1 Emergency Department, Clinical and Rehabilitation Cardiology Unit, San Filippo Neri Hospital, ASL Roma 1, 00135 Rome, Italy
2 ANMCO Research Center, Heart Care Foundation, 50121 Florence, Italy
3 Cardiology Department, Interventional Cardiology Unit, Ospedale Santa Corona, 17027 Pietra Ligure, Italy
4 Cardiac Rehabilitation Unit, Rehabilitation Clinic “Villa delle Magnolie", Castel Morrone, 81100 Caserta, Italy
5 CardioThoracic and Vascular Department, San Martino Policlinico Hospital, IRCCS for Oncology and Neurosciences, 16100 Genoa, Italy
6 Department of Biomedical Sciences for Health, IRCCS Ospedale Galeazzi-Sant'Ambrogio, University of Milan, 20161 Milan, Italy
7 Division of Cardiology, Ospedale Santa Maria delle Grazie Pozzuoli, 80078 Napoli, Italy
8 Cardiovascula Department, Clinical and Interventional Cardiology Unit, Istituto Clinico Sant'Ambrogio, 20149 Milan, Italy
9 Dipartimento Cardio-Toraco-Vascolare, U.O.C. Cardiologia, Azienda Ospedaliera San Camillo Forlanini, 00152 Roma, Italy
10 U.O.C. Cardiologia, Ospedale Garibaldi-Nesima, Azienda di Rilievo Nazionale e Alta Specializzazione “Garibaldi", 95122 Catania, Italy
11 De Gasperis Cardio Center, Niguarda Hospital, 20162 Milano, Italy
Academic Editor: Brian Tomlinson
Abstract
Therapeutic approaches based on gene silencing technologies represent a new opportunity to manage hypercholesterolemia. Inclisiran is a small interfering RNA that targets proprotein convertase subtilisin/kexin type 9 (PCSK9) mRNA. Clinical studies have demonstrated that inclisiran is effective, safe, and well-tolerated in reducing low-density lipoprotein cholesterol (LDL-C) in patients with familial hypercholesterolemia, atherosclerotic cardiovascular disease, and atherosclerotic cardiovascular disease risk equivalents. A meta-analysis of phase 3 trials demonstrated a 51% reduction in LDL-C levels at 18 months as compared with placebo. Adverse event incidence was found to be comparable in individuals treated with inclisiran and those receiving placebo, though the reactions at the site of injection were more common in patients receiving inclisiran as compared with those receiving placebo. The recommended inclisiran dose is 284 mg administered as a subcutaneous injection to be repeated after three months with a subsequent 6-month maintenance regimen. Overall, since the pharmacological efficacy of inclisiran in LDL-C reduction is comparable to that of monoclonal antibodies against PCSK9, the longer effect duration and the favorable safety profile may favor this newer approach for hypercholesterolemia management.
Keywords
- hypercholesterolemia
- LDL-cholesterol
- inclisiran
- siRNA
- cardiovascular disease
Dyslipidemia is a modifiable cardiovascular risk factor that contributes to atherosclerosis pathogenesis. Alongside healthy lifestyle interventions, lipid-lowering therapies represent the cornerstone of cardiovascular disease (CVD) risk reduction [1]. It has been estimated that 1 mmol/L (38.67 mg/dL) decrease in low-density lipoprotein cholesterol (LDL-C), the leading cause of atherosclerosis, reduces cardiovascular event incidence by 22% [2]. However, although there has been a progressive growth in the last decade in the therapeutic armamentarium available for reducing LDL-C, substantial CVD risk persists.
According to guidelines, statins represent the first line of therapy. At present, for patients who do not reach LDL-C therapeutic goals, the recommendation is to combine statins with ezetimibe and, if this combination is still insufficient to achieve the desired goal, to add monoclonal antibodies against proprotein convertase subtilisin/kexin type 9 (PCSK9) [3].
Two monoclonal antibodies, evolocumab and alirocumab, are currently available to treat patients at increased risk for CVD. By interfering with PCSK9 binding to the LDL receptor, they reduce the lysosomal breakdown of the LDL receptors in hepatocytes. The final effect is similar to that of statins, leading to LDL receptor upregulation and greater circulating LDL-C clearance. PCSK9 inhibitor monoclonal antibodies lower LDL-C by 50–60% and reduce the risk for CVD proportionally to the LDL-C reduction obtained [4, 5]. A further lipid-lowering strategy with less widespread use in clinical practice includes lipoprotein apheresis [6], a non-pharmacological extracorporeal intervention that is recommended in patients with homozygous or heterozygous (if uncontrolled with drugs) familial hypercholesterolemia.
Despite the different lipid-lowering treatments currently available, there are still several concerns about side effects, costs, and adherence issues that limit optimal lipid control. Observational studies have shown a significant gap between LDL-C levels achieved in clinical practice and the recommended goals [7, 8]. This gap is even greater in patient populations at higher CVD risk [7, 8].
Statins are associated with poor adherence and a high discontinuation rate, and a sizeable share of patients do not attain LDL-C goals. Although statin-induced myopathy risk has been shown to be low, in observational studies statin-associated muscle symptoms are more frequent than those reported in randomized clinical studies (RCTs) and often result in statin therapy discontinuation with a consequent increase in adverse cardiovascular event risk [9]. In large U.S. observational studies, around 10% of patients suspend a statin because of subjective complaints, the most common of which is muscle symptoms [10]. Ezetimibe is generally better tolerated than statins, but it leads to a lower reduction in LDL-C levels. Targeting PCSK9 has recently changed the paradigm of lipid-lowering therapy since it leads to a greater reduction in LDL-C. However, monoclonal antibody PCSK9 inhibitors are currently not used on a large scale mainly because of their high cost.
Innovative therapeutic approaches based on gene silencing technologies represent a new opportunity to manage CVD risk due to dyslipidemia [11]. This new therapeutic strategy uses antisense oligonucleotides (ASOs) or small interfering RNAs (siRNAs) for silencing gene expression. ASOs are nucleotide single-stranded chains that combine with their target RNA, leading to its degradation and thereby determining reduced protein synthesis. siRNA molecules are double-stranded sequences whose antisense strand, once inside the cell, binds to its target mRNA sequence and inhibits protein synthesis by inducing its cleavage. Inclisiran is a new lipid-lowering agent in a late stage of development that acts by silencing PCSK9 gene expression in the liver cells, therefore acting upstream with respect to monoclonal antibodies that antagonize circulating PCSK9 activity. This novel approach could fill the gap in achieving LDL-C target levels in patients at increased risk for CVD. Indeed, although there are still no outcome results for inclisiran, the clinical use of this new lipid-lowering agent has been recently approved in several countries.
This paper discusses available evidence on this new therapeutic option. We briefly illustrate its mechanism of action and report available data on its efficacy and safety. Finally, we discuss its current therapeutic applications.
Inclisiran molecule is a synthetic RNA whose target is the mRNA that encodes PCSK9. In order to prevent rapid siRNA molecule degradation in the bloodstream, some chemical modifications, including 20-O-methyl and 20-fluoro nucleotides and some phosphorothioate linkages that are used in substitution of phosphodiester linkages, are introduced.
Furthermore, to direct siRNA uptake within hepatocytes and avoid unintended effects on other cells, the sense strand is conjugated with N-acetylgalactosamine (GalNAc), which combines with the asialoglycoprotein receptor present on the surface of the liver cells. The asialoglycoprotein receptor in turn mediates inclisiran endocytosis. The stability of siRNA molecules stored in intracitoplasmatic compartments, which act as intracell drug depots, may explain the persistence of inclisiran action over time [12].
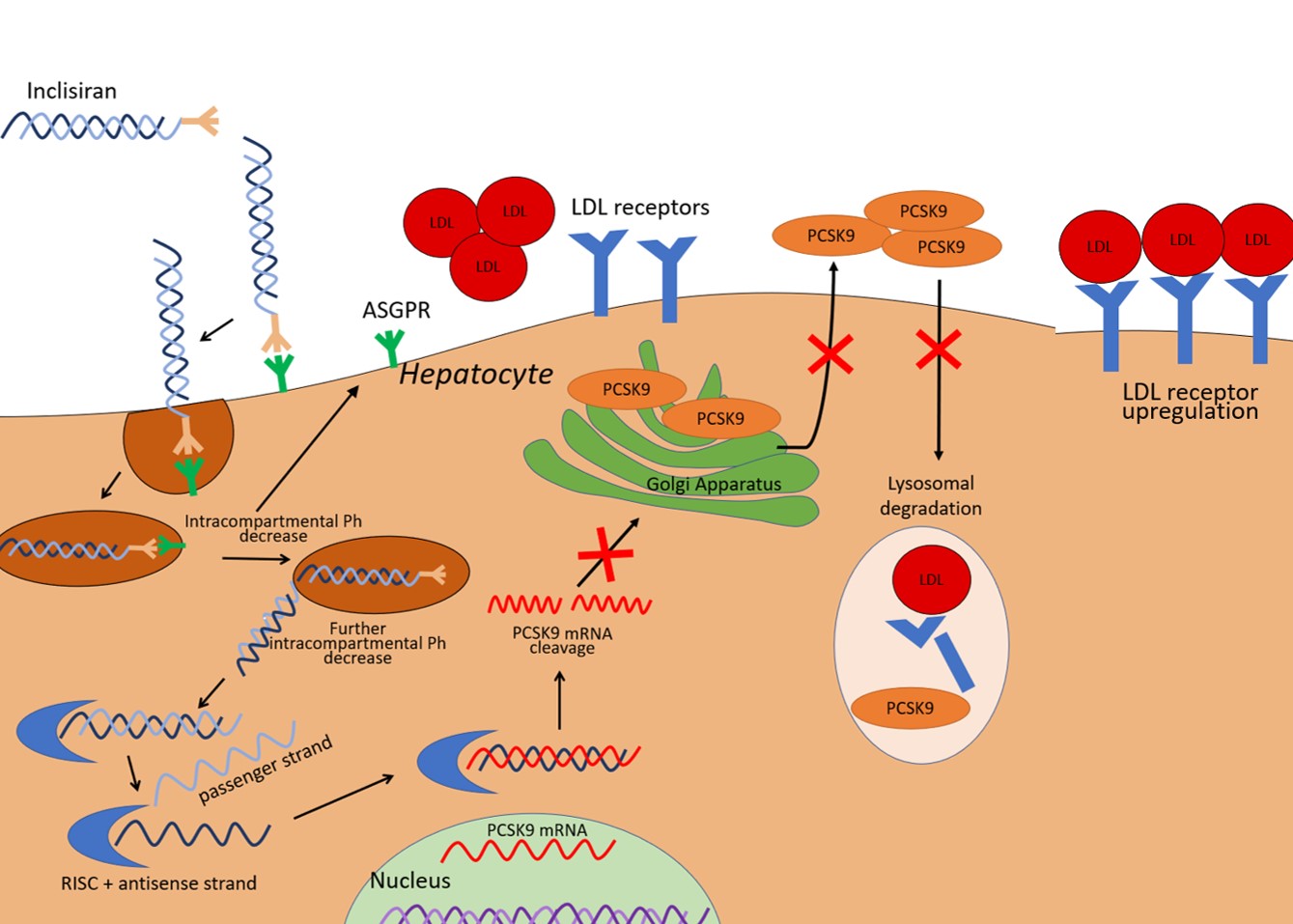
Inclisiran is composed of two complementary RNA chains, an antisense strand (also referred to as a guide strand) and a sense strand (also referred to as a passenger strand). The passenger strand allows siRNA to be integrated into the RNA-induced silencing complex and therefore dissociate from the antisense strand, which is then free to bind mRNA for PCSK9 (Fig. 1). The mRNA target is subsequently cleaved by argonaute-2, a protein component of the RNA-induced silencing complex (RISC). The result is the prevention of mRNA translation in PCSK9. A 66–74% reduction in circulating PCSK9 levels has been reported at 30 days after a single dose, with a similar magnitude of reduction persistent at 60 and 90 days [13]. Since PCSK9 has a central role in the recycling of LDL receptors, the consequence of lower PCSK9 hepatic expression is reduced LDL-receptor degradation and increased LDL particle clearance by hepatocytes, and thus a decrease in circulating LDL-C. Fourteen days after the first administration of inclisiran, a decrease in LDL-C levels of around 40% has been observed. After 30 days, the mean decrease in LDL-C was 45–51%. Reductions in nadir, ranging between 50 and 60%, were observed at 150 days with the two-dose regimen [13].
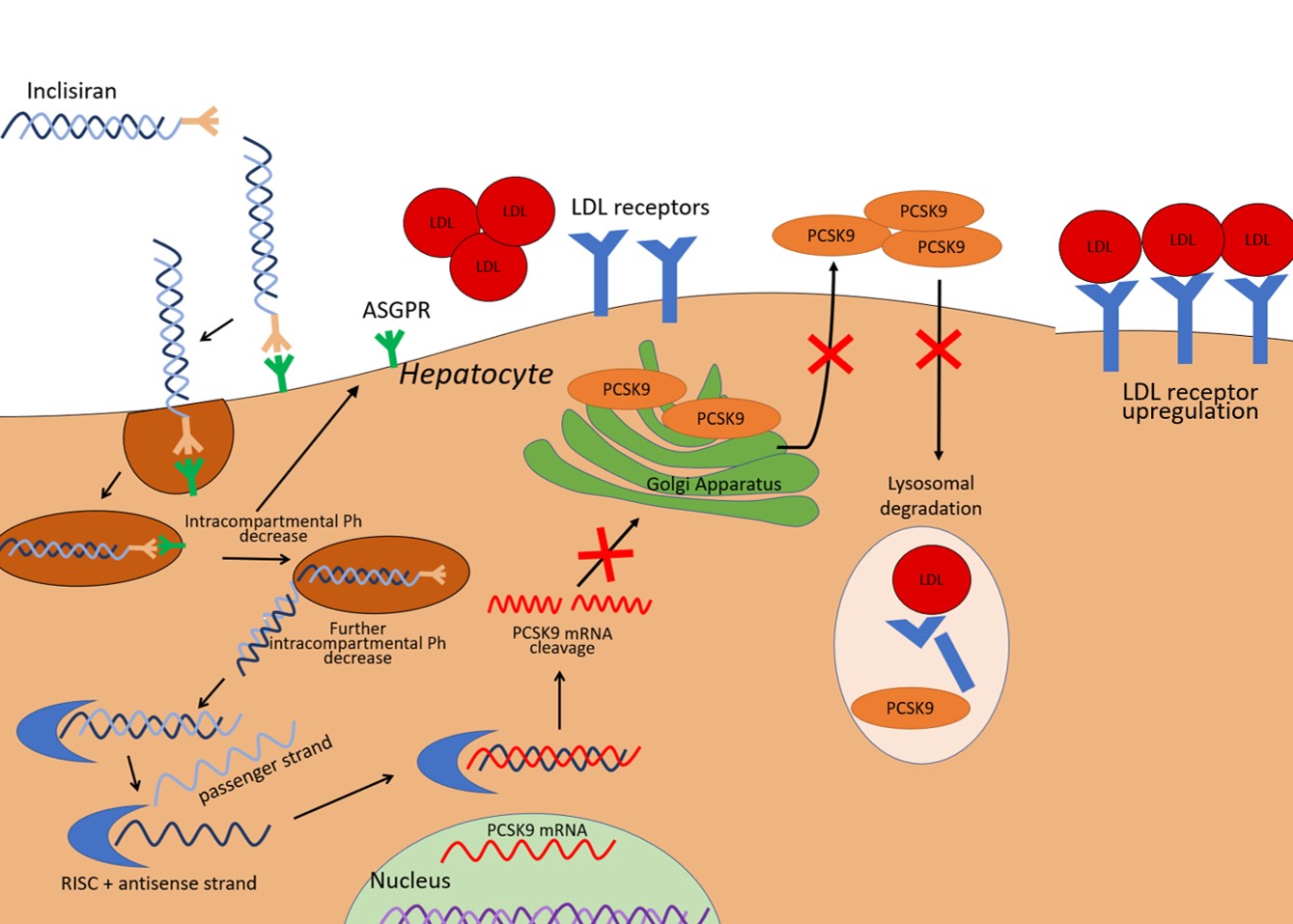 Fig. 1.
Fig. 1.Illustration of inclisiran effects on the liver cell. PCSK9 expressed by the liver mediates LDL-receptor degradation in lysosomes. Inclisiran is a small interfering RNA able to promote the degradation of the mRNA that encodes PCSK9, thereby it reduces PCSK9 availability and leads to an increased expression of LDL receptors. The result is an enhanced LDL-C clearance. ASGPR, asialoglycoprotein receptor; LDL-C, low-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; RISC, RNA-induced silencing complex; mRNA, messenger RNA.
After subcutaneous administration, inclisiran nadir plasmatic concentration is reached in 4 hours. It is subsequently quickly cleared from plasma by hepatocytes and after 48 hours it is undetectable in the bloodstream [14]. Like other siRNAs, inclisiran is characterized by a temporal pharmacokinetic and pharmacodynamic discrepancy with short duration plasma drug exposure (hours) and persistence of pharmacodynamic effects in the target cells for a long time (months) due to its storage in intracellular compartments.
Of note, even if PCSK9 inhibition does not reduce C reactive protein levels in clinical trials, pre-clinical and clinical studies suggest a potential role of PCSK9 in inflammation processes [15]. It has been demonstrated that PCSK9 may interact with receptors other than LDL receptors, including those that are expressed on macrophage cell surfaces and vascular smooth muscle cells, to further impact atherogenesis.
Kidney disease does not impact inclisiran safety or efficacy. Comparable effects in terms of LDL-C reductions have been reported in all renal disease states [16]. In mild or moderate liver dysfunction, the drug safety profile is comparable to that found in patients with normal liver function [17]. However, individuals with moderate liver dysfunction seem to have a lower percentage change in LDL-C. No data are available about inclisiran efficacy and safety in patients with severe hepatic impairment. In clinical practice, no dose adjustment is needed in patients with kidney disease or in those with mild-to-moderate liver dysfunction, while cautious use is recommended in severe liver dysfunction [16].
In the first preclinical studies (Table 1, Ref [18, 19]), siRNAs targeting PCSK9
mRNA were formulated in lipid nanoparticles. When tested in rodents and non-human
primates, these agents led to a decrease in circulating PCSK9 by 50–70% and in
LDL-C levels up to 60% [18]. The effects were dose dependent and were confirmed
in a phase 1 study [20]. These first anti-PCSK9 siRNAs were not chemically
modified or conjugated to GalNAc and were included in lipidoid nanoparticles to
facilitate liver delivery. However, these formulations needed intravenous
administration. This limitation led to lipidoid formulations being abandoned and
to the development of inclisiran, an siRNA administered by subcutaneous
injections. To increase molecule stability in the bloodstream and reduce the risk
of RNA strand degradation by circulating nucleases, some structural modification
to siRNA molecules, as described in the previous section, have been introduced.
Furthermore, the development of siRNA molecules conjugated with GalNAc allowed
oligonucleotide uptake to be directed within hepatocytes to avoid unintended
effects on other cells [19]. In preclinical studies, inclisiran was tested in
non-human primates and led to a substantial decrease in PCSK9 (
| Study, year | Experimental models | Design | Main Findings |
| Frank-Kamenetsky et al., 2008 [18] | Mice and rats | Different doses of the lipidoid-formulated siRNA molecule were intravenously injected into mice and rats. | siRNA displayed a dose-response decrease in PCSK9 levels. |
| Maximal PCSK9 mRNA silencing of 50–70% at a dose of 5 mg/kg. | |||
| The reduction in mRNA transcription translated into a 30% (for mice) and 60% (for rats) reduction in total plasma cholesterol. | |||
| Non-human primates | Animals were randomized to receive a 5 mg/kg dose of si-RNA molecule or to serve as controls | Inclisiran 5 mg/kg administered as a single dose resulted in a statistically significant decrease in LDL-C starting from day 3. | |
| LDL-C levels returned to basal values after 14–21 days. | |||
| A significant reduction in plasma PCSK9 concentration was observed. | |||
| Unpublished data reported by Fitzgerald et al., 2017 [19] | Non-human primates | Different doses of siRNA (inclisiran) subcutaneously administered to NHPs | With doses greater than 3mg/kg a substantial decrease in PCSK9 ( |
| LDL-C, low-density lipoprotein cholesterol; NHP, non-human primates; PCSK9, proprotein convertase subtilisin/kexin type 9; siRNA, small interfering RNA. | |||
In clinical studies, inclisiran has been shown to be safe and effective in inducing long-lasting reductions in PCSK9 and LDL-C circulating levels (Table 2, Ref [13, 19, 21, 22, 23, 24]).
| Study (year) [NCT registration number] | Number of patients | Population | Design | Study strategy (intervention duration) | Therapeutic regimen | Study start/ completion dates | Key findings |
| Fitzgerald et al., 2017 [19] [NCT02314442] | 69 | Healthy individuals with LDL-C |
RCT, single blind | Patients 3:1 randomly assigned to inclisiran or placebo (180 days) | Single-increasing-dose (25, 100, 300, 500, or 800 mg) vs a multiple-dose (four doses of 125 mg once a week, followed by two doses of 250 mg once a week, or two doses of 300 or 500 mg once a month) | December 2014/ November 2015 | In the single-dose group, inclisiran |
| Ray et al., 2017 [13] (ORION-1, 2017) [NCT02597127] | 501 | Patients at high risk for ASCVD with high LDL-C levels despite the maximum statin dose |
RCT, double-blind | 1:1 randomization to receive inclisiran or placebo (240 days) | Administration of a single dose of placebo vs inclisiran 200, 300, or 500 mg or two doses (at days 1 and 90) of placebo was two doses of inclisiran 100, 200, or 300 mg | January 2016/ June 2017 | At day 180, inclisiran single dose reduced LDL-C least-squares mean by 27.9–41.9% and by 35.5–52.6% after two doses. The greatest decrease in LDL-C was obtained with two-dose 300 mg regimen: 48% of patients reached LDL-C |
| Serious adverse events were observed in 11% of patients treated with inclisiran and in 8% of patients of the placebo group. Reactions at the site of injection were observed in 5% of patients treated with inclisiran | |||||||
| Ray et al., 2018 [21] [NCT02597127] | 501 | Patients at high risk for ASCVD | Prespecified analysis of an RCT study | 1:1 randomization placebo or inclisiran (210 days) | Administration of a single dose of placebo or inclisiran 200, 300, or 500 mg or two doses) of placebo or inclisiran 100, 200, or 300 mg (the first at day 1 and the second at day 90) | January 2016/ June 2017 | Inclisiran single dose decreased, non–HDL-C, VLDL-C, and apo B over 210 days. At day 180, a dose-dependent reduction in non–HDL-C was observed: –25% with single-dose of inclisiran 200 mg; –46% with two doses of inclisiran 300 mg. Apo B was also reduced (–23% and –41%) with the above-mentioned dose schemes. |
| Ray et al., 2019 [22] [NCT02597127] | 501 | Patients with high CVD risk | Prespecified analysis of an RCT study | Follow-up at 1 year of ORION-1 study patients (360 days) | Monthly check of LDL-C and PCSK9 levels. | January 2016/ June 2018 | Decrease in LDL-C after inclisiran single dose was 29.5%, 38.7% and 29.9%–46.4% after inclisiran two doses. The 2-dose regimen of inclisiran 300 mg led to the highest rate of responders and the greatest LDL-C decrease over one year. |
| Raal et al., 2020 [23] (ORION-9) [NCT03397121] | 482 | Patients with heterozygous FH | RCT, double-blind | 1:1 randomization to inclisiran or placebo (540 days) | Inclisiran 300 mg subcutaneous injections or placebo at day 1, 90, 270, and 450. | November 2017/ September 2019 | At day 510, a 39.7% LDL-C reduction was observed in the inclisiran group, with substantial decrease in LDL-C levels in all FH genotypes. |
| Ray et al., 2020 [24] (ORION-10) [NCT03399370] | 1561 | Patients with ASCVD and high LDL-C with maximum tolerated statin dose |
RCT, double-blind | 1:1 randomization to receive inclisiran or placebo (540 days) | Subcutaneous administration of inclisiran 284 mg or placebo at day 1, 90, and every six months thereafter over 540 days | December 2017/ September 2019 | At day 510, a 51.3% reduction in LDL-C was observed in the group treated with inclisiran. |
| Ray et al., 2020 [24] (ORION-11) [NCT03400800] | 1617 | Patients with ASCVD or ASCVD equivalent and high LDL-C despite maximum tolerated statin dose |
RCT, double-blind | 1:1 randomization to inclisiran or placebo (540 days) | Subcutaneous administration of inclisiran 284 mg or placebo at day 1, 90, and every six months thereafter over a period of 540 days | November 2017/ August 2019 | At day 510, a 45.8% LDL-C reduction was observed in patients treated with inclisiran. |
| ASCVD, atherosclerotic cardiovascular disease; Apo B, apolipoprotein B; CI, confidence interval; CVD, cardiovascular disease; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin-kexin type 9; RCT, randomized controlled trial; VLDL-C, very-low-density lipoprotein cholesterol. | |||||||
The ORION-1 phase 2 study was a multicenter RCT that enrolled 501 individuals
with an increased risk of CVD and high levels of LDL-C [13]. Participant mean age was
63 years, 35% were women, 69% had established atherosclerotic cardiovascular
disease (ASCVD), and 6% had familial hypercholesterolemia (FH) [13].
Seventy-three percent of included patients were treated with statins. Patients
were randomly assigned to receive placebo or 200, 300, or 500 mg of inclisiran in
one dose or two doses (at day 1 and day 90). After 180 days from the first
administration, the LDL-C percentage change with different inclisiran doses was
assessed. The greatest reduction in LDL-C level was observed with a two-dose
regimen of inclisiran 300 mg, with a 53% reduction (p
In the ORION-3 study, an open-label extension of the ORION-1 study, inclisiran was associated with a 51% reduction in LDL-C levels at day 210 and a 77% reduction in PCSK9 [26]. A persistent effect of inclisiran 300 mg was observed over about 22 months and the time-averaged reduction in LDL-C was around 60 mg/dL [26]. Over a follow-up of at least 3 years, no safety issues or laboratory test abnormalities, including hepatic and renal function examinations, were found to be associated with the treatment.
In phase 3 RCTs, inclisiran was tested in patients with FH (ORION- 9), ASCVD (ORION-10), and ASCVD or equivalent risk of ASCVD (ORION-11). Patients included in these studies required further reduction in LDL-C despite maximum tolerated statin treatment [23, 24].
ORION-9 was a double-blind RCT that evaluated inclisiran efficacy and safety in
patients with heterozygous FH and LDL-C levels
The ORION-10 and the ORION-11 studies were conducted in the U.S. and in
Europe/South Africa, respectively [24]. The first trial enrolled patients with ASCVD
and LDL-C levels
ORION-4 is a double-blind, phase 3 RCT [ClinicalTrials.gov Identifier:
NCT03705234] that aims to investigate inclisiran impact on clinical outcomes in
patients with ASCVD. Around 15,000 ASCVD patients aged
A meta-analysis of data of 3660 patients included in ORION-9, ORION-10, and
ORION-11 has shown that inclisiran treatment is associated with a 51% decrease
in LDL-C at 18 months (95% confidence interval, 48–53%; p
Two post-hoc aggregate analyses were carried out to assess inclisiran efficacy
and safety in two subpopulations of ASCVD patients, those with documented
cerebrovascular disease (CEVD) and those with polyvascular disease (PVD)
[30, 31]. Patients with CEVD were included if they had a previous ischemic stroke
and/or a stenosis greater than 70% of the carotid artery documented by
angiography or ultrasound and/or previous percutaneous or surgical carotid
revascularization. Patients with CEVD treated with inclisiran achieved a 55.2%
mean reduction in LDL-C at day 510 compared to placebo (p
Overall, available clinical findings support inclisiran use to lower LDL-C levels in patients at increased ASCVD risk, including patients with FH. Of note, currently there are no available data on studies comparing inclisiran treatment with other lipid-lowering treatment options such as ezetimibe, alirocumab, or evolocumab. Ongoing trials will assess inclisiran efficacy and safety in several different clinical settings, the impact on prognosis, and the persistence of LDL-C reduction with longer treatment duration. Indeed, although inclisiran demonstrated excellent lipid-lowering efficacy in phase 3 trials, we are still awaiting data on cardiovascular outcome that will be provided by ongoing studies. On the basis of current evidence, inclisiran clinical effectiveness is expected to be similar to monoclonal antibodies against PCSK9, which have a similar efficacy in reducing LDL-C levels.
Although data on cardiovascular outcome effects of lipid-lowering treatment with inclisiran are currently lacking, a significant reduction in cardiovascular event occurrence may be estimated by using the Cholesterol Treatment Trialist Collaboration meta-analyses results, which reported a substantial reduction (–22%) in cardiovascular risk per 38.67 mg/dL (1 mmol/L) decrease in LDL-C with statin use [32]. Due to the consistent LDL-C reduction achieved with inclisiran treatment, the first approval of inclisiran for clinical use was obtained even before the availability of the results of ongoing clinical trials assessing the clinical impact of treatment on cardiovascular outcomes [27, 28]. To date, several national and international drug agencies have approved its use in patients who do not achieve LDL-C therapeutic targets despite lifestyle interventions and the maximum tolerated dose of statins alone or in combination with other lipid-lowering treatments [14, 33, 34]. In clinical practice, inclisiran can be used to treat patients with hypercholesterolemia and statin resistance or intolerance [35]. The recommended inclisiran dose is 284 mg administered with subcutaneous injection. Subcutaneous administration is repeated at 3 months with a subsequent 6–month maintenance regimen. The dosing regimen is one of the main advantages of inclisiran treatment for lowering LDL-C. This regimen is expected to reduce undertreatment due to adherence issues that often affect currently available lipid-lowering therapies with a daily assumption regimen. It is recommended that inclisiran is administered subcutaneously by healthcare professionals, therefore a system of care that incorporates this treatment in the pathway of patients at increased ASCVD risk should be implemented.
No dose adjustment is needed in older patients or in patients with kidney disease or mild-to-moderate liver dysfunction. However, no data are available on its efficacy and safety in patients with end-stage kidney dysfunction or severe hepatic dysfunction. Since it is eliminated through the kidneys, hemodialysis sessions should be avoided in the 72 hours following administration. Unlike with monoclonal antibodies against PCSK9, no induction of antidrug antibodies interfering with drug efficacy or safety has been observed to date with inclisiran [36].
A potential limitation of inclisiran large-scale use is drug cost. It has been
estimated that inclisiran used in patients with ASCVD on top of standard of care
may be cost-effective for the U.S. healthcare system at annual prices between
Real-word clinical studies will provide information on inclisiran impact in patients with comorbidities that can affect inclisiran efficacy and safety. An additional aspect that still needs to be defined with additional clinical studies is the management of possible side effects that may persist as long as the drug action. However, the reversal of siRNA-induced gene silencing may be possible [38]. Real-world studies with long follow-up are needed to establish inclisiran long-term safety and possible side effects. Moreover, available evidence has been obtained by using inclisiran on top of standard lipid-lowering treatment, and therefore studies investigating inclisiran efficacy in monotherapy are also warranted. Further information critical for delineating tailored optimal lipid-lowering treatments will be provided by directly comparing inclisiran with monoclonal antibodies against PCSK9. A potentially relevant setting for inclisiran use is in younger people with an elevated risk of premature cardiovascular events. This population may benefit from intensive and early lipid-lowering treatment, and thus would be exposed to drug treatment for a longer time. The ongoing ORION-13 and ORION-16 studies (NCT04659863 and NCT04652726, respectively) will assess inclisiran safety and efficacy in adolescents (aged 12–17 years) with homozygous or heterozygous FH and high LDL-C levels already treated with standard lipid-lowering therapy [39]. Finally, the inclisiran-induced lipoprotein(a) reduction observed in phase 3 trials (19–22%) [24] also deserves further investigation to better define the magnitude of this reduction and its clinical impact [40].
Although CVD approaches with gene silencing therapies are still at an early stage, inclisiran represents a true milestone in this field. Nevertheless, since the pharmacological efficacy of inclisiran in LDL-C lowering seems to be similar to that of monoclonal antibodies against PCSK9, the longer durability of its effect, which allows a less frequent dosing regimen, and its favorable safety profile may favor this newer approach and contribute to increased treatment persistence and adherence and, finally, to LDL-C goal achievement. Furthermore, the infrequent dosing regimen and high drug tolerability make this treatment a potential optimal early and large-scale preventive intervention that is more acceptable than interventions with daily pill intake [41]. With inclisiran treatment, two major challenges of pharmacological therapies may be overcome: adherence and the interindividual variability of drug effect [42, 43]. Physicians should know emergent LDL-C-lowering therapeutic options, the mechanisms of action of these new treatments, and their indications and contraindications. Ongoing studies evaluating the effects of inclisiran on hard cardiovascular endpoints are also expected to confirm the therapeutic effect of this innovative drug.
FC, SADF—conceptualization, design, and revision; CB, FP, FM, VDM, GU, EC—extraction of data and drafting of the manuscript; AM, LDL, DG, MMG, FO—supervision and critical review. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Leonardo De Luca is serving as one of the Editorial Board members and Guest Editors of this journal. Stefania Angela Di Fusco, Francesco Perone, Vincenzo De Marzo and Furio Colivicchi are serving as Guest Editors of this journal. We declare that they had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Brian Tomlinson.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.