1 Taizhou Key Laboratory of Bone and Joint Degeneration Research, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, 317000 Taizhou, Zhejiang, China
2 College of Sports Medicine, Wuhan Sports University, 430079 Wuhan, Hubei, China
†These authors contributed equally.
Abstract
Peripheral nerve injury is a relatively common clinical condition that predominantly results from sensory, motor, and nutritional disorders. These can be due to aging, external forces, diseases, or changes in physical and chemical environments. Although interventions, including relevant drugs and surgeries, have led to advancements in peripheral nerve repair, achieving complete recovery remains a challenge. Untimely treatment and rehabilitation can lead to lifelong disabilities and neurological pain. Exercise is a low-cost intervention that plays an active role in the rehabilitation of patients with many diseases, including peripheral nerve injuries. This narrative review, conducted in accordance with the Scale for the Assessment of Narrative Review Articles guidelines, synthesized evidence from searches of PubMed, Scopus, Web of Science, and Google Scholar databases to summarize the molecular mechanisms of exercise and adjuvant therapies in peripheral nerve injury rehabilitation and the synergistic benefits of combined exercise and adjuvant therapy for peripheral nerve repair. This study revealed that the combination of exercise with either physical therapy or traditional Chinese medicine yielded superior therapeutic outcomes for peripheral nerve injuries attributable to aging, pathological conditions, and environmental factors. These benefits appear to be mediated by the suppression of oxidative stress and inflammatory responses, upregulation of neurotrophic factor expression, activation of autophagic pathways, modulation of endocrine homeostasis, and promotion of vascular network reconstruction. Furthermore, this study provides a theoretical foundation and a potential research direction for elucidating the targeted molecular mechanisms through which exercise ameliorates peripheral nerve injury.
Keywords
- combined modality therapy
- exercise therapy
- peripheral nerve injuries
- physical therapy modalities
- rehabilitation
- sports medicine
Peripheral nerve injury (PNI) is often accompanied by a long recovery period and possible sequelae, including pain, sensory abnormalities, and motor dysfunction. Accidental injury, body strain, aging, and disease are the primary causes of PNI. In the United States, the number of reported cases of PNI caused solely by sports injuries between 2008 and 2019 was 36.9 cases per million people [1]. Owing to the aging population, increased participation in sports and exercise activities, and war-related injuries, the number of PNI cases has increased annually. Surgical intervention and pharmacotherapy remain the primary treatment modalities for neural injury, although emerging therapeutic avenues—including fasting regimens and gut microbiome modulation, continue to be identified [2, 3, 4]. However, enhancing the recovery process of PNI and minimizing its associated sequelae have emerged as critical areas of global research. The regeneration and repair of PNI are intricately linked to multiple factors, including remyelination [5], axon regeneration [6], vascular reconstruction [7], suppression of inflammatory responses [8], regulation of reactive oxygen species (ROS) [9], supplementation of neurotrophic factors [10], and modulation of associated molecular signaling pathways. Currently, several pharmacological agents, such as methylcobalamin, B vitamins, aminopyridines, and exogenous neurotrophic factors, are used for neurological rehabilitation. However, their efficacy is frequently constrained by prolonged treatment durations and inconsistent therapeutic outcomes. Compared to the central nervous system, peripheral nerves have a relatively low degree of differentiation and a high possibility of returning to normal. When effectively amplified, the body’s recovery mechanisms, when effectively amplified, can enhance disease recovery and reduce the occurrence of sequelae. Exercise, the focus of our study, plays a well-documented and positive role in PNI repair [11]. However, the mechanism of PNI rehabilitation during exercise is complex. This review was based on the Scale for the Assessment of Narrative Review Articles (SANRA) criteria, which is used to evaluate exercise-based rehabilitation for PNI. The analysis encompasses its standalone therapeutic efficacy, synergistic effects when combined with other treatments, and the underlying molecular mechanisms. The primary objectives were to establish a theoretical foundation for research on exercise-accelerated neural recovery and to promote exercise engagement among individuals with neurological impairments. The potential implications of this approach extend to enhancing recovery trajectories while simultaneously alleviating the socioeconomic burden on affected families, healthcare systems, and society.
In 1943, Seddon classified nerve injuries into three categories: neuropraxia, axonotmesis, and neurotmesis. Currently, the five-degree classification system proposed by Sunderland remains the most widely adopted approach in clinical practice (Table 1) [12, 13]. In human, when the nerve length defect is
| Seddon classification | Sunderland classification |
I. Neurapraxia: The mildest form of nerve injury is where the nerve’s function is temporarily impaired, but the structure remains intact. There is no Wallerian degeneration, and recovery is usually spontaneous and complete. II. Axonotmesis: The axon is severed, but the endoneurial tube (the structure surrounding the axon) remains intact. Wallerian degeneration occurs in the distal segment of the nerve, and some degree of recovery can occur spontaneously, but functional recovery may be incomplete. III. Neurotmesis: The most severe type of nerve injury is where the entire nerve trunk is severed, including the axons, myelin sheaths, and the surrounding connective tissues. There is no spontaneous recovery, and surgical intervention is usually required for any chance of recovery. | I: There is a temporary conduction block with no axonal damage. II: Axons are severed, but the endoneurial tubes remain intact, allowing for some potential for spontaneous regeneration. III: The nerve fibers are completely transected, but the epineurium (the outermost connective tissue layer) remains intact, providing a pathway for regeneration. IV: The nerve is severely damaged with some fascicles (bundles of nerve fibers) being completely severed, while others may be intact. V: Complete transection of the nerve with no continuity between the proximal and distal stumps, requiring surgical repair for any chance of functional recovery. |
The beneficial effects of exercise in repairing PNI caused by external forces, such as traction, laceration, and compression are well-documented. Exercise has been shown to positively influence neurological recovery by promoting angiogenesis, facilitating axonal sprouting and elongation, enhancing neuromuscular junction reinnervation, and alleviating neuropathic pain [27, 28].
The degree of natural recovery post-PNI varies among mice of different ages, with newborn mice showing significantly better recovery than young mice. Aerobic running exercise intervention five times a week for 5 weeks can significantly accelerate the recovery of impaired neurological function, electrophysiology, and morphology in young rats, whereas such effects are markedly reduced in adult rats [29, 30]. Therefore, younger age demonstrated superior PNI recovery potential, and exercise interventions may further accelerate this regenerative process. This differential response could be attributed to factors such as the plasticity of peripheral nerves, metabolic activity of nerve cells, and rate of axonal regeneration.
Exercise intervention immediately after injury can effectively increase the regeneration of damaged axons [31], as confirmed in rat models of nerve injury [32]. A two-week running exercise intervention, initiated on day 3 post-PNI and conducted five times per week (1 h/session), significantly enhanced axonal regeneration [33]. However, when considering the induction of M2-type macrophages during Wallerian degeneration, it seems that exercise initiated on the 3rd day has a less pronounced effect on nerve regeneration compared to that begun on the 14th day [34]. Another study demonstrated that a 30-day swimming exercise intervention (1 h/day), initiated immediately, 14 days, or 30 days post-injury accelerated nerve regeneration and reversed synaptic loss induced by crush injury [35]. Assessments conducted using horseradish peroxidase neurohistochemistry and the modified Pal-Weigert method revealed that the positive effects of exercise on nerve regeneration may not be significantly discernible until the fourth week [36]. Current animal studies suggest that exercise interventions initiated immediately after PNI or at 3, 14, or 30 days post-injury, can promote nerve regeneration and repair [33, 34, 35]. Notably, multiple studies have reported positive effects of immediate exercise initiation [31]. However, clinical research on exercise timing in humans with PNI remains limited, making it difficult to establish specific optimal timeframes. This is an important area for future clinical investigation.
Short-term exercise can positively affect PNI, regardless of passive, active, or vibration stimulation [37, 38]. Although 5 weeks of whole-body vibration (WBV) intervention (5 sessions/week, 15 min/session, 15 or 30 Hz cycle) in sciatic nerve crush injury rats failed to demonstrate statistically significant improvements in nerve regeneration histomorphometrics [39], another study employing an eight-week WBV protocol (5 sessions/week, 20 min/session, 50 Hz) reported significant functional recovery following compressive nerve injury [37]. This discrepancy may be attributed to differences in the vibration parameters and intervention duration. Similarly, local vibration can achieve comparable effects, possibly due to the proliferation of Schwann cells and activation of Erk1/2 and Akt signaling pathways [40]. However, a previous study demonstrated that short-term exposure to vibrations following sciatic nerve transection and suturing did not promote nerve regeneration after repair [41]. The divergent outcomes observed with similar vibration interventions may stem from differences in vibration modalities and dosage parameters, as current evidence confirms that prolonged high-intensity mechanical vibration can induce neural damage.
Mice that performed voluntary movements regenerated sensory neuron axons, with the growth length of their nerve axons showing a positive correlation with the distance traveled during voluntary movement [42]. The improvement in nerve regeneration and remodeling of central neurons during active and passive exercises is likely due to the release of neurotrophic factors [43]. Low-intensity exercises can also effectively promote peripheral nerve axon elongation [44]. In addition, slow exercise before and after injury can increase the axonal growth of injured nerves [44, 45]; even in cases of repeated nerve compression, functional recovery may improve as a result of such physical activity [46]. These findings demonstrate that both active and passive exercises enhance PNI recovery, even at lower exercise intensities.
Studies have reported mixed results regarding the effects of exercise on nerve damage repair. Excessive swimming exercise has been demonstrated to positively impact nerve repair in some cases [47], while other study has indicated that it might not only fail to improve nerve damage repair but could also potentially worsen the extent of nerve injury [48]. This discrepancy may be attributed to variations in nerve types, PNI modeling methods, and differential effects of exercise on distinct body regions. For example, uphill treadmill exercise may foster the regeneration of exercise axons but appears to be less effective in achieving functional recovery [49]. In contrast, moderate-intensity running exercises have been found to enhance nerve crush injuries [50], suggesting that exercise efficacy depends on both modality and injury type. A study examining the effects of aerobic and resistance exercises on nerve regeneration suggested that aerobic endurance exercise can boost nerve damage repair, whereas resistance exercise, either alone or in combination with aerobic exercise, may delay functional recovery or have no effect on nerve regeneration [51]. This was further complicated by findings that showed no significant difference between weight-bearing and non-weight-bearing swimming exercises in their ability to improve nerve damage [52]. Another study reported that both aerobic and anaerobic exercises could promote recovery from sciatic nerve injury, with no significant differences observed between the various exercises performed [53]. Debate exists regarding the recovery of nerve damage through overload exercise, as research has indicated that an overload of physical activity at any stage of nerve regeneration can be detrimental [34].
In a comparative study on the efficacy of different exercises for PNI rehabilitation, short-term running exercises were found to have a better effect on nerve regeneration than long-term running, although this finding warrants further investigation [54]. Additionally, balance and coordination training has been investigated in nerve regeneration and shown to exert positive effects [55], although its impact on improving the neural ultrastructure appears less pronounced than that of aerobic endurance exercise [56].
Therefore, a conclusive comparison of the efficacy of various exercise methods in PNI repair may require more extensive research with larger sample sizes. The overall positive effect of exercise on nerve regeneration has already been confirmed. The specific type and intensity of exercise play pivotal roles in the observed outcomes of nerve regeneration. According to the current research findings, non-weight-bearing aerobic exercises appear to be particularly effective in repairing nerve damage.
Systematic reviews and analyses of relevant studies demonstrate that consistent moderate exercise can enhance the clearance of myelin debris, foster myelin formation and regeneration, and the promote expression of associated proteins [36, 57]. Repetitive hindlimb standing training in rats can reverse the reduction in nerve conduction velocity induced by crush injury [58]. Even in models of axotomy and repeated sciatic nerve crush injury, running exercise (
The mechanisms by which exercise affects peripheral nerve axon regeneration appear to differ according to sex. This effect was more pronounced in male mice during treadmill training. These differences may be related to testicular androgens [64]. The exercise environment can also affect neurological injury recovery; for example, swimming exercises in cold water have produced significant improvements [65, 66]. Therefore, environmental temperature modulation during exercise-mediated PNI recovery may accelerate rehabilitation by potentiating axonal regeneration and remyelination.
Disease-related PNI related to disease mainly results from hyperglycemia, hypoxia, radiation, and autoimmunity. The role of exercise in PNI repair in patients with chronic autoimmune diseases has been extensively investigated. The main mechanisms involved include improving the disease environment, increasing blood circulation, and improving immune function.
Diabetes mellitus, a disease caused by impaired blood sugar regulation leading to elevated systemic blood sugar levels, can induce various injuries, including PNI. In a diabetes model, a 16-week exercise intervention (30 min/session, 8 m/s speed, 0% incline) potentiated the beneficial effects of insulin therapy on the PNI [67]. Skin biopsy analyses in early stage diabetic patients revealed significantly increased nerve fiber density following exercise intervention compared to non-exercise controls [68]. Long-term exercise regimens have also been shown to prevent the development of diabetic peripheral neuropathy (DPN) [69]. For durations ranging from 8 to 12 weeks, moderate physical exercise in various forms, including aerobic, resistance, sensory, and gait training, has been shown to significantly increase the conduction velocities in the tibial, peroneal, and sural nerves of patients [70]. Notably, even in the well-established streptozotocin-induced diabetic rat model, aerobic running training not only significantly promotes axonal regeneration of the sciatic nerve but also increases myelin formation, inhibits myelin degradation, and prevents ultrastructural changes in axonal morphology [71, 72, 73]. However, other studies have shown that a 12-week program of aerobic exercise, isokinetic strength training, or a combination of both aerobic and isokinetic strength training does not alter the electrodiagnostic outcomes of sensory or motor nerves in patients with diabetes [74], but also suggest that larger sample sizes may be needed to support the current conclusions. Nevertheless, exercise may also suppress the progression of nerve damage [75], possibly due to increased levels of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) [76]. For individuals with type 2 diabetes, aerobic exercise positively affects nerve function and symptoms related to neuropathy, such as an increase in the branching of skin nerve fibers, and is associated with a lower rate of adverse events [77, 78]. In summary, exercise exerts beneficial effects on diabetes-associated PNI, primarily through the neuroprotective preservation of neural architecture, enhanced nerve conduction velocity, and elevated NGF levels. Additional mechanisms require further investigation (Fig. 1A).
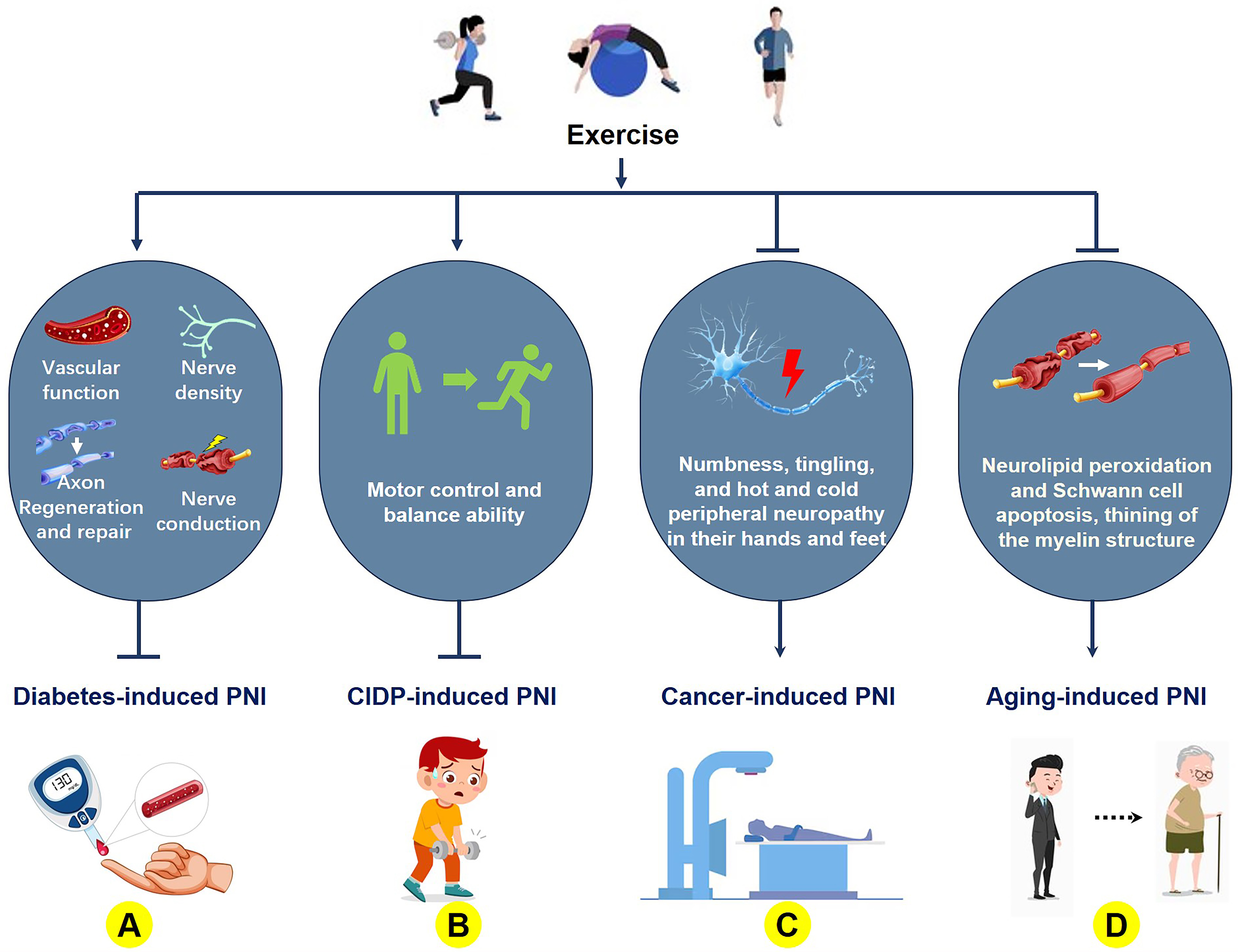 Fig. 1.
Fig. 1. The molecular mechanism of exercise improvements of aging- and disease-related peripheral nerve damage. (A) Exercise ameliorates diabetes-induced peripheral nerve injury (PNI) by enhancing vascular function, promoting axonal regeneration and repair, increasing synaptic density, and improving nerve conduction velocity; (B) Exercise improves PNI caused by chronic inflammatory demyelinating polyneuropathy (CIDP) through enhancing motor postural control and balance capacity; (C) Exercise mitigates PNI secondary to cancer and its treatments by suppressing distal limb numbness, tingling, and hot and cold peripheral neuropathy in their hands and feet; (D) Exercise attenuates aging-associated PNI via inhibiting lipid peroxidation in nerves, Schwann cell apoptosis, and myelin sheath thinning. The figure was created using Microsoft Office PowerPoint (Microsoft 365, Microsoft Corporation, Redmond, WA, USA).
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an autoimmune peripheral motor and sensory neuropathy characterized by chronic demyelination of the proximal peripheral nerves. CIDP primarily manifests as muscle weakness, respiratory involvement, and reflex loss. Although the mechanisms by which exercise improves peripheral nerve function in CIDP remain unclear, exercise has demonstrated significant efficacy in ameliorating clinical manifestations in patients with CIDP. Resistance and aerobic exercise interventions have been shown to improve muscle strength and aerobic capacity in patients with CIDP [79]. When combined with Russian current stimulation, exercise significantly increased the quadriceps strength and clinical performance [80] (Fig. 1B). After a 6-week clinical intervention, significant improvements in motor control and balance were reported [81]. Therefore, exercise can significantly enhance motor control abilities in patients with CIDP by improving peripheral nerve function. However, the mechanisms underlying nerve repair require further investigation to develop new therapeutic strategies for patients with CIDP.
Certain cancers can gradually erode the peripheral nervous system, and chemotherapy can exacerbate this damage. Exercise interventions are effective in mitigating such damage. An 8-week multimodal exercise intervention improved chemotherapy-induced peripheral neuropathy [82]. This finding was further validated in a cohort of 178 patients with chemotherapy-induced peripheral neuropathy secondary to cancer treatment [83]. Patients undergoing chemotherapy who received only 6 weeks of walking and resistance training experienced significantly reduced symptoms of numbness, tingling, and hot and cold peripheral neuropathy in their hands and feet [84]. In addition, endurance and balance training can improve chemotherapy-induced neuropathy [85], while regular swimming can alleviate neuroma-related pain, which may be related to increased adiponectin [86]. Overall, exercise ameliorated the symptoms of chemotherapy-induced PNI in patients with cancer (Fig. 1C). However, owing to the large differences in type, dose, and patient status, further research is required to determine the most beneficial exercise prescription [87, 88].
As age increases, peripheral nerve function undergoes degenerative changes. Exercise not only slows age-related cognitive decline [89] but also plays a crucial role in the repair of PNIs. After 10 months of swimming exercise in rats aged 17–27 months, a 10-month swimming exercise intervention prevented the degeneration of peripheral nerves associated with aging [90], reduced neurolipid peroxidation and Schwann cell apoptosis, and demonstrated thickening of the myelin structure, thereby achieving nerve protection [91]. Simultaneously, low-intensity aerobic exercise in aged rats can enhance peripheral nerve health by increasing the tibial nerve blood vessels, which diminish with age to protect these blood vessels [92] (Fig. 1D). Therefore, the ameliorative effects of exercise on age-related PNI primarily involve the modulation of peripheral nerve biological mechanisms through enhanced myelination and vascular protection. However, implementation of specific exercise regimens may require individualized clinical information and monitoring to ensure effectiveness.
Exercise has become an important intervention in the clinical rehabilitation of patients with PNI. Its effectiveness is not only reflected in improving physical function and nerve regeneration but also involves complex molecular mechanisms. These mechanisms include the regulation of neurotrophic factors, oxidative stress, sex hormones, inflammation, vascular regeneration, autophagy, and neural Erk1/2 and Akt/mechanistic target of rapamycin (mTOR) pathways.
Neurotrophic factors (NTFs), which comprise a series of proteins that support nerve survival, growth, development, and repair, play indispensable roles in PNI repair. The observed improvement in PNI with running exercises may be closely related to increased concentrations of glial cell line-derived neurotrophic factor (GDNF), BDNF, and insulin-like growth factor 1 (IGF-1) [93]. Voluntary exercise also increases brain-derived BDNF levels and improves neuroplasticity, thereby aiding recovery after PNI [94]. However, BDNF secretion typically decreases following exercise cessation, as evidenced by a significant decrease in its levels [95]. The role of exercise in reducing PNI-related pain may be related to reduced glial cell responses to sciatic nerve pain, increased BDNF expression in nonglial cells, and suppression of the BDNF/Akt/mTOR signaling pathway, which inhibits autophagy and regulates microglial polarization (Fig. 2A) [96, 97]. Exercise ameliorates neural damage by preserving or restoring synaptic inputs to the axonal motor neurons. Conditional knockout of BDNF results in a significant reduction in synaptic contact, and transplantation of BDNF-knockout neurons greatly reduces the effect of exercise on axonal regeneration [98]. The Val66Met BDNF polymorphism is linked to activity-dependent impairments in BDNF secretion, and exercise does not enhance the axonal regeneration capacity of heterozygous and homozygous Val66Met BDNF polymorphic mice after nerve injury through the promotion of BDNF secretion [99]. Similarly, Schwann cells lacking BDNF expression fail to recapitulate the regenerative potential observed in their wild-type counterparts, as demonstrated in transplantation experiments [100]. Study has shown that exercise may increase the number of tropomyosin receptor kinase B (TrkB) receptors in the growth cones of regenerating axons [59]. TrkB inhibition partially offsets the positive effects of exercise; however, pharmacological activation of TrkB does not completely mimic the effects of exercise [101]. While the importance of BDNF in PNI repair has been confirmed, one study indicated that BDNF mRNA levels significantly increased after sciatic nerve injury, and exercise reduced the increase in mRNA levels to promote nerve regeneration and recovery [102]. Additionally, exogenous nerve NGF inhibits p38 mitogen-activated protein kinase (p38 MAPK) activation, protects hypoglossal neurons, and promotes nerve regeneration [103]. Variations in neurotrophic factors may help distinguish between types of nerve injuries [104]. Therefore, exercise-induced upregulation of NTFs in the neural and somatic tissues may represent a critical therapeutic strategy for PNI recovery.
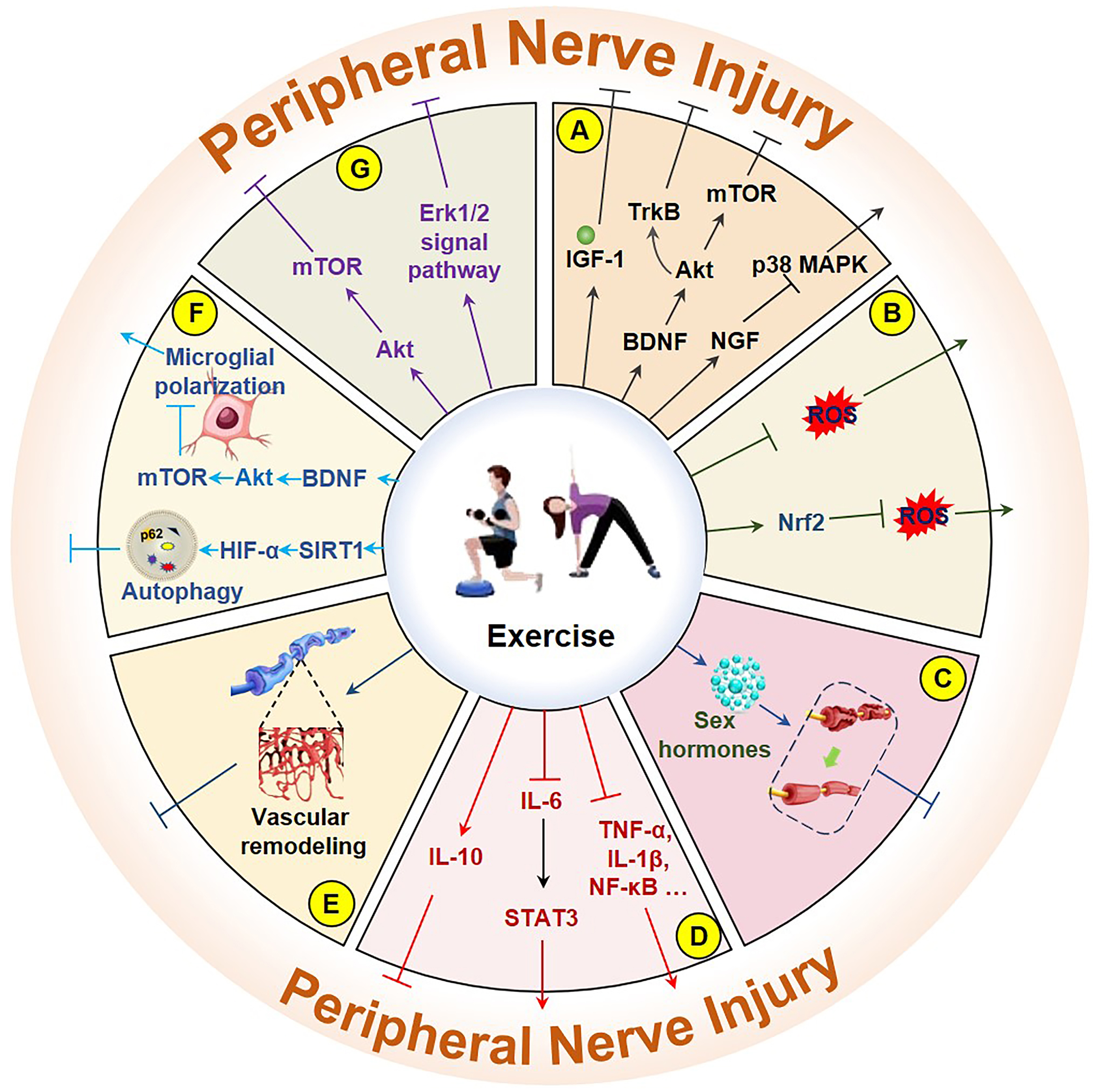 Fig. 2.
Fig. 2. Molecular mechanisms of exercise improving PNI. (A) Exercise mitigates neuronal damage by increasing IGF-1, BDNF/Akt/mTOR, BDNF/TrkB, and NGF/p38 MAPK. (B) Exercise mitigates neuronal damage by directly reducing ROS and by activating the Nrf2/ROS pathway. (C) Exercise improves neuronal damage by increasing sex hormones. (D) Exercise improves neuronal damage by promoting IL-10, inhibiting the IL-6/STAT3 signaling pathway and inflammatory factors (NF-
ROS can damage cell function and survival as byproducts of mitochondrial energy production. PNI is often accompanied by an increase in ROS levels, and the antioxidant effect of exercise plays a positive role in the repair and improvement of both the peripheral and central nervous systems [105, 106]. Aging-induced PNI is more common in older adults, and aerobic exercise can protect peripheral nerves by attenuating oxidative reactions and pathological changes [91]. The inhibition of ROS generation plays a protective role [107]. In addition, preconditioning exercise activates nuclear factor erythroid 2-related factor 2 (Nrf2), which in turn reduces ROS levels and relieves neural pain (Fig. 2B) [108]. Forced exercise can also increase total antioxidant capacity and reduce TNF-
Sex differences appear to influence the effect of exercise on peripheral nerve axon regeneration, with a greater effect previously reported in males during treadmill training. However, this difference was not observed in castrated male mice, suggesting a potential role for testicular androgens [64]. When testosterone is administered within the normal physiological range, it reduces dendritic atrophy and increases excitability [112], an effect that appears to be comparable to the effects of exercise [113]. The regenerative effects of exercise or electrical stimulation on nerves are weakened after blocking androgens [114]. Additionally, the anti-androgen flutamide has been shown to significantly attenuate exercise-induced preservation of synaptic connectivity following sciatic nerve transection [115]. Importantly, flutamide-mediated androgen receptor blockade similarly diminishes the regenerative effects of exercise and electrical stimulation on axonal growth, with comparable outcomes observed in orchiectomized animal models [116]. In addition, increased testosterone levels can accelerate peripheral nerve regeneration [117], a mechanism that may be related to intraneuronal BDNF levels and TrkB receptor regulation [118]. However, systemic androgen receptor blockade significantly decreased exercise-induced axonal regeneration in both sexes. Although the effect of exercise on PNI repair is more pronounced in male mice, it also plays an important role in neural repair in female mice [119]. However, to achieve the same effect, females may need a higher training intensity. Notably, the advantage of this intervention was lost in castrated male mice during treadmill training [64]. Administering estradiol increases the number of axonal regenerations in peripheral neurons and shows effects similar to those of exercise [120]. Thus, sex hormones may play a positive role in PNI repair, with androgens particularly important for nerve repair (Fig. 2C).
PNI is accompanied by inflammation, which is an important link between PNI and repair. Proinflammatory factors play a role in nerve damage repair by recruiting macrophages, which aid in the repair and reduction of inflammation through the action of anti-inflammatory factors. Exercise accelerates the regeneration of damaged nerves by activating macrophages [121]. Exercise before and after nerve injury significantly reduces nuclear factor kappa-light-chain-enhancer of activated B cells (NF-
Nervous tissue damage is often accompanied by vascular tissue damage, posing a significant obstacle to nerve tissue repair. Because peripheral nerves have a poor vascular supply, vascular remodeling is crucial for accelerating the healing process and quality by providing nutrients and bioactive substances. Furthermore, exercise can promote vascular regeneration of damaged nerves, thereby accelerating the regeneration and repair of nerve axons [27]. Regular moderate aerobic exercise improves neurological degeneration in aged rats by increasing the thickness of myelin around myelinated fibers and by dilating capillaries [128]. In terminal diabetes mellitus-related microcirculatory disorders, exercise significantly improved blood circulation and oxygen concentration in the feet by increasing microvessel density (Fig. 2E). Exercise positively affects nerve terminal diseases [129, 130, 131] and significantly improves nerve conduction velocity [132]. Therefore, improving circulation by enhancing neurovascular remodeling through exercise positively affects PNI repair.
Autophagy, as a scavenging process in the body, removes aging damaged organelles and misfolded proteins in cells and plays an active role in nerve injury recovery. In models of nerve injury, sirtuin 1 (SIRT1)/HIF-1
Exercise also involves the regulation of related classical molecular signaling pathways in the PNI repair process. In animal models of sciatic nerve injury and diabetes-related PNI, treadmill exercise activated p-Erk1/2 levels in Schwann cell and improved nerve damage [131]. Conversely, the Erk1/2 inhibitor PD98059 significantly inhibited axonal regeneration [136]. In a postoperative sciatic nerve model, running training promoted sciatic nerve regeneration by activating the Erk1/2 signaling pathway [137]. This activation may also activate the Akt/mTOR signaling pathway [138]. Therefore, the molecular mechanism by which exercise regulates the Erk1/2 and Akt/mTOR signaling pathways to enhance recovery from PNI injury deserves further investigation (Fig. 2G).
Exercise plays an active role in the repair and regeneration of PNI. Furthermore, a combination of various therapies may enhance the benefits of exercise for PNI treatment. In physical therapy, the integration of therapeutic ultrasonography (TU), light, electricity, and heat, along with traditional Chinese medicine practices such as acupuncture, and modern interventions, such as stem cell transplantation, may be beneficial (Table 2, Ref. [110, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155]).
| PNI model | Exercise and dosage | Combination treatment and dosage | Microstructure or molecular mechanism changes | Ref |
| Rat (sciatic nerve transection and suture repair) | TR: 2 h/time/every day, for 4 weeks | ES: 20 Hz (3 V, 0.1 ms); 1 h/day, for 4 weeks | Myelinated fibers, muscle reinnervation, and function | [139] |
| Rat (neuropathic pain of sciatic nerve constriction injury) | TR: 20 min/time/day for 8 days | tDCS: 20 min/time/day for 8 days | BDNF, IL-1β, IL-4 | [140] |
| Rat (sciatic nerve section and suture repair) | TR: 1 h/time, 1 time/day for 4 days | ES: 20 Hz (3 V, 0.1 ms), 4 h, 1 time | GDNF, BDNF, and function | [141] |
| Human (brachial plexus injury and transplantation) | Shrugging, abduction, and other movements (200 repetitions/time, 6 times/day, for 1 month); lift the forearm of affected limb (25–30 min/time, 2 times/day; for 1 month) | LFES: 25–30 min/time, 2 times/day, for 1 month | Nerve conduction velocity and amplitude | [142] |
| Rat (surgery for sciatic nerve injury) | Swimming: 10 min/time, 5 times/week, for 4 weeks | LFES: 20 min/time, 5 times/week, for 4 weeks | Function | [143] |
| Mouse (sciatic nerve section and suture repair) | TR: 1 h/time/every 3 days, for 2 weeks | ES: 20 Hz, 1 h/time/every 3 days, for 2 weeks | Regeneration of axons | [144] |
| Rat (sciatic nerve chronic constriction injury) | TR: 14–16 m/min, 0.5 h, 5 times/week, for 4 weeks | TU: 1 MHz, 1-W/cm2 intensity, and 100 Hz frequency, 5 min/day, for 4 weeks | IL-10 | [145] |
| Rat (sciatic nerve chronic constriction injury) | TR | TU | IL-10 | [146] |
| Rat (surgery for sciatic nerve injury) | Aerobic exercise: 10 min/time/day, for 2 weeks | Laser: 5 min/time/day, for 2 weeks | Neuroregeneration and neurological function | [147] |
| Rat (surgery for sciatic nerve injury) | Swimming: 10 min/time in 1st week, 20 min/time in 2nd week, 30 min/time in 3rd week, 40 min/time in 4th week, 1 time/day, for 4 weeks | Laser: 8 s/time in 1st week, 16 s/time in 2nd week, 24 s/time in 3rd week, 24 s/time in 4th week, 1 time/day, for 4 weeks | Area, diameter, thickness, axons, and myelin sheaths of nerve fibers | [148] |
| Rat (sciatic nerve crush injury) | TR | Electroacupuncture | Neuroregeneration, myelin recovery, and function | [149] |
| Rat (sciatic nerve crush injury) | TR: 30 min/time/day, for 21 days | Gallic acid: 200 mg/kg weight/d, 21-day | Motor coordination | [150] |
| Rat (sciatic nerve crush injury) | TR: 14–16 m/min, 30 min/time/day, 5 time/week, for 3 weeks | Curcumin: 60 mg/kg/day, for 2 weeks (IP.) | Nerve conduction velocity | [151] |
| Rat (sciatic nerve crush injury) | TR: 14–16 m/min, 30 min/time, 1 time/day, 5 time/week, for 3 weeks | Curcumin: 60 mg/kg/day, for 2 weeks (IP.) | Neuropathic pain | [152] |
| Rat (surgery for sciatic nerve injury) | RE: 60% 1 RM and increased by 10%/week, 8 repetitions/time, 3 times/day, for 3 weeks | Bowdichia virgilioides hydroethanolic extract: 200 mg/kg (p.o.) | MDA | [110] |
| Rat (diabetes-induced sciatic nerve damage) | Swimming: 30 min/day, 5 days/week, for 5 weeks | Quercetin: 30 mg/kg/day, for 5 weeks (p.o.) | MDA, PC, nitrites, iNOS, and sciatic nerves injury | [153] |
| Human (sciatic nerve transection) | Pilates and exercises | Peripheral nerve from sural and autologous cultured Schwann cells | Sensory and motor recovery | [154] |
| Mice (sciatic nerve transection) | TR: 10 m/min, 30 min/time, 2 repetitions/time, 3 times/week, for 8 weeks | Schwann cell graft | Function and morphology | [155] |
Abbreviations: CAT, catalase; ES, electrical stimulation; iNOS, inducible nitric oxide synthase; IP, intraperitoneal; LFES, low-frequency pulse electrical stimulation; LLLT, low-level laser therapy; MDA, malondialdehyde; p.o per os (by mouth); RE, resistance exercise; RM, repetition maximum; SOD, superoxide dismutase; tDCS, transcranial direct current stimulation; TR, treadmill; TU, therapeutic ultrasound;
In conventional physical therapy, neuromuscular electrical stimulation promotes nerve regeneration [156]. In a rat sciatic nerve injury suture model, a combination of exercise and electrical nerve stimulation significantly improved synapse regeneration and neuromuscular innervation in the initial stage and reduced the occurrence of hyperreflexia [139]. This combination therapy also alleviated pain resulting from nerve damage by regulating BDNF, IL-1
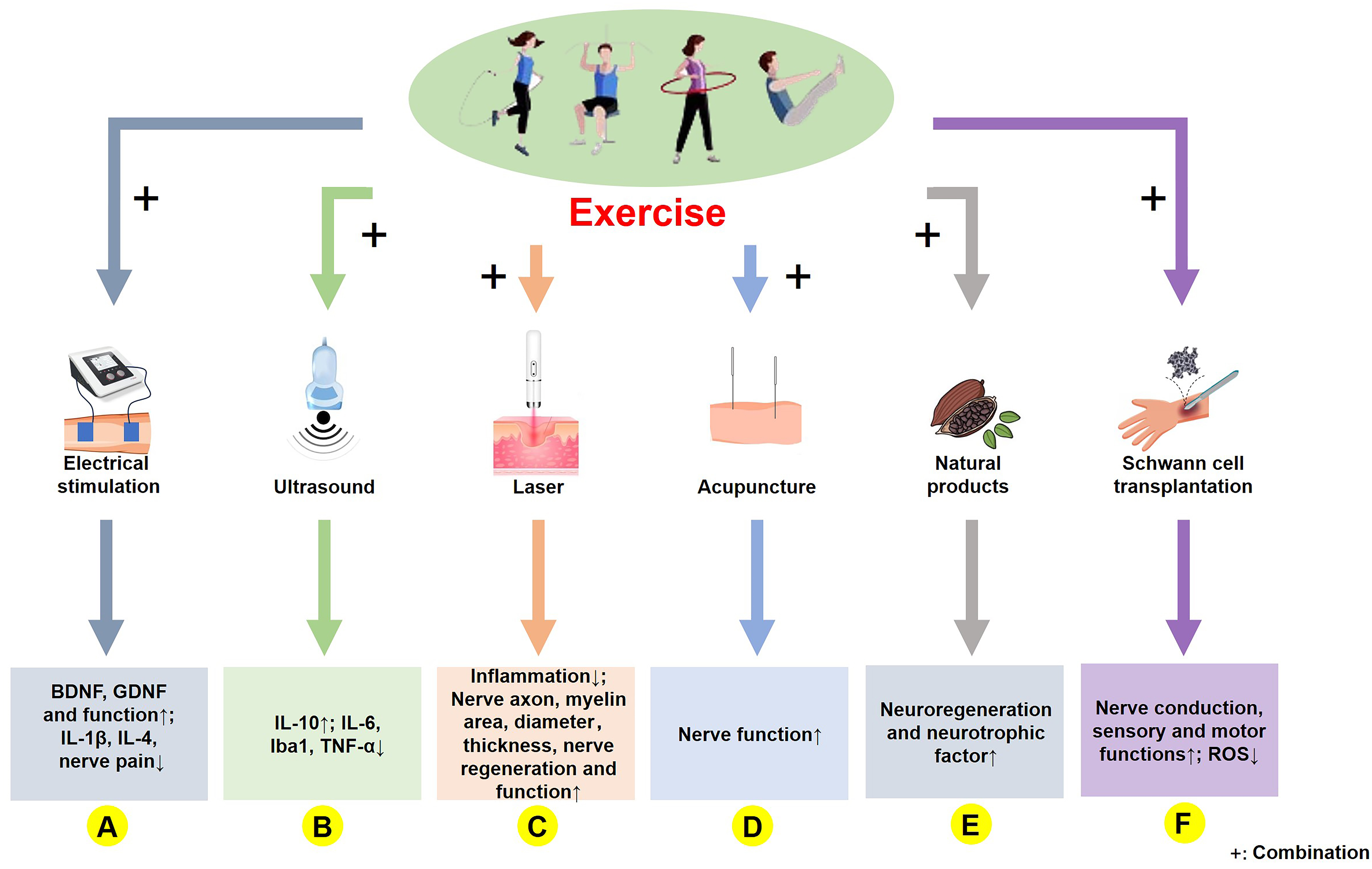 Fig. 3.
Fig. 3. Molecular mechanism of exercise combined with other treatments in PNI rehabilitation. (A) Exercise combined with electrical stimulation upregulates BDNF and GDNF levels, enhances motor function, and reduces IL-1
TU can improve microcirculation by penetrating the surface tissues and heating deeper tissues. In a rat model of chronic constriction injury of the sciatic nerve, the combined intervention of TU and exercise alleviated pain resulting from nerve injury by suppressing IL-6 and Iba1 and upregulating IL-10 [145]. Similarly, running exercise combined with TU showed an improved reduction in IL-6 and TNF-
As a highly penetrating and noninvasive physical therapy method, high-energy lasers are commonly used for skeletal muscle and nervous system rehabilitation, and laser therapy is widely recognized. In a sciatic nerve crush injury model, a 14-day regimen of low-level laser therapy significantly promoted recovery from nerve injury [159]. In the same rat model of sciatic nerve injury, low-intensity laser therapy alone augmented the morphometric area of nerve myelin, without exerting a positive impact on the functional recovery of the nerve [160]. This suggests that both the laser treatment parameters and PNI models can significantly influence the extent of recovery. However, low-power laser irradiation combined with exercise significantly reduces inflammation, increases nerve regeneration, and improves nerve function [147], indicating that the integration of exercise with laser therapy may have synergistic effects on both nerve injury and functional recovery. This was further confirmed in a rat sciatic nerve crush injury model in which laser therapy was combined with swimming exercises. The main findings showed that neither exercise nor laser therapy alone had significant effects on the morphology and function of the injured nerve, whereas the combined intervention significantly improved both nerve morphology and function (Fig. 3C) [148]. However, it has been reported that intervention using laser therapy does not significantly improve the PNI [160], whereas laser therapy combined with exercise leads to improvement. Overall, exercise combined with laser treatment may accelerate recovery from PNI.
Acupuncture is a traditional Chinese therapy that is widely used to treat various diseases. In a rat model of sciatic nerve injury, the combination of combining electroacupuncture with exercise was more effective than either treatment alone. This combined therapy not only promoted better recovery of myelinated axons but also enhanced their innervation and muscle activity (Fig. 3D) [149]. In a rabbit model of sciatic nerve injury, combining electroacupuncture with treadmill exercise significantly enhanced axonal regeneration compared to either treatment alone [161]. While exercise and acupuncture may positively affect PNI, relevant studies are scarce.
Natural products, which are biologically active substances found in nature, are widely used in disease management and healthcare. In a rat model of sciatic nerve crush injury, a 21-day regimen of gallic acid (200 mg/kg) combined with aerobic treadmill exercise significantly improved nerve conduction function [150]. Curcumin improved myelination and functional recovery in a sciatic nerve compression model by reducing oxidative stress [162]. In a chronic compression nerve injury model, a combination of curcumin and exercise effectively prevented electrophysiological disorders of the sciatic nerve [151]. Moreover, when comparing curcumin and exercise, a combination treatment was more effective in reducing pain [152]. A combined intervention of Bowdichia virgilioides hydroethanolic extract and resistance exercise reduced oxidative stress associated with PNI [110]. In diabetic nerve injury models, exercise improved nerve damage by reducing oxidative stress via interactions with quercetin (Fig. 3E) [153]. A combination of movement and natural products may have a synergistic effect on the PNI, and an in-depth exploration of this mechanism is a future research direction.
Schwann cells are present in both myelinated and unmyelinated nerve fibers. They promote axonal regeneration and myelination by secreting neural factors. In 2016, Levi et al. [154] were the first to demonstrate that transplanting Schwann cells through autologous culture could accelerate recovery from PNI. After sciatic nerve transection and Schwann cell transplantation, there was a significant increase in the nerve function index and number of neurons (Fig. 3F) [155]. Exercise can improve nerve regeneration after nerve injury, which can also be improved by Schwann cell transplantation [100]. Therefore, combining exercise with Schwann cell transplantation may enhance PNI repair. Although research in this area is limited, this could offer a promising approach for neurosurgical and rehabilitation studies.
The rehabilitation process for a PNI caused by trauma or disease is complex and involves multiple physiological processes and molecular mechanisms. Exercise plays a critical role in accelerating recovery and reducing long-term complications. Current evidence suggests that non-weight-bearing aerobic exercise, early exercise intervention, temperature-modulated exercise, and a combination of exercise and other treatment modalities may enhance rehabilitation outcomes. However, clinical research on such combined therapeutic regimens remains limited, particularly regarding exercise combined with physical therapy, exercise environment optimization, and traditional Chinese medicine, all of which demonstrate substantial clinical potential. Therapeutic strategies targeting multiple pathological mechanisms, including inflammation suppression, oxidative stress reduction, neurotrophic factor upregulation, cellular autophagy modulation, vascular remodeling facilitation, and hormonal homeostasis regulation, may offer novel research avenues for PNI rehabilitation.
ZWZ and JLH performed the literature search, wrote the manuscript and created the figures. JWS and HZ were responsible for the conception and design of the manuscript. All authors contributed to editorial changes in the manuscript and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



