1 Department of Cardiovascular and Pulmonary Sciences, Catholic University of the Sacred Heart, 00168 Rome, Italy
2 Department of Cardiovascular Medicine, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, 00168 Rome, Italy
Academic Editor: Jerome L. Fleg
Abstract
Despite ischemic heart disease (IHD) has been commonly identified as the consequence of obstructive coronary artery disease (OCAD), a significant percentage of patients undergoing coronary angiography because of signs and/or symptoms of myocardial ischemia do not have any significant coronary artery stenosis. Several mechanisms other than coronary atherosclerosis, including coronary microvascular dysfunction (CMD), coronary endothelial dysfunction and epicardial coronary vasospasm, can determine myocardial ischemia or even myocardial infarction in the absence of flow-limiting epicardial coronary stenosis, highlighting the need of performing adjunctive diagnostic tests at the time of coronary angiography to achieve a correct diagnosis. This review provides updated evidence of the pathophysiologic mechanisms of myocardial ischemia with non-obstructive coronary arteries, focusing on the diagnostic and therapeutic implications of performing a comprehensive invasive functional evaluation consisting of the assessment of both vasodilation and vasoconstriction disorders. Moreover, performing a comprehensive invasive functional assessment may have important prognostic and therapeutic implications both in patients presenting with myocardial ischemia with non-obstructive coronary arteries (INOCA) or myocardial infarction with non-obstructive coronary arteries (MINOCA), as the implementation of a tailored patient management demonstrated to improve patient’s symptoms and prognosis. However, given the limited knowledge of myocardial ischaemia with non-obstructive coronary arteries, there are no specific therapeutic interventions for these patients, and further research is warranted aiming to elucidate the underlying mechanisms and risk factors and to develop personalized forms of treatment.
Keywords
- INOCA
- MINOCA
- pathophysiology
- diagnosis
- therapy
Ischemic heart disease (IHD) is the leading cause of disability and mortality
worldwide and, traditionally, it has been identified with the presence of
obstructive coronary artery disease (OCAD) (defined as any coronary artery
stenosis
Coronary arterial circulation can be described as two contiguous districts with different size and functions.
The proximal district is represented by the epicardial vessels, conductance
arteries with cross-sectional diameter
Conversely, the distal compartment is represented by the coronary
microcirculation including all vessels with cross-sectional diameter
Although coronary angiography remains the gold-standard for the diagnosis and
exclusion of OCAD, the inability to directly visualize coronary microcirculation
(beyond its resolution limit of 0.5 mm) substantially limits its diagnostic
accuracy for coronary vascular disorders [23]. However, the filling of coronary
vasculature by the angiographic contrast medium can still provide limited
information regarding the function of coronary microcirculation. A retarded
filling of the distal coronary vessels (the so-called coronary slow-flow) in the
absence of OCAD has been used in previous studies as an indicator for CMD, with
diagnostic criteria varying from a thrombolysis in myocardial infarction (TIMI)
flow grade
To overcome the limitations of coronary angiography, several invasive and
non-invasive methods have been studied and validated to evaluate microvascular
function. Despite the obvious limitations (besides low risks), the invasive
approach remains the gold standard for the evaluation of the coronary artery
vasculature offering several advantages such as the possibility to exclude
haemodynamically significant epicardial CAD (i.e., by demonstrating a fractional
flow reserve (FFR) value
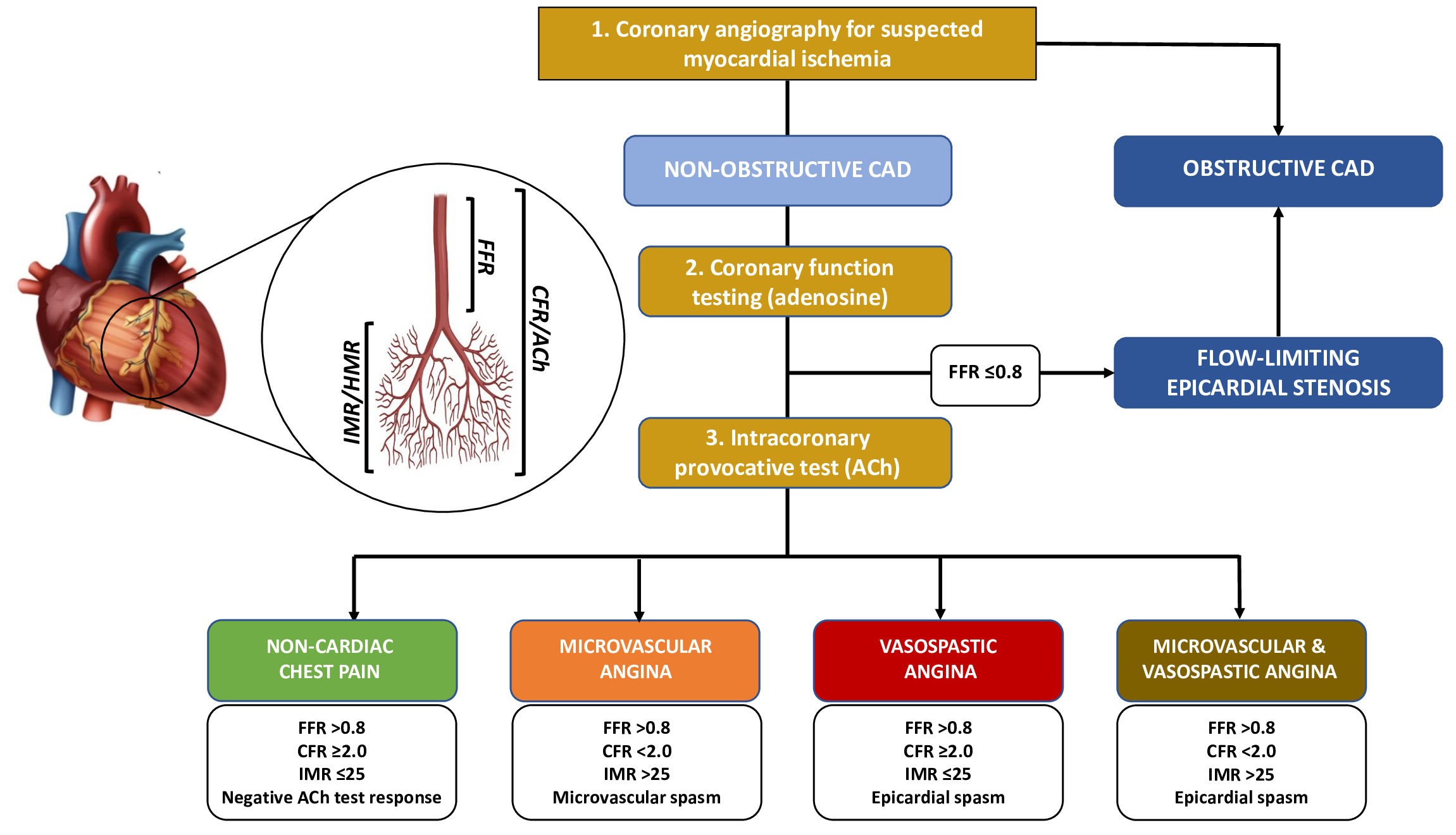 Fig. 1.
Fig. 1.Diagnostic algorithm for a comprehensive invasive functional assessment in patients with suspected myocardial ischaemia. FFR, Fractional Flow Reserve; CFR, Coronary Flow Reserve; IMR, Index of Microvascular Resistance; HMR, Hyperaemic Microvascular Resistance; ACh, Acetylcholine; CAD, Coronary Artery Disease.
The CFR is a physiological index that represents the vasodilator capacity of the
entire coronary tree (i.e., both the epicardial vessels and the microvasculature)
and evaluates the ratio of maximum hyperaemic to basal CBF and the extent to
which myocardial coronary flow can increase above its baseline value in response
to increased demand through the dynamical reduction of vascular resistance [5, 18, 26]. CBF can be estimated by measuring CBF velocity with an intracoronary
Doppler flow wire (usually placed in the distal left anterior descending artery
because of the large percentage of subtended myocardium and coronary dominance)
or using a temperature sensor-tipped guidewire by measuring the saline bolus
transit time at rest and in response to continuous infusion of pharmacological
stressors (i.e., adenosine) to induce maximal hyperaemia with the thermodilution
technique [27]. CFR can be impaired in patients with CMD and a defective
hyperaemic microvascular dilatory response as well as in those with epicardial
OCAD and, therefore, an impaired CFR (defined as a value
However, some limitations of CFR must be acknowledged: first, it is an indirect estimation of true coronary flow; second, a steady-state hyperaemia with adenosine is required for its calculation, and its administration may cause adverse effects; third, thermodilution can overestimate CFR at higher values and is partially dependent on operator’s injections, thus determining a large intra-observer variability; finally, obtaining a stable signal during Doppler-based measurements may be challenging.
Recently, a novel method based on continuous thermodilution with stable hyperaemia achieved by intracoronary infusion of saline at room temperature has been introduced and validated to directly quantify absolute CBF and resistance, offering the potential advantages of being operator independent, highly reproducible, and not requiring adenosine administration. However, since normal values of absolute CBF and resistance are still a matter of debate, further research are needed before the implementation of absolute CBF measurement in daily clinical practice [32].
The index of microvascular resistances (IMR) is a dimensionless index that can
be obtained by thermodilution as the product of the distal coronary pressure and
mean transit time of a saline bolus during maximal hyperaemia induced by
adenosine. Compared with CFR, IMR is independent on epicardial vascular function
and hemodynamic conditions, thus providing a more reproducible assessment of the
microcirculation [33, 34]. The cut-off of
The diagnosis of coronary vasomotor disorders is usually achieved by performing an intracoronary provocative test with pharmacologic vasoactive agents that can trigger a vasoconstrictive response at the epicardial and/or microvascular level in susceptible individuals [36, 37].
The most used vasoactive provocative agent to explore endothelium-dependent vasodilation in clinical practice is acetylcholine (ACh): given that ACh indirectly induces vasodilation by stimulating the release of NO from endothelial cells and directly promote VSMCs contraction, the resulting effect of intracoronary ACh administration can be either vasodilatation (healthy endothelium) or coronary spasm (endothelial dysfunction or increased VSMCs reactivity) [38]. The safety of intracoronary provocative tests with ACh has been widely investigated both in patients with INOCA and MINOCA with a relatively low risk of transient complications (mainly represented by transient bradyarrhythmia and supraventricular tachycardia) and performing a provocative test may have relevant prognostic implications, as a positive ACh test (either for microvascular or epicardial spasm) is associated with a higher risk of future cardiovascular events [9]. In particular, a recent study demonstrated that ACh provocation testing is associated with a low risk of mild and transient complications (mainly represented by transient bradyarrhythmia) but patients with a positive test had a higher incidence of major adverse cardiovascular and cerebrovascular events (defined as the composite of cardiovascular death, nonfatal MI, hospitalisation due to unstable angina, and stroke/transient ischaemic attack) compared to those with a negative result at a median follow-up time of 22 months [39].
As an alternative to ACh, ergonovine (ER) can be used as vasoactive agent for intracoronary provocative test. ER mainly acts through serotonergic receptors (5-HT2) on VSMCs, inducing an endothelium-independent vasoconstriction in susceptible vessels. Moreover, ER may also cause the release of relaxing prostanoids from the healthy endothelium, a process that can be compromised in the presence of endothelial dysfunction, in which ER favours vasoconstriction [40].
Epicardial spasm is diagnosed when the typical ischemic symptoms (i.e., chest
pain) are reproduced by ACh infusion in association with ischemic
electrocardiographic (ECG) changes (e.g., ST segment depression or elevation) and
coronary artery spasm defined as transient total or subtotal coronary artery
occlusion (
The term INOCA identifies a significant proportion of patients (up to 50% and particularly women) referred for coronary angiography because of stable, chronic ischaemic symptoms (stable angina or angina equivalent) and/or signs of ischemia on non-invasive testing (i.e., exercise stress test, stress echocardiography or nuclear imaging) and found to have normal or near-normal coronary arteries [3, 5].
In the most recent ESC guidelines, performing an invasive coronary function testing for measurement of CFR and IMR/HMR have a Class IIa (“should be considered”) recommendation in patients with INOCA. On the contrary, performing an intracoronary provocative test with ACh have a Class IIb recommendation (“may be considered”) for the diagnosis of MVA and a Class IIa if VSA is suspected [3]. However, the rationale for performing adjunctive functional tests in these patients is strong and threefold: diagnosis, treatment and prognostic implications. Indeed, a comprehensive functional assessment, including both coronary function testing and provocative test, allows the identification of specific endotypes within the heterogeneous population of INOCA characterized by distinct mechanisms and/or responses to medical therapy (i.e., MVA, VSA, both MVA and VSA, or none) (Table 1) [36]. The “Coronary Microvascular Angina” (CorMicA) trial demonstrated that performing an invasive functional assessment in INOCA patients and implementing a consequent tailored medical therapy are associated with significant improvements in patients’ outcomes in terms of reduction of angina severity and quality of life [41]. Indeed, if the specific endotype is not correctly identified nor an appropriated medical therapy is not instituted, INOCA patients may often experience recurrent angina, with hospital readmission and repeated invasive procedures. This is particularly relevant, as INOCA patients are usually younger than patients with OCAD [36]. Therefore, in patients with signs and/or symptoms of myocardial ischemia without angiographic evidence of OCAD, coronary angiography should be considered incomplete without adjunctive diagnostic tests aiming to assess the presence of coronary vascular dysfunction.
| (A) Microvascular Angina | (B) Vasospastic Angina | (C) Both Microvascular and Vasospastic angina | (D) None/non-cardiac chest pain |
| Evidence of CMD defined as any of: | Normal CFR ( |
Evidence of CMD and epicardial spasm | Normal CFR ( |
| Normal IMR ( |
Normal IMR ( | ||
| Abnormal CFR ( |
Epicardial spasm | Neither microvascular nor epicardial spasm | |
| Abnormal IMR ( | |||
| Microvascular spasm | |||
| INOCA, Ischemia with Non-Obstructive Coronary Arteries; ACh, Acetylcholine; CMD, Coronary Microvascular Dysfunction; CFR, Coronary Flow Reserve; IMR, Index of Microvascular Resistance. | |||
Treatment of INOCA patients should target the underlying risk factors and pathogenic mechanism of the different endotypes. However, to date, there are no disease-modifying therapies specific to INOCA, and there is a strong need for further research to address this unmet clinical need.
Traditional cardiovascular risk factors, such as hypertension, dyslipidaemia, smoke habit, and diabetes, are all relevant contributor to the development of coronary microvascular and vasospastic dysfunction as well as to determine a structural remodelling of the coronary circulation. In particular, hypertension has been strongly associated with the adverse remodelling of coronary microvasculature and, therefore, an optimal control of blood pressure is fundamental to prevent the progression of CMD and obtain a reduction in angina frequency and intensity [36]. The choice of the best medications should be based upon the predominant endotype (e.g., VSA, MVA or both).
Standard anti-ischemic medications often obtained disappointing results in patients with INOCA. Long-acting nitrates may help to reduce angina episodes, but their efficacy in reducing MACE and improving CMD was not demonstrated [42]. Furthermore, they may worsen anginal symptoms in MVA due to a stealing effect [43]. Short-acting nitrates, although useful to treat acute anginal attacks especially if an abnormal vasodilator reserve is present, are usually only partially effective [20]. Calcium-channel blockers (CCBs) are particularly effective in presence of both microvascular and epicardial spams, and most experts’ consensus indicate CCBs as the first-line agents when the presence of vasomotor disorders is either suspected or documented [36]. In particular, CCBs demonstrated to improve angina status and reduce the rate of MACE in patients with VSA [44].
If MVA is associated with an abnormal CFR and/or an increased IMR, thus
suggesting the presence of adverse arterial remodelling,
In patients with MVA and effort-induced angina with evidence of increased
adrenergic activity,
Statins demonstrated to reduce angina recurrence and the rate of MACE in patients with epicardial spasm as well as to improve endothelial dysfunction and CFR in patients with CMD, probably due to their anti-inflammatory and anti-oxidant properties [47].
Nicorandil, a vasodilator drug that induces the relaxation of coronary VSMCs by stimulating guanylyl cyclase and increasing cyclic guanosine monophosphate (cGMP) levels as well as by inducing the activation of K+ channels and hyperpolarization, was proven to prevent exercise induced myocardial ischemia (i.e., both time to 1-mm ST depression and total exercise duration at treadmill exercise test) in patients with CMD without modifying the heart rate variability, thus suggesting a direct vasodilatory effect on coronary microvasculature [48].
Ranolazine, an inhibitor of the late inward sodium current that enhances myocyte relaxation and ventricular compliance by reducing intracellular calcium levels, improved anginal symptoms and myocardial perfusion reserve in patients with MVA and a severely reduced CFR owing to an impaired vasodilation [49].
Ivabradine, a heart-rate-lowering agent that acts by selectively inhibiting the cardiac pacemaker current (If), could likely improve persistent anginal symptoms in selected patients, but its role in MVA is still controversial and barely investigated [50].
Fasudil is a selective Rho-kinase inhibitor that induces vasodilation by reducing the phosphorylation of the myosin light chain phosphatase, thus increasing phosphatase activity and preventing VSMCs contraction. Recent evidence demonstrated its efficacy in preventing coronary spasm and myocardial ischemia in patients with evidence of epicardial and/or microvascular spasm as well as to reduce microvascular resistances in patients with increased IMR [51, 52, 53].
Finally, Zibotentan is a potent and selective oral antagonist of endothelin A receptors that could be beneficial by contrasting the increased vasoconstrictive response of coronary microcirculation to endothelin in patients with MVA. To this aim, the ongoing Precision Medicine With Zibotentan in Microvascular Angina (PRIZE) randomized controlled trial (NCT04097314) will evaluate whether the add-on treatment with Zibotentan could improve treadmill exercise times in patients with MVA and impaired exercise intolerance [54].
The Women’s IschemiA TRial to Reduce Events In Non-ObstRuctive CAD (WARRIOR) is
a multicenter, prospective, randomized, blinded outcome trial evaluating a
strategy of intensive medical therapy compared with usual care in 4422
symptomatic women with INOCA (NCT03417388). The hypothesis is that an intensive
medical therapy consisting of high-intensity statin, maximally tolerated
ACEi/ARBs, and aspirin could reduce the primary outcome of first occurrence of
MACE defined as the composite of all-cause death, non-fatal MI, non-fatal stroke,
or hospitalization for chest pain or heart failure by 20% compared to usual care
consisting of symptom control and primary risk reduction at
Moreover, it is important to highlight the prognostic implications of performing an invasive functional assessment, as INOCA patients are at increased risk for future cardiovascular events (including acute coronary syndromes, heart failure hospitalization, stroke and repeated cardiovascular procedures) [56]. Upon the results of invasive coronary function testing, patients with CMD may be further classified according to IMR into distinct ‘structural’ (low CFR, high IMR/HMR) and ‘functional’ (low CFR, normal IMR/HMR) endotypes, with distinct underlying pathophysiological process that could represent therapeutic targets in the future. The ‘structural CMD’ endotypes are more frequently associated with an increased risk of acute coronary syndromes and mortality, while ‘functional CMD’ is associated with an increased risk of hospitalizations for recurrent angina [57].
Finally, even in patients with clear angiographic evidence of OCAD, the presence
of coexistent coronary microvascular abnormalities both at epicardial and/or
microvascular level may determine myocardial ischemia in territories supplied by
healthy coronary arteries as well as contribute to reduce CFR and may worsen
myocardial ischemia in territories supplied by arteries with significant CBF
reduction due to the presence of epicardial coronary stenosis. These mechanisms
may partially justify the results of the recent International Study of
Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA)
trial demonstrating the limited prognostic efficacy of revascularization in
subjects with inducible moderate-to-severe ischemia and
MINOCA accounts for up to 6–8% of patients presenting with acute MI and is defined as the evidence of MI with normal or near-normal coronary arteries at coronary angiography without any alternative diagnosis for clinical presentation (e.g., sepsis, pulmonary embolism, tachyarrhythmias, myocarditis and Takotsubo syndrome). A variety of pathogenetic mechanisms may result in MINOCA (i.e., coronary plaque rupture/erosion, spontaneous coronary artery dissection, epicardial/microvascular spasm, and coronary embolism) and MINOCA should be considered a heterogeneous working diagnosis requiring a comprehensive assessment aiming to investigate the potential underlying aetiologies [59, 60, 61].
Despite some initial concerns, it has been widely demonstrated that performing an intracoronary provocative test with ACh for coronary vasomotor evaluation in the acute phase is safe and allows the detection of coronary vasoconstriction disorders at either epicardial or microvascular level as well as the start of a tailored medical therapy. Moreover, the presence of coronary functional alterations has also prognostic implications, as a positive ACh test (either at epicardial or microvascular level) identifies a high-risk subset of patients with an increased risk of future cardiovascular events [9, 10, 38]. To date, the management of MINOCA is still scarcely supported by evidence-based literature and current guidelines do not specifically provide recommendations regarding acute and long-term management of MINOCA (Table 2).
| Clinical presentation | Therapeutic implication | Prognostic implications |
| INOCA | Identification of specific endotypes (MVA, VSA, both MVA and VSA, none) and the start of a tailored medical therapy according to the different endotypes | A tailored medical therapy based upon the specific underlying mechanism of INOCA demonstrated to improve clinical outcomes. |
| Consider ACEi and statins in all patients | Patients with CMD may be classified according to IMR into ‘structural’ (low CFR, high IMR/HMR) and ‘functional’ (low CFR, normal IMR/HMR) endotypes. | |
| VSA | CCBs (1st-line agents, demonstrated to improve angina status and reduce the rate of MACE) | Higher risk of MACE, especially within 3 months of symptoms onset or even in asymptomatic patients. |
| Long-acting nitrates (no efficacy in reducing MACE and improving CMD) | Smoking cessation and CCBs therapy are the most determinant prognostic factors. | |
| Nicorandil | IMR | |
| Fasudil (especially if increased IMR) | ||
| Avoid | ||
| MVA Microvascular spasm | CCBs | Generally better prognosis compared with epicardial spasm. |
| Nicorandil | ||
| Fasudil (especially if increased IMR) | ||
| Nitrates may aggravate symptoms due to a stealing effect. | ||
| MVA Functional CMD | More frequently associated with chest pain hospitalizations. | |
| MVA Structural CMD | CCBs | More frequently associated with acute coronary syndromes and deaths. |
| Nicorandil | ||
| Ranolazine (especially if markedly reduced CFR) | ||
| Ivabradine | ||
| VSA and MVA Mixed type | CCBs | Particularly high risk of MACE. |
| Nicorandil | ||
| Fasudil | ||
| MINOCA | Performing an invasive provocative test for coronary vasomotor evaluation allow the detection of coronary vasoconstriction disorders and the start of a tailored medical therapy. | MINOCA patients with a positive intracoronary provocative test (i.e., epicardial or microvascular spasm) are at higher risk for future cardiovascular events. |
| The presence of myocardial bridge should induce to perform an invasive provocative test in MINOCA patients. If positive, avoid |
A positive intracoronary provocative test in patients with myocardial bridge and MINOCA is associated with a worse medium-long term outcome. | |
| INOCA, Ischemia with Non-Obstructive Coronary Arteries; MVA, Microvascular Angina; VSA, Vasospastic Angina; ACEi, Angiotensin Converting Enzyme inhibitors; CMD, Coronary Microvascular Dysfunction; CFR, Coronary Flow Reserve; IMR, Index of Microvascular Resistance; HMR, Hyperaemic Microvascular Resistance; CCBs, Calcium Channel Blockers; MACE, Major Adverse Cardiovascular Events; MINOCA, Myocardial Infarction with Non-Obstructive Coronary Arteries. | ||
Moreover, the effects of secondary preventive treatments beneficial in
myocardial infarction due to OCAD are still largely unknown in MINOCA, with few
prospective trials exploring this fields [62]. In particular, the ongoing
“Randomized Evaluation of Beta Blocker and ACEi/ARBs treatment of MINOCA
patients” (MINOCA-BAT) clinical trial aims to determine whether
Mocardial bridging (MB) is a congenital coronary anomaly in which a segment of an epicardial coronary artery extends intramurally through the myocardium for a portion of its length below a muscular bridge. The prominent angiographic finding revealing the presence of MB is the dynamic compression during systole of the involved epicardial coronary artery [69].
Even if initially considered a benign condition, recent evidence showed that
patients with MB without angiographic evidence of OCAD undergoing intracoronary
provocative test with ACh may frequently present endothelial dysfunction either
at epicardial or microvascular level. Moreover, the presence of coronary
vasomotor disorders in these patients is an important yet often overlooked cause
of MINOCA [70]. Therefore, the presence of MB should hint to perform an
intracoronary provocative test with ACh, particularly in patients presenting with
an acute clinical presentation. Indeed, a positive result in these patients has
relevant prognostic implications as it has been associated with an increased rate
of MACE at follow-up. Finally, a positive provocative test result in patients
with MB has also therapeutic implications, as
This review demonstrates that performing a comprehensive invasive functional assessment consisting of the assessment of both vasodilation and vasoconstriction disorders at the time of coronary angiography is important for the decision making in patients with IHD as it allows to evaluate the whole coronary vascular tree from epicardial vessels to coronary microcirculation and to establish a correct diagnosis. Moreover, this review provides evidence that, even in the absence of OCAD, the presence of coronary vascular alterations (i.e., CMD and epicardial coronary spasm) can be accurately detected by performing an invasive functional assessment and are associated with adverse outcomes in both INOCA and MINOCA patients. Furthermore, the implementation of a tailored patient management demonstrated to improve patient’s symptoms and prognosis. However, the limited knowledge of myocardial ischaemia with non-obstructive coronary arteries precludes specific therapeutic interventions and, therefore, further research is warranted aiming to elucidate the underlying mechanisms and risk factors and to develop personalized forms of treatment.
IHD, Ischemic Heart Disease; OCAD, Obstructive Coronary Artery Disease; CMD, Coronary Microvascular Dysfunction; MI, Myocardial Infarction; INOCA, Ischemia with Non-Obstructive Coronary Arteries; MINOCA, Myocardial Infarction with non-Obstructive Coronary Arteries; VSA, Vasospastic Angina; VSMCs, Vascular Smooth Muscle Cells; CBF, Coronary Blood Flow; MVA, Microvascular Angina; NO, Nitric Oxide; TIMI, Thrombolysis In Myocardial Infarction; CFR, Coronary Flow Reserve; IMR, Index of Microvascular Resistances; HMR, Hyperaemic Microvascular Resistance; ACh, Acetylcholine; ECG, Electrocardiographic; MACE, Major Adverse Cardiovascular Events; CCBs, Calcium-Channel Blockers; ACEi, Angiotensin Converting Enzyme inhibitors; ARBs, Angiotensin-II Receptor Blockers; cGMP, cyclic Guanosine Monophosphate; DAPT, Dual Antiplatelet Therapy; MB, Myocardial Bridging.
RR, CS, AC—extraction and drafting of the manuscript; RR, RAM—analysis of data, manuscript revision; RR, RAM—design and revision. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

