1 McKinney Family Medicine, McKinney, 75070, TX, USA
2 Baylor University Medical Center, Dallas, 75226, TX, USA
3 Baylor Heart and Vascular Institute, Dallas, 75226, TX, USA
4 Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, 75226, TX, USA
Abstract
There is an emergency need for early ambulatory treatment of Coronavirus
Disease 2019 (COVID-19) in acutely ill patients in an attempt to reduce disease
progression and the risks of hospitalization and death. Such management should
be applied in high-risk patients age
Keywords
- SARS-CoV-2
- COVID-19
- multidrug
- hospitalization
- mortality
- ambulatory
- antiviral
- zinc
- hydroxychloroquine
- ivermectin
- doxycycline
- azithromycin
- vitamin
- corticosteroid
The epidemic viral outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection Coronavirus Disease 2019 (COVID-19) is advancing across the United States unabated despite public policy measures focussed on contagion control (McCullough et al., 2020). The United States has a current 877 deaths per million inhabitants despite having technically advanced hospitals and to date sufficient capacity to handle the surges of patients requiring hospitalization (Worldometer, 2020). Conversely India, a country with broad implementation of early COVID-19 treatment has 102 deaths per million (Worldometer, 2020). The regulatory agencies as well as the National Institutes of Health have had their principal areas of focus being late stage hospitalized patients and vaccine development (COVID-19 Treatment Guidelines, 2020). This has left a void for the role of early ambulatory treatment of COVID-19 at home. Such management has the goals of lessening the intensity and severity of symptoms and preventing hospitalization and death. There are currently no approved drugs or drug combinations in the U.S. indicated for the ambulatory treatment of COVID-19 or its complications. In the absence of conclusive randomized trials of single drugs and combination regimens, clinicians faced with large numbers of ill patients have responded with innovative empiric approaches that attempt to reduce the progression of SARS-CoV-2 infection, improve symptoms, avoid complications, and reduce the risk of complications and death. The mechanisms by which a multidrug approach would globally improve outcomes could be to address viral replication, cytokine storm, and thrombosis. This report discloses real world data and the clinical outcomes of early ambulatory treatment of acute COVID-19 in patients at high risk for hospitalization and death.
Beginning in March 2020, a team of primary care providers consisting of a
lead physician (BCP) and four advanced practice practitioners (CR, VP, ES, CH)
responded to urgent visits by patients with suspected SARS-Co-V infection and
symptomatic COVID-19. All patients underwent standard informed consent for care
and were under the direct management of licensed medical personnel including a
senior attending physician (BCP). Contemporary real-time polymerase chain
reaction (PCR) assay tests from anterior nasal swab samples were obtained. It
was understood at that time period that COVID-19 test results could be falsely
negative, particularly in the setting where a patient had the characteristic
symptoms of the syndrome (Woloshin et al., 2020). They additionally had an
assessment according to the severity of symptoms and scored as depicted in Table 1. The treatment regimens are given in Table 2. All patients received empiric
treatment on the first day of presentation in most cases before COVID-19 test
results with standard office practice and contagion control measures (Fiorillo et al., 2020). According to clinical judgment and planned use of
hydroxychloroquine (HCQ) a 12-lead electrocardiogram was obtained to evaluate the
QTc interval. For patients with high severity of symptoms, urgent in-clinic
administration of albuterol nebulizer, inhaled budesonide, and intravenous volume
expansion with supplemental parenteral thiamine 500 mg, magnesium sulfate 4
grams, folic acid 1 gram, vitamin B12 1 mg (Flannery et al., 2017).
Additionally, for the severely ill population dexamethasone 8 mg and ceftriaxone
1 gram was administered intramuscularly (Table 2). All patients had in-person
or telemedicine followup at 48 hours and as needed after that point which was
part of the general consent for treatment. Univariate statistics were reported
with means
| Symptom | Points |
| Fever | 1 |
| Fever at night | 1 |
| Fatigue | 1 |
| Body aches | 1 |
| Cough | 1 |
| Difficulty breathing | 1 |
| Additional symptoms | 1 |
| Probability of COVID-19: 0-1 points = low, 3-4 points = moderate, 5+ points = high |
| Agent | Rationale |
| Zinc | Inhibits SARS-CoV-2 RNA synthesis |
| Hydroxychloroquine 200 mg po bid | Inhibits endosomal transfer of virions, anti-inflammatory |
| Ivermectin (200 mcg/kg) usual dose 12 mg po qd × 3 days | Attenuates importin |
| Azithromycin 250 mg po bid | Covers respiratory bacterial pathogens in secondary infection |
| Doxycycline 100 mg po bid | Covers respiratory bacterial pathogens in secondary infection |
| Inhaled budesonide, Dexamethasone 8 mg IM | Treats cytokine storm |
| Folate, thiamine, vitamin 12 | Reduce tissue oxidative stress |
| Intravenous fluid | Intravascular volume expansion |
A total of 922 patients were evaluated between the ages of 12 and 89 years.
The mean age was 50.5
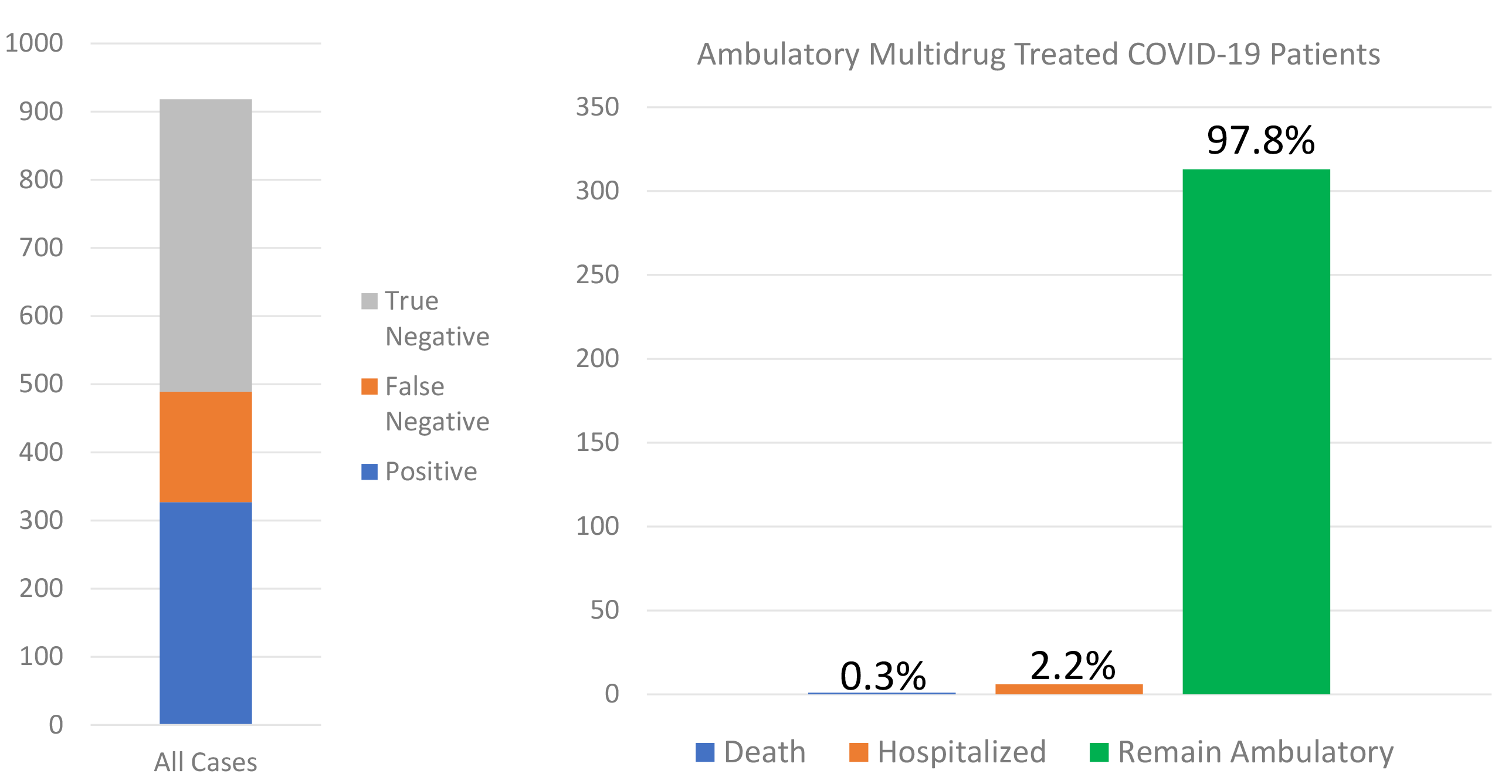 Fig. 1.
Fig. 1.The SARS-CoV-2 nasal PCR test results are shown on the left and among the 320 cases that were confirmed positive and were high risk, the outcomes of hospitalization and death are shown on the right.
The observations in this report suggest that primary care physicians can take an organized, empiric approach to acutely ill patients with COVID-19 with very low rates of subsequent hospitalization and death. The execution of this program was heavily dependent on telemedicine technology (Cervino and Oteri, 2020). Our observations suggest a majority of hospitalizations could be avoided and the spread of SARS-CoV-2 can be reduced with a first treat-at-home approach featuring telemedicine during follow-up (Gambardella et al., 2020; Tolone et al., 2020). We leveraged of agents that were commercially available and had a reasonable chance of therapeutic gain with acceptable safety. Because multiple agents are used empirically and in combination given the context of an emergency pandemic, it is impossible to retrospectively stratify for each component and analyze individual effects. We addressed viral replication, cytokine storm, and tissue damage due to oxidative stress utilizing vitamins, micronutrient supplements, and prescription medications (Zhang et al., 2020). Additionally, we encouraged the use of renin-angiotensin system inhibitors based on their theoretical effect over the long term for upregulation of the angiotensin converting enzyme 2 receptor which, despite being the entry receptor for the SARS-CoV-2 also protects lungs in preclinical models of adult respiratory distress syndrome (Lo et al., 2020; Palazzuoli et al., 2020). Our approach was later supported by concurrent analyses and subsequent published reports (Lo et al., 2020; Palazzuoli et al., 2020; Derwand et al., 2020). The observed rates of these outcomes are considerably lower than reported in other studies in our region. A recent report from Methodist hospital in Houston reported that patients with progressive symptoms when hospitalized suffered a 5.8% mortality rate was despite the use of HCQ, remdesivir, convalescent plasma, and anticoagulants (Vahidy et al., 2020). Undoubtedly a portion of the mortality benefit of outpatient therapy is reducing the need for supplemental oxygen and mechanical ventilation. A recent series from Italy has demonstrated that there is a graded increase in death rates with 7.4% for whom no oxygen was required, 12.9% for oxygen-requiring, and 23.0% for mechanically ventilated patients (Palazzuoli et al., 2020). Our data suggest the advancement of early home use of off-target antiviral agents (zinc, HCQ, ivermectin, azithromycin, doxycycline), antibiotics, corticosteroids, and in the future empiric anticoagulants could markedly reduce the risk for hospitalization and potentially reduce overall death rates before and during hospitalization (McCullough et al., 2020).
Our report has all the limitations common to the reporting of clinical practice outcomes. During this time there was an evolving set of SARS-CoV-2 assays, and hence when assessed in context to the clinical syndrome, we experienced both false positive and negative testing as reported. Follow-up was performed by usual practice call logs and electronic medical systems and is temporally truncated to the time of this report.
In conclusion, empiric multidrug treatment for ambulatory COVID-19 according to
age, comorbidities, and initial severity of symptoms is feasible with close
follow-up. Our data suggest that such a strategy is associated very low rates of
hospitalization in high-risk patients who receive early outpatient treatment.
This may be due to symptom relief attributed to medications, supportive
parenteral volume expansion, micronutrient supplementation, and compassionate
care delivered by in person visits and telemedicine. The rates of death in our
study indicate that early multidrug therapy is associated with
BCP, CR, VP, ES, CH, contributed patient data, PAM drafted the first version, and all authors contributed edits to the final version.
All patients provided informed consent for treatment according to good clinical practice.
There are no acknowledgments to disclose.
Nothing to disclose. Authors had access to the data and wrote the manuscript.

