1 Athens Microneurosurgery Laboratory, National and Kapodistrian University of Athens School of Medicine, 10676 Athens, Greece
2 Department of Neurosurgery, National and Kapodistrian University of Athens School of Medicine, 10676 Athens, Greece
3 Centre for Spinal Studies and Surgery, Queens Medical Centre, Nottingham University Hospital, NG7 2UH Nottingham, UK
4 Section of Neurosurgery, Dartmouth-Hitchcock Medical Center, Lebanon, NH 03756, USA
5 Department of Neurosurgery, Toronto Western Hospital, University Health Network, Toronto, ON M5T 1P5, Canada
†These authors contributed equally.
Abstract
The objective of this study was to map the topography, morphology, connectivity, and correlative anatomy of the ansa lenticularis (AL) in the human brain since there is a paucity of direct structural evidence from cadaveric studies.
Twenty normal adult formalin-fixed cerebral hemispheres were treated with Klingler’s method and subsequently explored through the fiber microdissection technique. Basal and medial dissections focusing on the anterior perforated substance, subthalamic, and mesencephalic areas were carried out in a stepwise manner. Hemispheric asymmetries were recorded, and the spatial relationship of the AL with the surrounding fiber tracts and nuclei was investigated.
The AL and its segments were consistently identified sweeping around the cerebral peduncle in a medial direction posterior to the anterior commissure and dorsal to the optic tract and ansa peduncularis after arising from the ventral-posterior margin of the globus pallidus. Then it made an almost right angle to reach the thalamus (dorsal segment), subthalamic nucleus (dorsal segment), red nucleus (middle segment), and substantia nigra (ventral segment), respectively. Additionally, the dorsal segment of the AL intermingled with the fasciculus lenticularis (FL) at the level of the zona incerta (ZI) to form the thalamic fasciculus (H1 field of Forel), which travelled slightly lateral to the cerebellothalamic fibers, ascended through the prerubral field, and terminated in the area of the anterior and ventral thalamus.
We provide structural evidence of the topography, morphology, and connectional anatomy of the ansa lenticularis. From our review of the literature this is the first cadaveric study using white matter microdissection to delineate the comprehensive composition of the ansa lenticularis. Fiber microdissection studies are integral for the extrapolation of accurate anatomical conclusions which subsequently inform clinical practice. Combined with tractography and histology, these studies enhance our understanding of delicate pathways that can act as surgical targets in fields such as stereotactic neurosurgery.
Keywords
- ansa lenticularis
- basal ganglia
- fiber tracts
- deep brain stimulation
- neuroanatomy
By the end of the 19th century, neuroanatomists, including Louis Pierre Gratiolet, Theodore Meynert, Auguste Forel, and Constanin von Monakow, had engaged with the anatomy of complex subthalamic pathways. Von Monakow was the first to describe a group of fibers participating in basal ganglia connectivity known as the pallidofugal pathways consisting of the subthalamic fasciculus, lenticular fasciculus (or fasciculus lenticularis), and ansa lenticularis (AL)—a term he coined [1, 2]. According to von Monakow, the AL consisted of a dorsal and a ventral pathway interconnecting the globus pallidus to the thalamic region. During the first half of the 20th century, Vogt’s study of the AL described this tract as one of two main components of the pallidothalamic pathways; the other component is the lenticular fasciculus [3]. The pallidothalamic pathway would become the main gateway of the cortico-basal ganglia-thalamo-cortical pathway and the principal output of the globus pallidus internus (GPi). Additionally, the nigrothalamic tract has been marked as another important output pathway of the basal ganglia via its connectivity of the substantia nigra to the thalamus [4]. It is now understood that, through the pallidothalamic and nigrothalamic tracts, the basal ganglia are involved in various roles including motor, limbic, and associative functions [4, 5]. Yet, for the purposes of this study, we will focus on the pallidothalamic tract of the ansa lenticularis given the paucity of literature on this structure.
In a more complex approach, later researchers, including Klingler and Gloor, described the AL as a fiber tract connecting the globus pallidus not only to thalamic and subthalamic areas, but also to mesencephalic structures like the red nucleus and substantia nigra [6]. Greater interest in the anatomy of the pallidothalamic connectivity was sparked during the second half of the 20th century with the evolution of stereotactic neurosurgery techniques targeting certain nuclei in the pathway for the treatment of movement disorders and neuropsychiatric disorders [2]. Further investigation has led to an evolution in the anatomical understanding of the complex pallidothalamic pathways over time. Near the end of the 20th century, novel evidence of the pertinent anatomy was derived mainly from histologic and non-human primate studies such as the review by Nauta and Mehler (1966) [7] which utilized degeneration and tracer techniques. Yet there are three caveats with this method. First, it often leads to the vague extrapolation of findings from animal models to the human brain. Second, the design makes it such that a three-dimensional perspective of the spatial subcortical relationships cannot be appreciated. Third, although the accuracy in identifying the origin of fibers is high, there are limitations in tracing fiber projections beyond that.
Since then, the recent advent of sophisticated tractographic protocols has blazed new trails in deciphering the anatomy of complex regions and pathways in the human brain, but, as already emphasized in the literature, these imaging techniques offer indirect structural evidence and have to be validated by cadaveric studies, which are a valuable tool to appreciate and illustrate the complex 3-D subcortical anatomy [4]. In this context, Klingler’s technique has been revitalized as a direct anatomical method to assess and validate diffusion tensor imaging (DTI) findings [8]. With this in mind, we aimed to offer a focused overview of the intrinsic and correlative anatomy, morphology and connectivity of the ansa lenticularis using Klingler’s method with white matter micro-dissection technique. Our ultimate goal was to explore the microsurgical features of the ansa lenticularis and to enhance understanding of the elegant architecture of this elusive tract in hopes that this will aid future targeting protocols in fields such as stereotactic neurosurgery for the management of neurologic disease.
Twenty (20) normal adult cadaveric formalin-fixed hemispheres (10 right and 10 left) (Science Care, Phoenix, AZ, USA) were treated according to Klingler’s preparation and subsequently studied using the white matter microdissection technique with the aid of a surgical microscope (OPMI Carl Zeiss Meditec AG, Wetzlar, Hesse, Germany) as previously described [9, 10, 11, 12, 13]. Our dissection tools included various micro-dissectors, micro-forceps and micro-scissors as well as a number 10 blade scalpel. At each dissection step, multiple photographs were obtained from different angles using a Nikon DSLR with macro-lenses (AF-S DX Micro NIKKOR 40mm f/2.8G, Shinagawa, Tokyo, Japan).
In all specimens, we carried out focused stepwise dissections of the medio-basal cerebral surface extending to the area of the anterior perforated substance, anterior commissure and thalamic/subthalamic region. Our goal was to record the topography of the AL while investigating its intrinsic anatomy and spatial relationship to adjacent structures including: the anterior commissure (AC), the ansa peduncularis (AP) with its subcomponents (i.e., amygdalo-septal, amygdalo-thalamic and amygdalo-hypothalamic fibers), the optic tract (OT), the mammillo-thalamic tract, the post-commissural fornix, the nucleus accumbens (NAcc), substantia innominata (SIn), the fasciculus lenticularis (FL), and the internal capsule (IC). Left-right asymmetries were examined. Additionally, in each specimen, the entry zone of the AL to the globus pallidus (GP) and the subthalamic/mesencephalic area were meticulously isolated and recorded. Finally, the patterns of the proposed segmentation of the AL were reassessed based on our anatomical data.
Before starting the dissection process, a number 10 blade scalpel was used to make a sharp cut along the cingulate sulcus, the collateral sulcus, and the anterior orbital lobule. This preparatory step allowed delineation of the area of interest and facilitated specimen manipulation during the rest of the procedure. We started the dissection at the area of the posterior/posteromedial orbital lobule. Upon removing the cortex and superficial white matter of this area, the nucleus accumbens was exposed as a continuous layer of gray matter (Fig. 1). The cortex and superficial white matter of the anterior uncus and the part of the planum temporale adjacent to the limen insulae were then removed to reveal the amygdala. During this step, we made sure not to damage the underlying fibers of the ansa peduncularis that converge on the amygdaloid nucleus. Those fibers were exposed at the next dissection step.
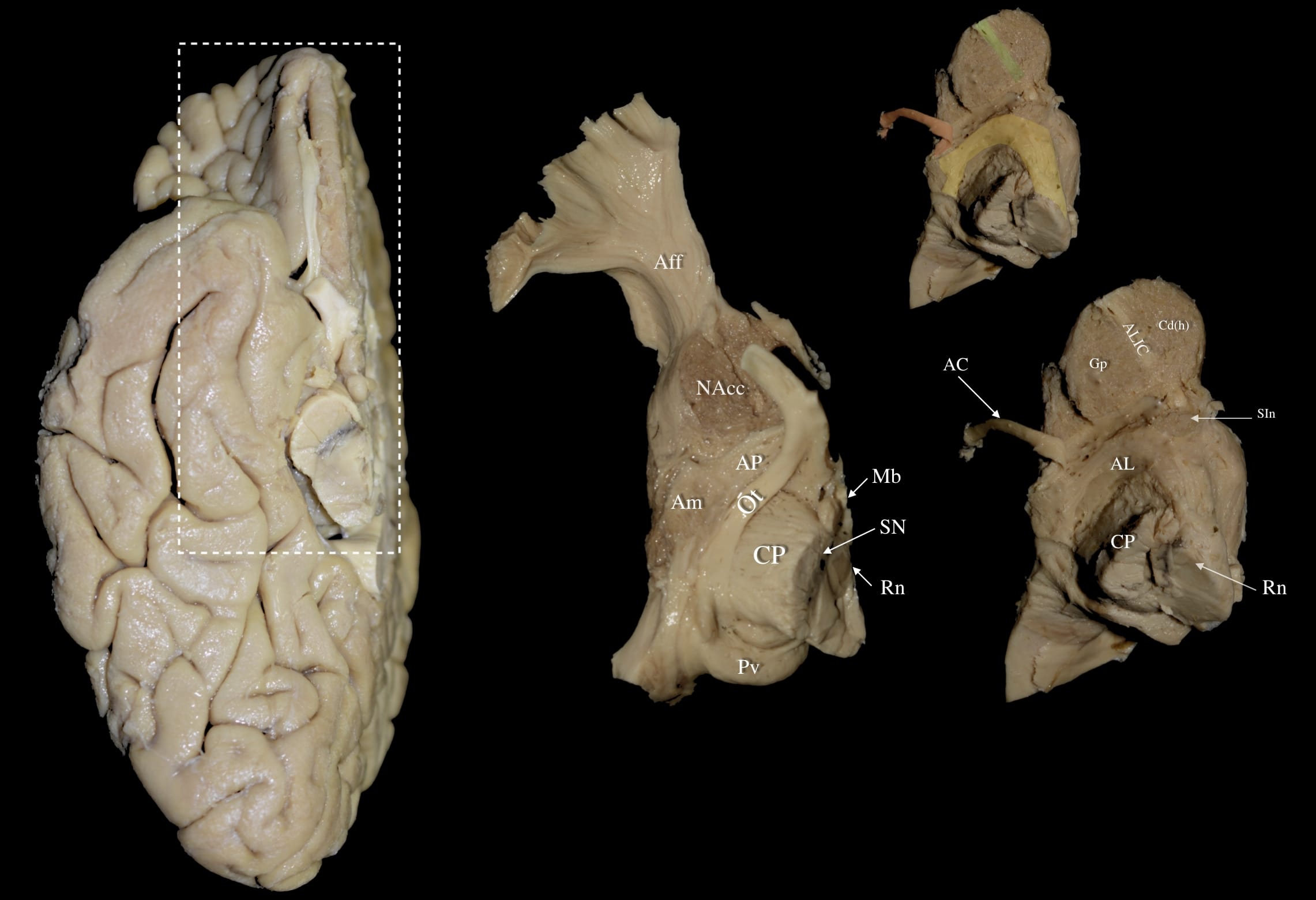 Fig. 1.
Fig. 1. Right hemisphere: Stages of a stepwise dissection of the medio-basal cerebral surface focused in the area of the midbrain, anterior perforated substance, anterior commissure and posterior/posteromedial orbital lobule. Left Panel: Right hemisphere basal view. White dashed box demarcates the dissected area of interest. Middle Panel: Dissection of the posterior/posteromedial orbital lobule and removal of the cortex and superficial white matter in this area exposes the nucleus accumbens (NAcc). Further removal of the anterior uncus and adjacent cortex of the planum temporale reveals the amygdala (Am) and part of the underlying ansa peduncularis (AP) fibers. The superficial lamina terminalis is carefully dissected to expose the anterior AP, primarily composed of amygdalo-septal fibers. Right Panel: Following careful dissection of the Am and AP fibers, the inferior posterior edge of the globus pallidus (Gp) and the anterior portion of the ansa lenticularis (AL) (highlighted in yellow on upper right panel) can be appreciated. Removal of the optic tract further exposes the posterior AL as it curves around the cerebral peduncle. Dissection at the NAcc level gradually reveals the head of the caudate nucleus (Cd (h)) medially and the anterior globus pallidus laterally, with the anterior limb of the internal capsule (ALIC) fibers (highlighted in green on upper right panel) running between them. At this stage, the underlying substantia innominata (SIn) and the anterior commissure (AC) fibers (highlighted in red on upper right panel) dorsal to the SIn can be appreciated. Aff, Accumbofrontal Fasciculus; AC, Anterior Commissure; AL, Ansa Lenticularis; ALIC, Anterior Limb of Internal Capsule; Am, Amygdala; AP, Ansa Peduncularis; CP, Cerebral Peduncle; Gp, Globus Pallidus; Mb, Mammillary Body; NAcc, Nucleus Accumbens; Ot, Optic Tract; Pv, Pulvinar; Rn, Red Nucleus; SIn, Substantia Innominata; SN, substantia nigra.
At this stage, the superficial layer of the lamina terminalis that is slightly posterior to the olfactory triangle was carefully dissected to reveal the anterior aspect of the ansa peduncularis, consisting mostly of amygdalo-septal fibers. Extending the dissection further posteriorly to the level of the optic tract revealed the remaining portion of the ansa peduncularis, which is composed of amygdalo-thalamic and amygdalo-hypothalamic fibers, and the most anterior fibers of the ansa lenticularis. These fibers terminated laterally at the superior and medial aspect of the amygdala.
By carefully retracting the amygdala along with the fibers of the ansa peduncularis, one can unveil the infero-posterior edge of the globus pallidus as well as the fibers of the anterior part of the ansa lenticularis. Removing the optic tract exposed the posterior part of the ansa lenticularis that was seen curving around the cerebral peduncle. Attention was turned to the nucleus accumbens, and dissection of its gray matter disclosed the head of the caudate nucleus medially and the anterior part of the globus pallidus laterally. Between these two anatomical structures, the fibers of the anterior limb of the internal capsule were appreciated. At this stage, the underlying SIn and the AC fibers dorsal to the SIn can be appreciated.
The ependymal layer lining the intraventricular surface of the caudate nucleus and thalamus were peeled away, and the post-commissural fornix terminating at the mammillary body was identified and retracted (Fig. 2). Upon retracting the post-commissural fornix, the amygdalo-thalamic and amygdalo-hypothalamic fibers of the ansa peduncularis bent posterior to the anterior commissure and radiated to the thalamus and hypothalamus, respectively. In contrast, the amygdalo-septal fibers coursing anterior to the anterior commissure terminated in the septal area. At this point, a diligent dissection of the amygdalo-thalamic and amygdalo-hypothalamic fibers was carried out to reveal the distinct fibers of the ansa lenticularis terminating in the area of the thalamus/hypothalamus/subthalamic nucleus, red nucleus, and medial part of the substantia nigra (Fig. 3). Of note, dorsal to the AL was a group of fibers originating from the ventral tegmental area and projecting towards the ventral striatum; these fibers form the mesolimbic pathway (not pictured).
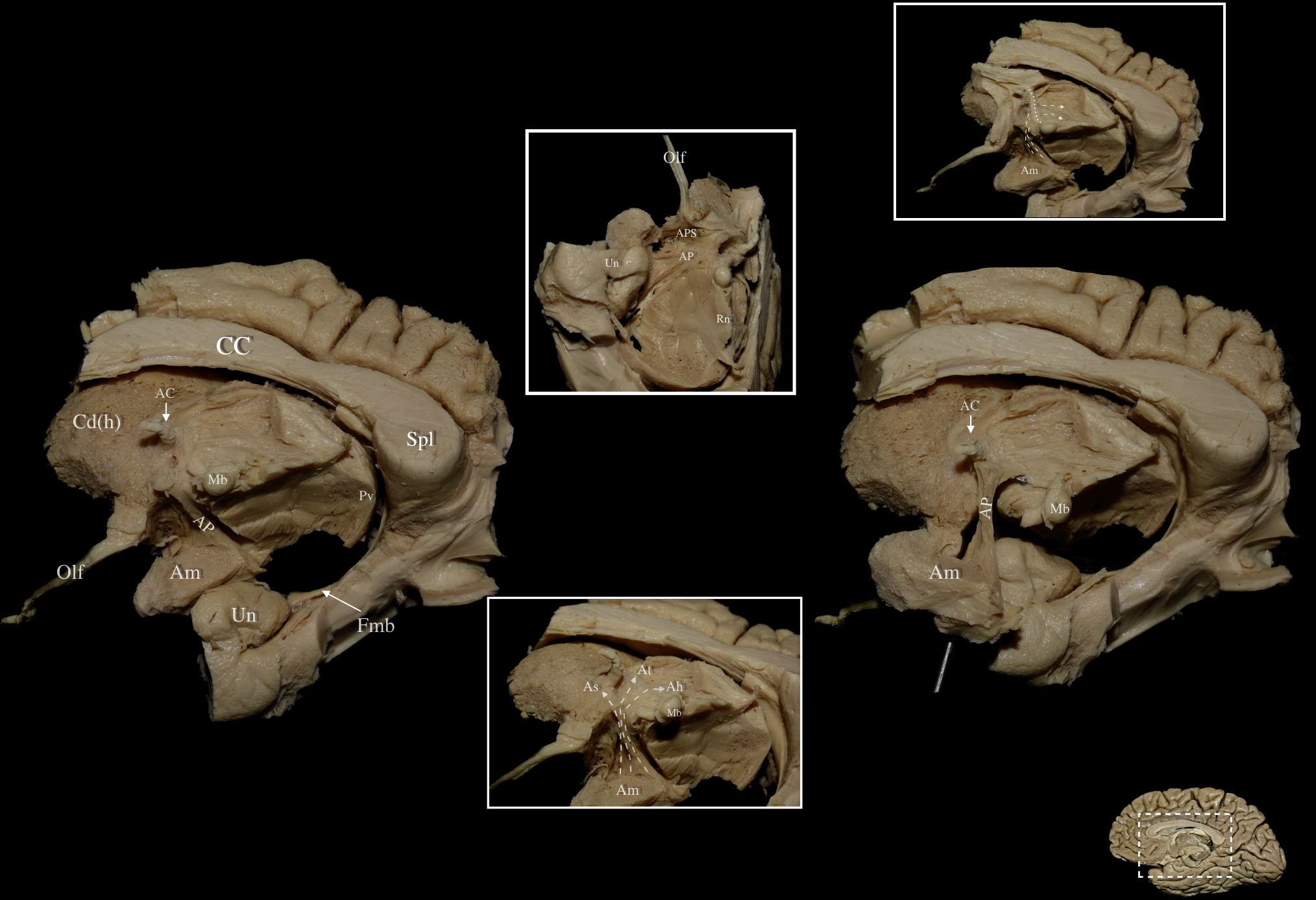 Fig. 2.
Fig. 2. Right hemisphere: Medial view of the central core. Left & Right Main Panels: Medial view of the ansa peduncularis and associated anatomical structures following sequential dissection. The ependymal layer lining the intraventricular surface of the caudate nucleus, thalamus, and post-commissural fornix has been removed, revealing the ansa peduncularis (AP). Middle Upper Inlet: Basal view following removal of posterior/posteromedial orbital lobule, optic tract, anterior uncus, and post-commissural fornix revealing the underlying AP. Middle Lower Inlet: Medio-basal view of a right hemisphere. The courses of the amygdalo-septal (As) as well as the amygdalo-thalamic (At) and amygdalo-hypothalamic (Ah) fibers of the AP are demonstrated via the dashed arrows. Amygdalo-septal fibers can be seen running anteriorly towards the septal region while At and Ah fibers can be seen coursing posterior to the anterior commissure. Right Upper Inlet: Medial view of a right hemisphere demonstrating the spatial relationship of the ansa peduncularis with the mammillothalamic tract. Fibers of the ansa peduncularis (dashed arrows) can be observed running lateral to the mammillothalamic tract (dotted line). Right Lower Corner: Medial view of a right hemisphere. White dashed box demarcates the dissected area of interest in the right medial hemisphere. Ah, Amygdalo-Hypothalamic; APS, Anterior Perforated Substance; At, Amygdalo-Thalamic; As, Amygdalo-Septal; CC, Corpus Callosum; Cd (h), Head of Caudate Nucleus; Fmb, Fimbria; Olf, Olfactory Tract; Spl, Splenium; Un, Uncus.
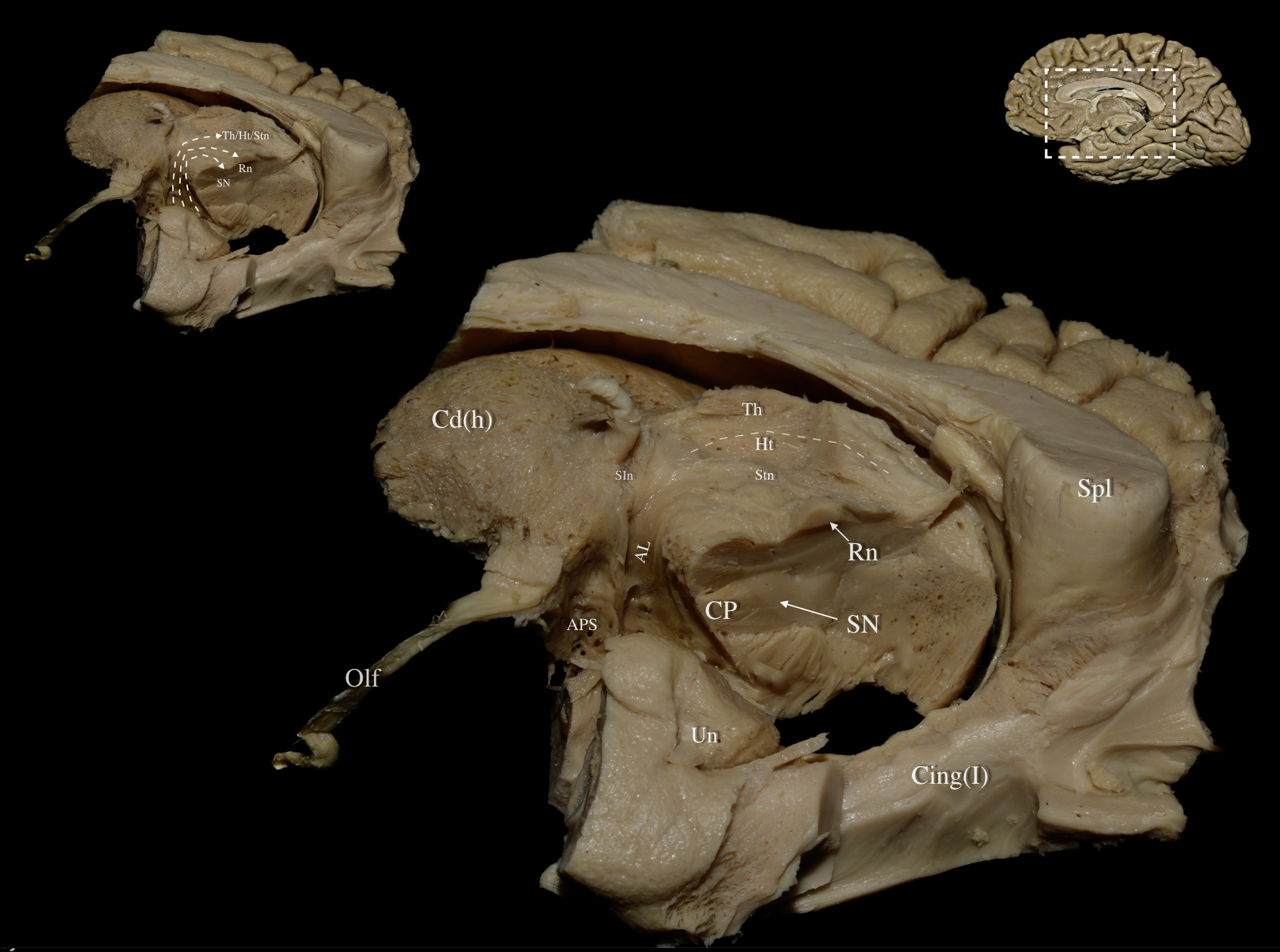 Fig. 3.
Fig. 3. Right hemisphere: Mediobasal view of the central core. Left Upper Panel: The fibers of the ansa lenticularis traveling to the thalamus/hypothalamus/subthalamic nucleus, red nucleus, and medial part of the substantia nigra are demonstrated in dashed lines. Main Panel: The optic tract, paraterminal gyrus, cingulate cortex, prefrontal cortex, underlying U-fibers, callosal radiations, cingulum fibers, and the shell of the ventral striatum have been resected exposing the medial part of the head of caudate. Further meticulous dissection of the medial thalamic and hypothalamic regions delineates the termination of the ansa lenticularis (AL) fibers within the thalamus, hypothalamus, subthalamic nucleus, red nucleus, and medial substantia nigra. Right Upper Panel: Medial view of a right hemisphere. White dashed box demarcates the dissected area of interest. Cing (I), Cingulum Bundle-V; CP, Cerebral Peduncle; Ht, Hypothalamus; SN, Substantia Nigra; SIn, Substantia Innominata; Stn, Subthalamic Nucleus; Th, Thalamus; Un Uncus.
Under high magnification, the dissection process ended by removing the descending fibers of the posterior limb of the internal capsule (PLIC) at the posterior aspect of the GPi. In this manner, we exposed a slender group of fibers originating from the inferomedial surface of the GPi, crossing perpendicular to the fibers of the PLIC, and joining the thalamic fibers of the AL above the subthalamic nucleus. This fiber pathway is the fasciculus lenticularis (or lenticular fasciculus), the second component of the pallidothalamic pathway.
During the study, the ansa lenticularis was consistently identified as a fiber tract emerging from the apex of the globus pallidus, curving around the cerebral peduncle, and turning steeply towards the medial thalamic/subthalamic/mesencephalic area (Fig. 4). The AL originates at the ventral-posterior margin of the globus pallidus. The anterior two-thirds of the ansa lenticularis travel adjacent to the anterior commissure and can be assigned as the dorsal and middle segments, which terminate in the areas of the thalamus/subthalamic nucleus and medial red nucleus respectively (Fig. 5). These segments lie dorsal to the fibers of the ansa peduncularis. The posterior third of the ansa lenticularis curves around the cerebral peduncle and can be assigned as the ventral segment which terminates in the medial aspect of the substantia nigra. The pallidothalamic fibers assigned to the dorsal segment merge with the fibers of the fasciculus lenticularis before terminating in the thalamus.
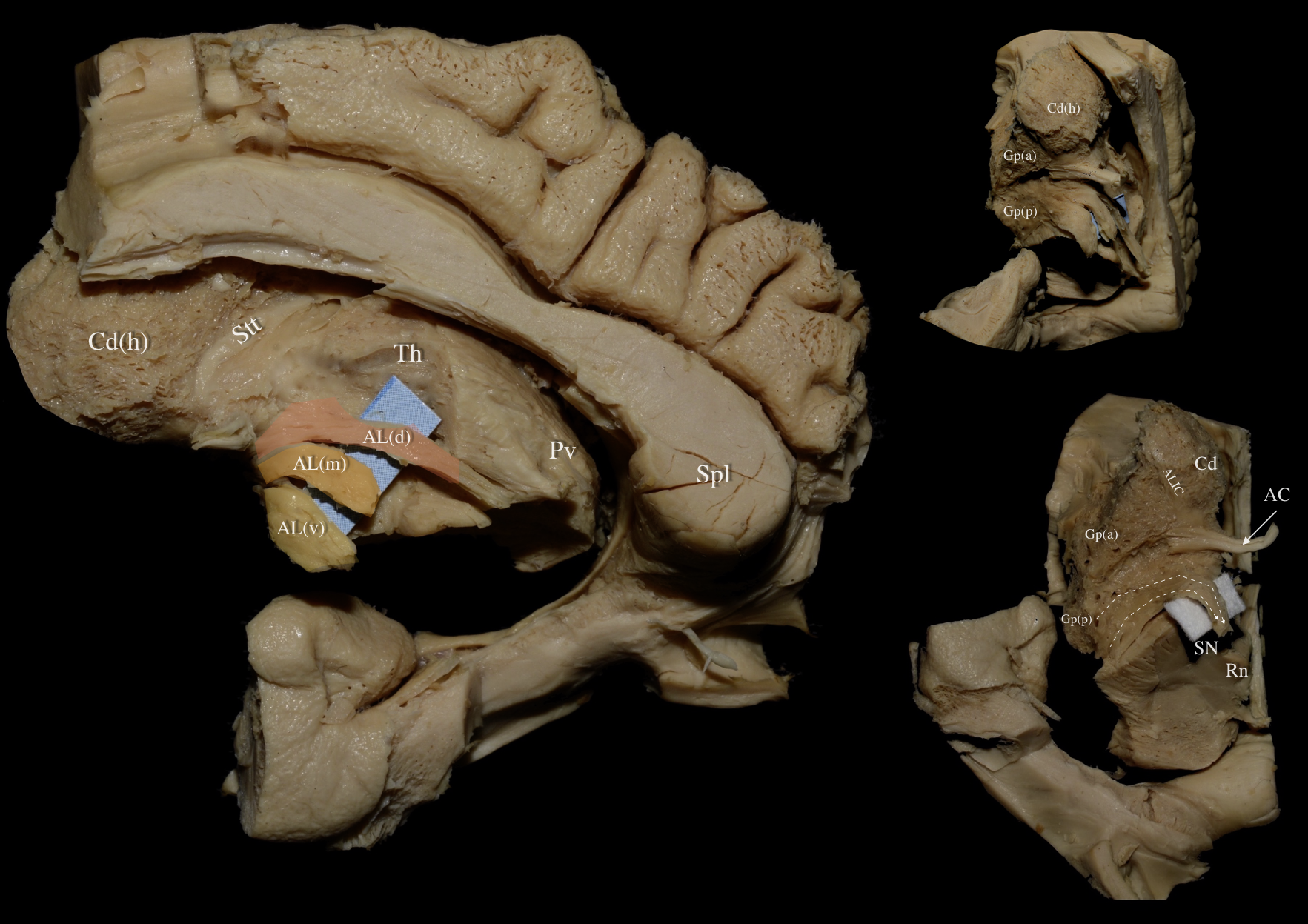 Fig. 4.
Fig. 4. Right hemisphere: Segments of the ansa lenticularis. Left Panel: The optic tract, fornix, paraterminal gyrus, cingulate cortex, prefrontal cortex, underlying U-fibers, callosal radiations, cingulum fibers, the shell of the ventral striatum, ependyma of the third ventricle, medial thalamic and hypothalamic regions have been resected exposing the medial part of the head of caudate, the stria terminalus thalami (Stt), thalamus (Th), and AL. The ventral, middle and dorsal segments are represented by yellow, orange and red coloring respectively. Right Upper Panel: Focused dissection at the level of the nucleus accumbens. Progressive dissection of the gray matter of the nucleus accumbens discloses the head of the caudate nucleus superomedially and the anterior (Gp (a)) and posterior (Gp (p)) parts of the globus pallidus inferolaterally. Right Lower Panel: Fibers of the anterior limb of the internal capsule (ALIC) can be appreciated between the head of the caudate nucleus and the globus pallidus. Fibers of the ansa lenticularis (dashed arrows) can be observed running inferior to the AC. AL (d), dorsal Segment of Ansa Lenticularis; AL (m), middle segment of Ansa Lenticularis; AL (v), ventral segment of Ansa Lenticularis; ALIC, Anterior Limb of Internal Capsule; Cd, Caudate Nucleus; Cd (h), Head of Caudate; Gp (a), anterior Part of Globus Pallidus; Gp (p), posterior part of Globus Pallidus; SN, Substantia Nigra; Stt, Stria Terminalis Thalami; Th, Thalamus.
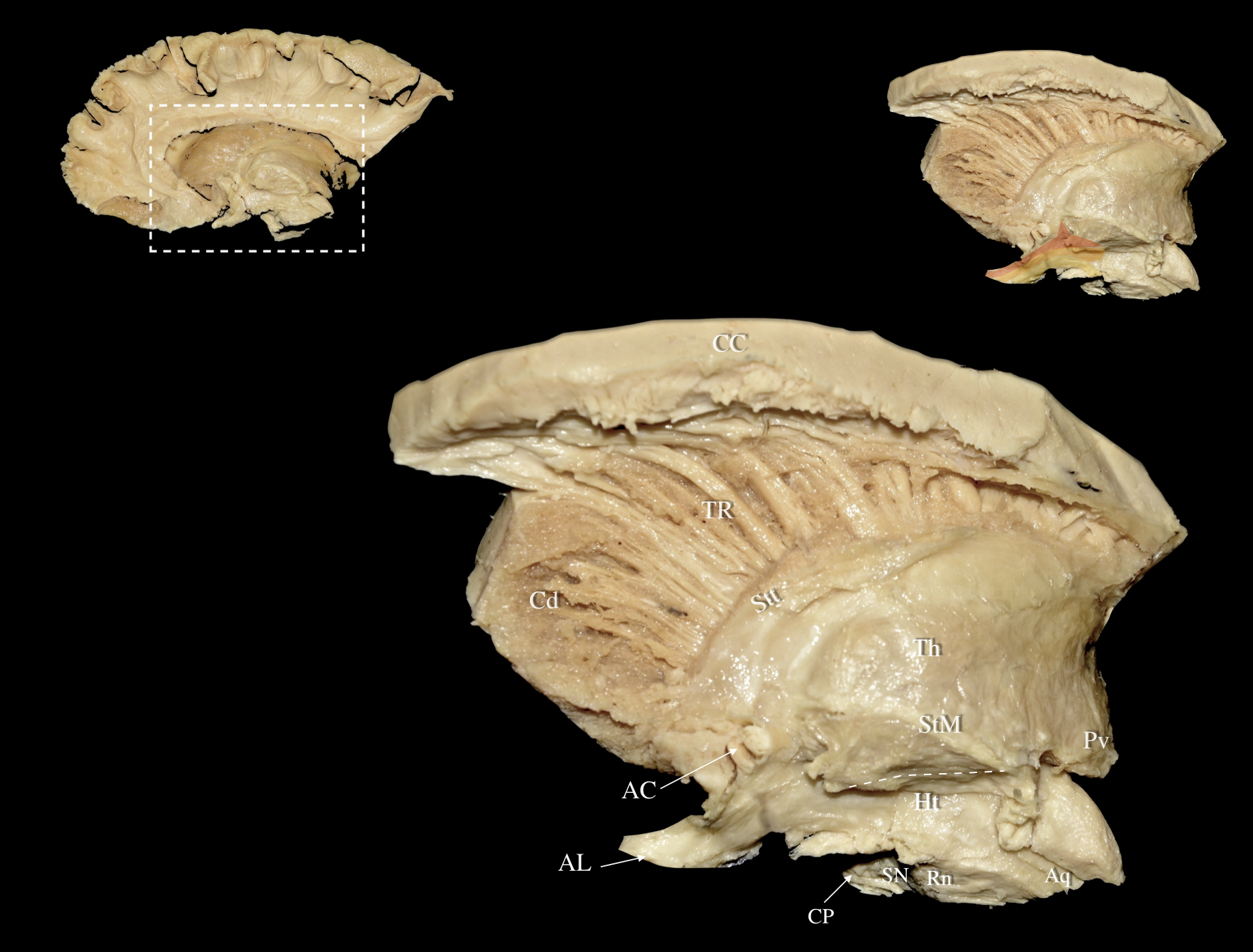 Fig. 5.
Fig. 5. Right hemisphere: Medial views of a progressive nuanced dissection. Left Upper Panel: Medial view of a right hemisphere. The cortex, underlying U-fibers, cingulum, fornix, and optic tract have been removed revealing the underlying callosal radiations, ventral striatum, and central core. White dashed box demarcates the dissected area of interest. Middle Panel: Medial view of a right hemisphere. Meticulous resection of the ventral striatum and medial part of the caudate nucleus reveals the thalamic radiations (TR) and fibers contributing to the anterior limb of the internal capsule as well as the ventral allocation of ansa lenticularis fibers. Right Upper Panel: The ventral allocation of ansa lenticularis fibers and their relationship to diencephalic structures, thalamic radiations, and thalamo/hypothalamotegmental fibers can be appreciated. The ventral, middle and dorsal segments of the ansa lenticularis are represented in yellow, orange and red respectively. Aq, Aqueduct; Cd, Caudate Nucleus; CP, Cerebral Peduncle; Ht, Hypothalamus; StM, Stria Medularis Thalami; Stt, Stria Terminalis Thalami; Th, Thalamus; TR, Thalamic Radiations.
The AL is confined within a square (“AL square”) delineated by four imaginary lines (Fig. 6):
(1) A sagittal line passing through the external geniculate body and parallel to the interhemispheric fissure laterally
(2) A sagittal line passing through the medial aspect of the red nucleus and parallel to the interhemispheric fissure medially
(3) A coronal line passing through the middle of the optic chiasm and perpendicular to the interhemispheric fissure anteriorly
(4) A coronal line passing through the middle of the cerebral peduncle and perpendicular to the interhemispheric fissure posteriorly
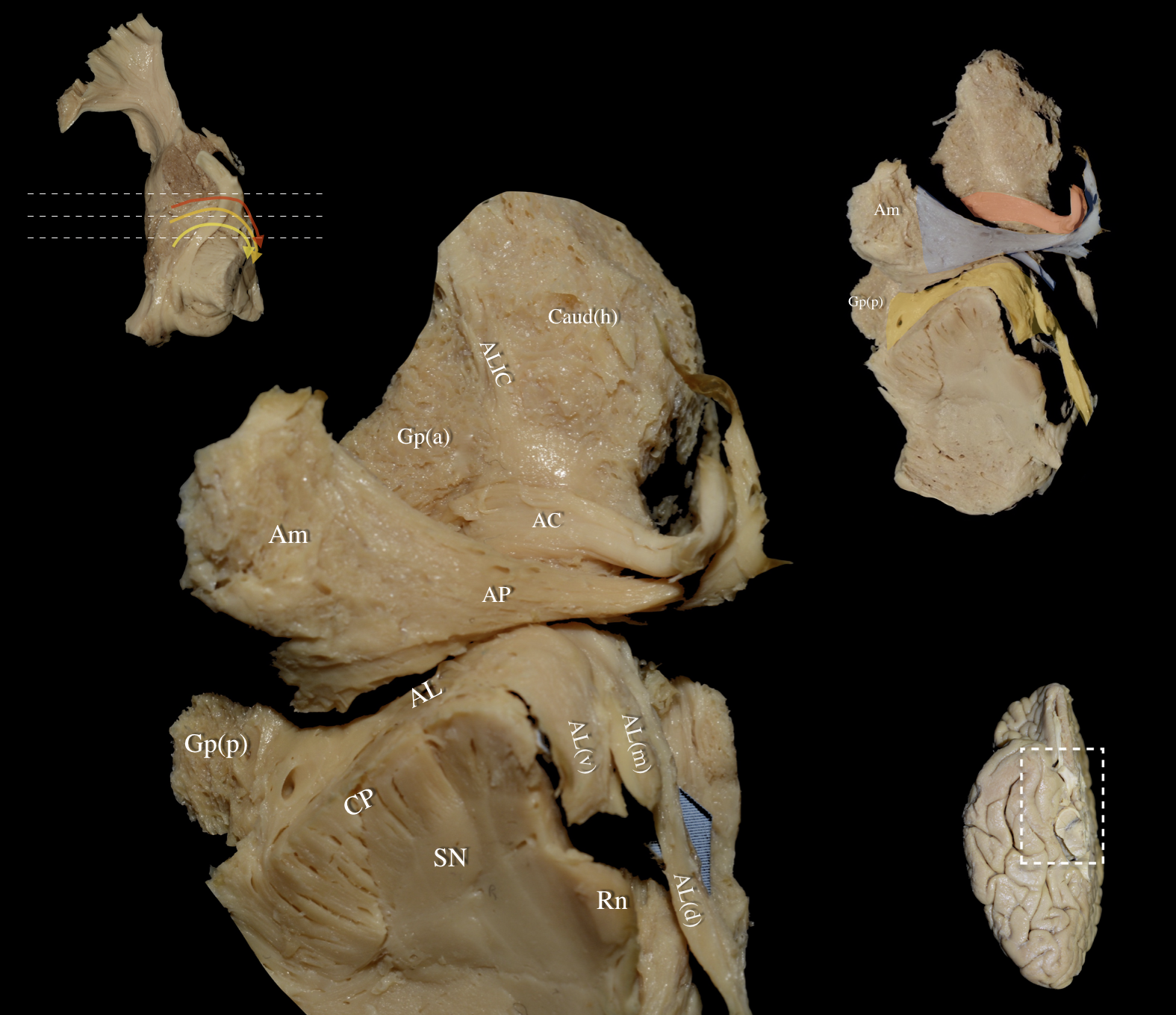 Fig. 6.
Fig. 6. Right hemisphere: Basal view of the central core demonstrating the segmentation pattern and topography of the three different segments of the AL. Left Upper Panel: Basal view of a right hemisphere. In this dissection, the optic nerve, chiasm, and optic tract have been preserved, and the relationship of the AL to the optic nerve is demonstrated. Dashed lines represent different rostro-caudal levels from top to bottom. The ventral, middle and dorsal segments of the AL are represented by the yellow, orange and red curved arrows respectively. Left Main Panel is a magnified view of the Right Upper Panel: Basal view of a right hemisphere. Removal of the olfactory bulb/tract, medial/lateral olfactory striae, olfactory tubercle, paraterminal gyrus, gyrus rectus, orbital gyri (anterior, posterior, medial and lateral), and the underlying U-fibers exposes the uncinate fasciculus, inferior occipitofrontal fasciculus, cingulum, and forceps minor. After resecting the ventral striatum and identifying the nucleus accumbens, cortical microdissection exposed the ventral aspect of the ALIC running between the head of the caudate nucleus (Caud (h)) and the globus pallidus (Gp (a)). The AC is highlighted in red, and the AP is highlighted in blue. Further dissection of the hypothalamus (lateral preoptic area, supraoptic, retrochiasmatic nucleus), column of the fornix, anterior olfactory nucleus, and mammillary bodies, exposes the AL—highlighted in yellow—running between the posterior aspect of the globus pallidus (Gp (p)) and the medial thalamic/subthalamic/mesencephalic area. Right Lower Panel: Right hemisphere basal view. White dashed box demarcates the dissected area of interest. AL (d), dorsal segment of Ansa Lenticularis; AL (m), middle segment of Ansa Lenticularis; AL (v), ventral segment of Ansa Lenticularis; ALIC, Anterior Limb of Internal Capsule; Caud (h), Head of Caudate nucleus; CP, Cerebral Peduncle; SN, Substantia Nigra.
The fibers of the dorsal and middle segment of the AL (Fig. 7):
(1) Reside in the same plane and slightly posterior to the fibers of the anterior commissure at the level of the anterior half of the AL square
(2) Travel dorsal to the fibers of the AP
(3) Curve ventral to the substantia innominata
(4) Run parallel to, and ventral to, the mesolimbic fibers
(5) Are located lateral to the amygdalo-thalamic and amygdalo-hypothalamic components of the AP and the post-commissural fornix
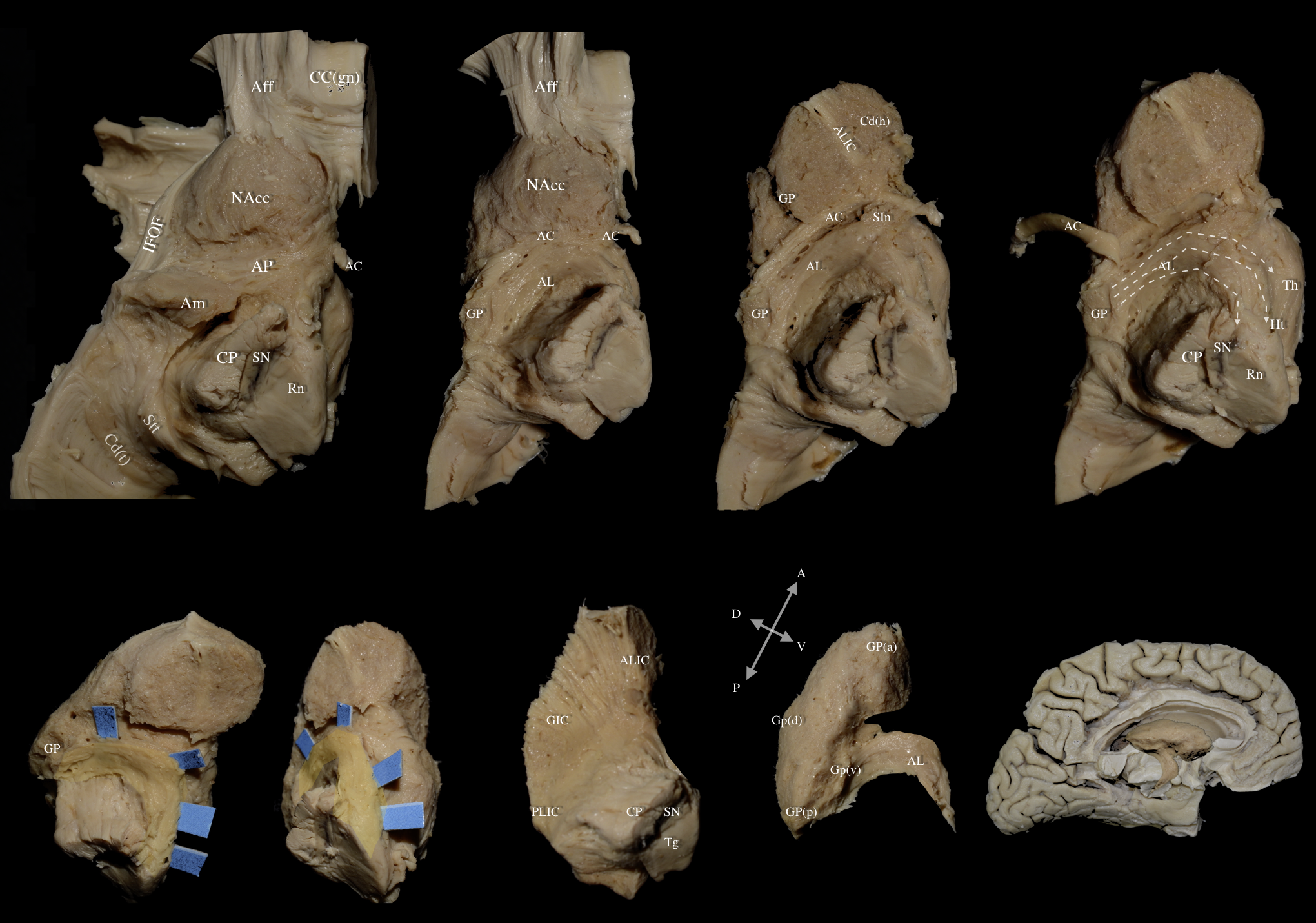 Fig. 7.
Fig. 7. Left hemisphere: Views of multilayered dissection depicting the relationship of the AL with major association fiber tracts. Top Row Panel: Progressive dissection from medial to lateral cerebral hemisphere. Last image in the top panel (on the far right) shows dashed arrows which represent the fibers of the dorsal (top), middle, and ventral (bottom) segments of the AL. Bottom Row Panel: The AL is highlighted in yellow and demarcated with the blue pads. The first three images in the bottom panel show the AL in different orientations. The last two images in the bottom panel showcases the AL as it emerges from the ventral-posterior margin of the globus pallidus. The second to last image on the bottom row is a 90° counterclockwise orientation of the central core demonstrated in the last image on the bottom row. Aff, Accumbofrontal Fasciculus; CC (gn), Genu of the Corpus Callosum; Cd (t), Tail of Caudate Nucleus; CP, Cerebral Peduncle; GIC, Genu of Internal Capsule; GP (d), dorsal segment of Globus Pallidus; GP (v), ventral segment of Globus Pallidus; IFOF, Inferior Fronto-Occipital Fasciculus; NAcc, Nucleus Accumbens; PLIC, Posterior Limb of Internal Capsule; SIn, Substantia Innominata; SN, Substantia Nigra; Stt, Stria Terminalis Thalami; Tg, Tegmentum.
The fibers of the ventral segment of the AL:
(1) Reside in the same plane as the anterior commissure, dorsal and perpendicular to the optic tract at the level of the posterior half of the AL square
(2) Run anterior to, and following the curve of, the cerebral peduncle
(3) Make a steep turn lateral to the amygdalo-hypothalamic fibers and the post-commisural fornix before reaching the substantia nigra
The AL curves around the cerebral peduncle anterior and dorsal to the fibers of the fasciculus lenticularis (FL)—also known as the H2 field of Forel—that cross the PLIC in a perpendicular trajectory (Fig. 8). The AL and FL intermingle at the level of the zona incerta (ZI) and form the thalamic fasciculus (H1 field of Forel). The thalamic fasciculus travels slightly lateral to the cerebellothalamic fibers and ascends through the pre-rubral field, after which both fiber groups terminate in the anterior and ventral thalamus.
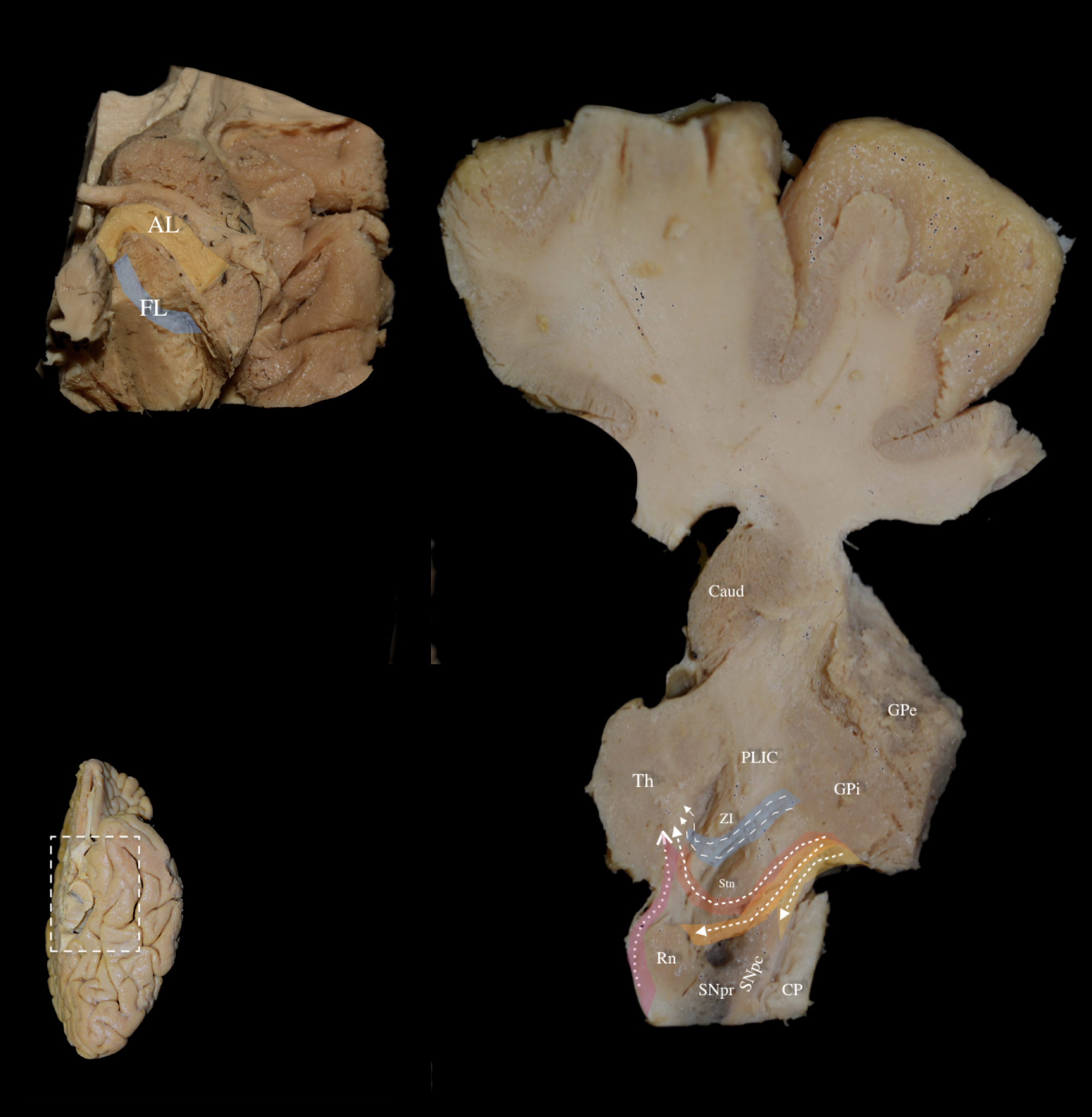 Fig. 8.
Fig. 8. Left hemisphere: Basal view. Left Lower Panel: Left hemisphere, basal view. White dashed box demarcates the dissected area of interest. Left Upper Panel: Basal view of a left hemisphere. Following exposure of the AL, removal of the posterior limb of the internal capsule (PLIC) fibers under high magnification exposed the fasciculus lenticularis (FL), running between the inferomedial globus pallidus internus (GPi), and STN. Right Panel: Oblique axial section at the level of the PLIC demonstrating the relationship between the zona incerta (ZI), FL (highlighted in blue), and AL (highlighted in yellow/orange/red to represent the ventral/middle/dorsal segments respectively). The ansa lenticularis forms a ventral “loop” around the internal capsule, while the fasciculus lenticularis takes a more dorsal direct route through the internal capsule. The left upper panel shows part of the AL loop, and the right panel shows the other part of the AL loop that combines with the FL at the level of the zona incerta (ZI) to form the thalamic fasciculus (H1 field of Forel). The thalamic fasciculus travels slightly lateral to the cerebellothalamic fibers (highlighted in light purple) and ascends through the pre-rubral field after which both fiber groups (thalamic fasciculus and cerebellothalamic fibers) terminate in the anterior and ventral thalamus. Caud, Caudate Nucleus; CP, Cerebral Peduncle; GPe, external Globus Pallidus; FL, fasciculus lenticularis; PLIC, Posterior Limb of Internal Capsule; SNpr, Substantia Nigra pars reticulata; SNpc, Substantia Nigra pars compacta; Th, Thalamus; ZI, Zona Incerta.
The ansa lenticularis is typically described as one of the two main pallidothalamic tracts of the human brain; the other being the fasciculus lenticularis or lenticular fasciculus [6, 14]. Early studies approached the ansa lenticularis as a purely pallidothalamic connection merging into the fiber system of the ansa peduncularis. According to this view, the ansa peduncularis consists of four main components: (a) the amygdalo-septal fibers or diagonal band of Broca that connects the amygdala and temporal cortex to the septal area, (b) the amygdalo-thalamic fibers or extracapsular thalamic peduncle or inferior thalamic peduncle connecting the amygdala and temporal cortex to the thalamus (c) the amygdalo-hypothalamic fibers connecting the amygdala to the hypothalamus and (d) the ansa lenticularis connecting the globus pallidus to the thalamic region [15, 16]. However, modern researchers have consistently recorded that the ansa lenticularis also projects to the subthalamic nucleus, red nucleus, and the substantia nigra. Taking it a step further, a recent study by Li and colleagues [17] used generalized q-space sampling tractography, a sophisticated diffusion model, to identify four subcomponents within the AL: the fibers travelling from globus pallidus to thalamus (GPT), the fibers coursing from globus pallidus to substantia nigra (GPSN), the fibers running from globus pallidus to red nucleus (GPRN), and the fibers running from globus pallidus to subthalamic nucleus (GPST).
An ambiguous point in the pertinent literature concerns the connectivity pattern of the AL in the area of the globus pallidus. Some authors agree with the observation that the fibers of the AL originate mainly from the ventral area of the globus pallidus internus, but others place the origin of the fibers in the lateral aspect of the GPi [2, 14, 18]. In this regard, the current study aims to shed light on four obscure issues.
The first is the relationship of the fibers of the ansa lenticularis to those of the ansa peduncularis. According to our findings, the fibers of these two pathways can be easily dissected apart and therefore differentiated as they are found to course in different planes. The amygdalo-septal, amygdalo-thalamic (assigned to the extracapsular thalamic peduncle), and amygdalo-hypothalamic fibers of the ansa peduncularis travel in a more superficial plane than the fibers of the ansa lenticularis and project to different cerebral areas.
The second issue concerns the segmentation pattern of the AL. Our findings are in agreement with the segmentation pattern proposed by Li and colleagues [17]. We have consistently identified a ventral segment previously described as GPSN terminating in the medial substantia nigra, a middle segment previously described as GPRN terminating in the red nucleus, and a dorsal segment including the GPST and GPT terminating in the subthalamic nucleus and the area of the thalamus, respectively [17].
The third issue relates to the origin of the AL in the GP (GPEZ). AL was consistently found to originate at the posterior half of the ventral margin of the GPi. We did not disclose any fibers residing at the lateral aspect of the GPi. Interestingly enough, we found the fibers of the ventral segment to originate posteriorly in the GPEZ in relation to those of the middle and dorsal segment of the AL.
The fourth, and last, issue is the apparent paucity of cadaveric, three-dimensional direct structural evidence regarding the anatomy of the AL and adjacent fiber tracts [17]. Improving our anatomic understanding particularly from a microsurgical point-of-view requires an “in-situ” examination of the implicated fiber groups in order to grasp and systematically record the complex topography and spatial subcortical relationships. As mentioned above, traditional histologic images offer excellent detail but only at a two-dimensional level and thus fail to shape a mental map of the areas of interest. To our knowledge, this is the first dedicated cadaveric study implementing the white matter micro-dissection technique to demonstrate and illustrate the anatomy of the ansa lenticularis.
Before the introduction of deep brain stimulation (DBS), direct surgical lesioning of the thalamus (thalamotomy) and GPi (pallidotomy) were mainstay procedures. The first recorded surgical treatment of psychomotor diseases through lesioning of the ansa peduncularis can be traced back to 1954 with the use of “ansotomy” for the treatment of paralysis agitans [19]. Lesioning of the pallidothalamic tract by means of ultrasound thermocoagulation has also been recently revisited for the treatment of tremor, dyskinesia and akinesia in patients with Parkinson’s disease (PD) as well as for the treatment of dystonia [20]. The ansa lenticularis has been targeted in DBS procedures as the principal output of the GPi for the treatment of dystonia with good results [21]. Stimulation and lesioning of the Forel fields has also been recently used successfully as an alternative for improving unresponsive motor symptoms, including gait and balance disorders, in patients with PD and dystonia, respectively [22, 23]. Generally, the principle of lesioning the pallidothalamic fibers for the treatment of PD or essential tremor is based on the increase in disinhibition of an overinhibited thalamus [22, 24]. This result can be achieved with a significantly smaller lesion in the area of the ansa lenticularis than in the thalamus or GPi, thus avoiding the sensorimotor symptoms that can be elicited when lesioning these latter areas [24].
Review of the pertinent literature reveals growing evidence supporting the theory that the pallidothalamic pathways represent an important target for stereotactic neurosurgery [20]. Nevertheless, the subthalamic area is an extremely complex and highly dense area where the fine anatomy has to be understood at the most detailed level prior to introducing new techniques and targets. The origin and exact hodotopy of the fibers and their trajectories should be understood as well as their anatomy with respect to adjacent pathways and structures [25]. Therefore, the anatomy of the ansa lenticularis has to be initially recorded in relation to prominent structures including the anterior commissure, the mammillary body, the optic tract and the post-commissural fornix and before it can be conveyed to stereotactic atlases [26]. From there, the delicate difference between the trajectory and location of adjacent fiber systems including the ventral amygdalofugal pathway, the fasciculus lenticularis, and the mesolimbic fibers, among others, has to be firmly understood in order to optimize targeting [27]. Finally, anatomical evidence has to be integrated into the planning of stimulation or lesioning targets according to the existing theoretical concepts [28, 29]. Histology, high-resolution magnetic resonance imaging (MRI), tractography and white matter dissection function as complementary tools to provide sound data that may inform and modify surgical strategies and planning [17].
The fiber micro-dissection technique based on Klingler’s method has been established as a gold-standard method to examine and validate anatomical evidence that derives mainly from tractography studies [30, 31]. Klingler’s preparation entails the fixation of hemispheres in formalin solution followed by a freeze-thaw-process. Zemmoura and colleagues [32] proved this technique helps preserve the anatomical integrity of the axons while facilitating the separation of the fiber tracts in a laboratory setting under the operating microscope. In recent literature, the fiber dissection technique has been repeatedly accredited with high accuracy in illustrating the fiber trajectory as well as termination pattern and offers a high spatial resolution even in complex anatomical areas such as the subthalamic area and mesencephalon [9].
However, the fiber micro-dissection method is an expensive and extremely time-consuming technique requiring patience and excellent three-dimensional anatomical perception and dexterity. In this regard, it is highly dependent on the experience and skillset of the operator. Additionally, poor specimen quality and inadequate or incorrect preparation can reduce the accuracy of the dissection and affect repeatability of the process. Finally, the spatial resolution provided by the fiber dissection technique is lower in comparison to histology, optical coherence tomography, and polarized light imaging.
By using the fiber microdissection technique, we explored and recorded the topographic anatomy and subcortical silhouette of a major pallidothalamic pathway: the ansa lenticularis. To our knowledge this is the first focused and dedicated microanatomical study in the pertinent literature that provides direct structural evidence on the architecture and connectivity of this elusive tract. Combining our results with data from different modalities—histology, high-resolution MRI, tractography, and diffusion models—will offer a more accurate understanding of the functional anatomy implemented in pallidothalamic connectivity, which would inform surgical technique and practice, particularly in the field of stereotactic neurosurgery, for neurologic diseases such as tremor, PD, and others.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
SK, AK, CK conceived the project and methodology. SK, GPS, ED, EN, LA, EC, GS performed literature search and completed data extraction and statistical analysis. SK, AK, GPS, and SAI wrote the paper. SK, SI, GS, AK, ED, EN, LA, EC, GS, LE, CK interpreted the data and provided input on analysis and paper revision. CK, LTE provided supervision for the project. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study received approval from the ethical board at the University of Athens (01.14.2019/protocol number: 067), and all procedures performed adhered to the ethical standards of the institutional and/or national research committee, as well as the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.








