated with CVD risk. These
measures include insufficient increase in heart rate during exercise
(chronotropic incompetence) and a slow heart rate recovery following exercise,
and abnormal blood pressure responses during and after exercise. A prospective
study on 1910 male veterans showed that failure to achieve at least 80% of
age-predicted maximal heart rate during treadmill exercise testing was associated
with a 2.8-fold (p
Higher CRF in individuals who already have had a clinical CVD event is an
important prognostic factor. In both women and men completing supervised cardiac
rehabilitation following a clinical coronary event,
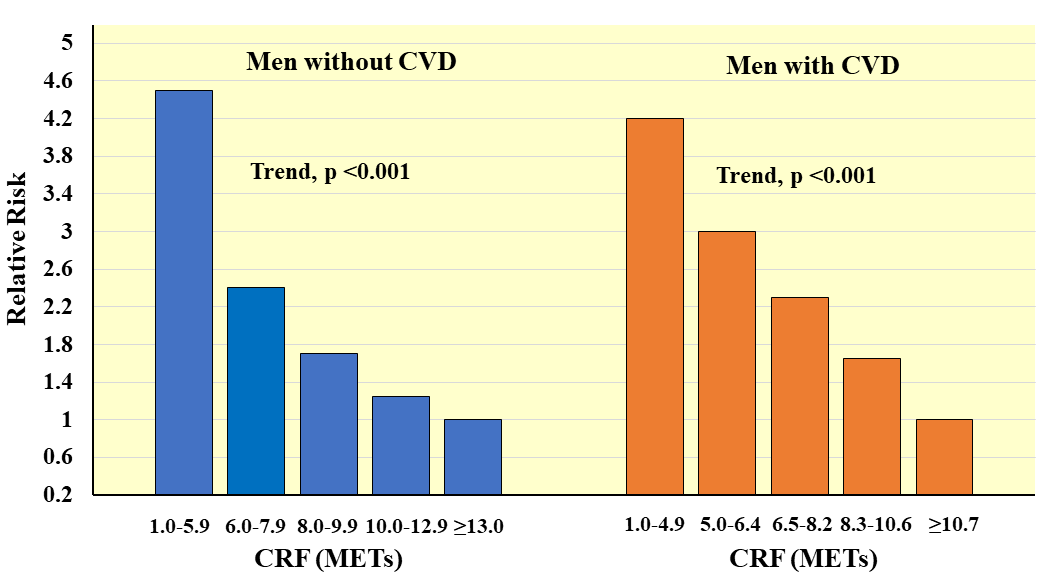 Fig. 10.
Fig. 10.Prospective association between CRF and all-cause mortality in men with and without CVD. Relative risks are adjusted for age. Adapted from Meyers J et al., New England Journal of Medicine. 2002; 346: 793–801. [42].
Studies evaluating longitudinal changes in CRF in relation to CVD outcomes
provide a stronger test of the hypothesis than do those based on only a single
assessment of CRF. Changes in CRF over two assessments are associated with
significantly lower risks of developing major CVD risk factors including
hypertension, diabetes, elevated cholesterol, and metabolic syndrome, to a large
extent independent of changes in body weight [82, 124]. In a follow-up on 2014 men
ages 40–50 at first of two maximal cycle ergometry assessments, the
multivariable-adjusted relative risks over incremental quartiles of CRF change
were 1.00, 0.64, 0.53, and 0.40 (p
One argument for CRF being a better reflection of exposure to sedentary
lifestyles than self-reported or device-measured PA is less misclassification due
to reporting biases and incomplete assessment of PA behavior [12]. Because CRF
represents an integrated response in several biological systems, including
genetics, required to support PA at given levels of effort, CRF might offer a
broader representation of the underlying construct at a physiological level. A
meta-analysis on observational studies that related either CRF or PA with
incident CVD events showed that for both CRF and PA exposures there was a
significant inverse pattern of association with CVD risk (p
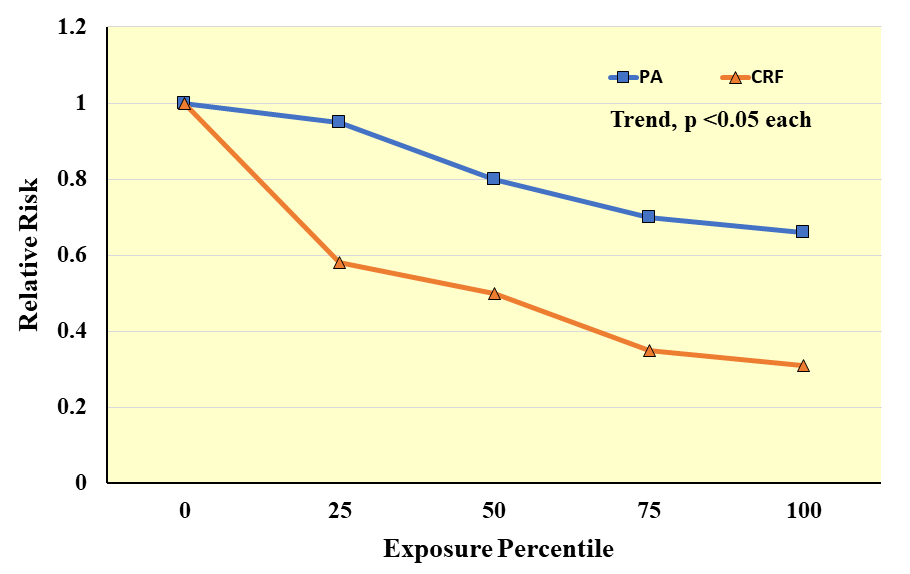 Fig. 11.
Fig. 11.Meta-analysis results of observational studies on cardiorespiratory fitness (CRF) or physical activity (PA) exposures in relation to the relative risk of clinical CVD events. Exposure percentiles are ranked lowest (0) to highest (100) on the x-axis. Adapted from Williams PT. Medicine & Science in Sports & Exercise. 2001; 33: 754–761. [127].
Few investigations have included assessments of both CRF and PA in the
same study group. In adults ages 18–95 years,
In the preceding sections it was clear that CRF is associated with one’s
propensity for adverse CVD outcomes. In an office setting, healthcare providers
typically rely on multiple risk factor scoring algorithms to determine their
patient’s short-term probability of a clinical CVD event and, in turn, guide
decisions on initiation and intensity of primary preventive measures [7]. A small
number of studies have attempted to quantify the additional prognostic value of
adding a measure of CRF to conventional office-based CVD risk calculation (e.g.,
Framingham Risk Score). Table 3 (Ref. [133]) summarizes results of a study on 41,708 men who
were without clinical CVD at the time of baseline examination that included a
maximal treadmill fitness test [133]. After 17 years follow-up, each 1-unit
increment in Framingham risk score (10-Year predicted probability) was associated
with a 6% higher relative risk of CVD and CHD mortality (p
| CVD mortality | CHD mortality | |||
| (1307 deaths) | (792 deaths) | |||
| FRS alone* | ||||
| FRS (per 1-unit increment) | 1.06 (1.04, 1.07) | 1.06 (1.05, 1.08) | ||
| FRS plus CRF* | ||||
| FRS (per 1-unit increment) | 1.03 (1.02, 1.06) | 1.02 (1.01, 1.06) | ||
| CRF (per 1-MET decrement) | 1.24 (1.21, 1.27) | 1.27 (1.22, 1.32) | ||
| Likelihood ratio statistic | 214.6 (p |
165.7 (p | ||
| Framingham risk score (10-year probability) | ||||
| 10–20% | ||||
| (Low risk) | (Intermediate risk) | (High risk) | ||
| CVD death | ||||
| CRF (per 1-MET decrement) | 1.21 (1.15, 1.27) | 1.15 (1.10, 1.22) | 1.18 (1.10, 1.25) | |
| CHD death | ||||
| CRF (per 1-MET decrement) | 1.21 (1.12, 1.28) | 1.22 (1.14, 1.29) | 1.16 (1.08, 1.27) | |
| Data are hazard ratio (95% confidence interval). *Model also includes age, examination year, and family history of CVD. Adapted from LaMonte MJ et al., Circulation. 2005; 112(Suppl II): II-829. [133]. | ||||
The overview presented here on CRF and CVD prevention was not an exhaustive review of the published scientific literature nor did it address all possible mechanisms by which greater CRF might enhance cardiovascular health. The exemplar studies discussed were selected to make specific points but may not represent the range of available findings in a given area. Future studies that include both a performance-based measure of CRF and a well-documented assessment of PA would be helpful to clarify the extent to which PA and CRF confer independent cardiovascular benefits, especially in older adults whose maximal CRF is limited. Continued efforts to identify an absolute level of CRF where CVD risk reduction would be expected in apparently healthy adults, and to identify the PA dose required to achieve that level of CRF, is critical to enhancing future public health recommendations on lifestyle behaviors.
As depicted conceptually in Fig. 3 and supported by evidence summarized herein,
CRF is a modifiable factor associated with multiple paths in CVD incidence and
prognosis. The gold standard measure of CRF is the maximal oxygen uptake
(
MJL is the sole author and is responsible for conceptualization; content design; writing original draft; review and editing; approval of the published version of the manuscript.
Not applicable.
Not applicable.
This work was supported by NHLBI contract 75N92019R0031 and research grants HL151885, HL153462, HL150170, HL130591.
The author declares no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.











