1 Center of Reproduction, The Second People’s Hospital of Changzhou, The Third Affiliated Hospital of Nanjing Medical University, Changzhou Medical Center, Nanjing Medical University, 213100 Changzhou, Jiangsu, China
2 Department of Pharmacology, Nanjing Medical University, 211166 Nanjing, Jiangsu, China
3 Laboratory of Neurological Diseases, The Second People’s Hospital of Changzhou, The Third Affiliated Hospital of Nanjing Medical University, 213100 Changzhou, Jiangsu, China
4 Department of Clinical Pharmacy, The Affiliated Hospital of Jiangsu University, Jiangsu University, 212001 Zhenjiang, Jiangsu, China
†These authors contributed equally.
Abstract
Dyslipidemia during midlife represents a significant risk factor for neuropathological alterations associated with cognitive decline. Given the currently incurable nature of dementia, implementation of preventive strategies and early therapeutic interventions prior to disease progression are paramount. Emerging evidence suggests that hyperbaric oxygen (HBO) therapy exhibits neuroprotective properties in various neurological conditions. However, whether HBO treatment modulates lipid metabolism dysregulation and subsequent neurodegeneration remains unanswered. This investigation aimed to elucidate the therapeutic potential of HBO treatment in ameliorating cerebral dysfunction and metabolic perturbations using apolipoprotein E (ApoE)-deficient (ApoE-/-) mice.
ApoE-/- mice received HBO treatment for 10 consecutive days, and then behavioral assessment tests were performed. Serum and brain tissue were collected to measure oxidative stress levels and inflammatory factors.
Compared with ApoE-/- group, cognitive declines was significantly reversed in mice of the ApoE-/-+HBO mice. The blood lipid profiles of ApoE-/- mice were also improved after HBO treatment, accompanied by a reduction in body weight. Moreover, HBO treatment was found to ameliorates neuronal injury and amyloid-β deposition in the hippocampus of ApoE-/- mice. Further studies have revealed that the benefits of HBO treatment occurred through the reduction of inflammatory factors and attenuation of oxidative stress.
These findings indicate that HBO treatment effectively improves the intracerebral microenvironment of ApoE-/- mice, providing a novel regulatory mechanism of protection against dyslipidemia-associated brain deficits by HBO treatment.
Keywords
- hyperbaric oxygenation
- apolipoprotein E
- dyslipidemias
- cognitive dysfunction
- inflammation
Alterations to the plasma lipid profile characterize dyslipidemia, defined as an elevation of serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C) or reduced high-density lipoprotein cholesterol (HDL-C). The prevalence of dyslipidemia is rising among young people as living standards rise. Dyslipidemia is a chronic systemic condition that has the capacity to significantly contribute to various serious health complications, including obesity, atherosclerosis, and stroke [1, 2]. Existing evidence indicates comorbidity or some form of connection between dyslipidemia and central nervous system disorders, such as dementia and Parkinson’s diseases [3, 4, 5]. A comprehensive assessment of this relationship is hypothesised to help with the development of viable preventative and treatment strategies.
Hyperbaric oxygen (HBO) treatment, a non-invasive method, has become a useful treatment for neurological disorders such dementia, traumatic brain injury, and cerebral ischemia [6, 7, 8]. Previous research has shown that breathing pure oxygen at pressures of more than one absolute atmospheric pressure (ATA) raises arterial and cerebral oxygen tension, which enhances oxygen delivery to the brain [9, 10]. Numerous studies have shown that HBO treatment exerts positive therapeutic effects by modulating the release of inflammatory cytokines [11, 12, 13]. Although HBO has been shown to alleviate diminished blood flow, hypoxia, and neuroinflammation in the brain, its effect on dyslipidemia and neurodegeneration are not yet to be fully understood.
Apolipoprotein E (ApoE), predominantly expressed in astrocytes, controls phospholipid and cholesterol transport and redistribution within the central nervous system [14]. Hypercholesterolemia and atherosclerosis are observed in ApoE-deficient (ApoE-/-) mice. Numerous investigations have demonstrated that hypercholesterolemia exacerbates the accumulation of amyloid beta (A
Currently, there are few lipid management interventions in the prevention and treatment of dementia and brain degeneration. Changes in lipid metabolism undoubtedly affect the microenvironment of brain tissue, although the precise role remains unknown. This study was aimed to assess the association between specific changes in lipid metabolism and lesions in brain tissue, excluding age-related variables, thereby contributing to the clinical management of dyslipidemia and brain degeneration.
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University. Male ApoE-/- mice (C57BL/6J background) and wild-type (WT) C57BL/6J mice (eight months old) were obtained from Gempharmatech Co., Ltd. (Nanjing, Jiangsu, China). Animals were maintained under specific pathogen-free conditions with controlled 12-hour light/dark cycles.
Mice were stratified into three cohorts (n = 7 per group): WT group: Age-matched C57BL/6J mice serving as baseline controls, an ApoE-/-group: Untreated ApoE-/- mice maintained on a standard diet and an ApoE-/- +HBO group: ApoE-/- mice subjected to HBO therapy for 10 consecutive days.
HBO treatment was conducted using a rodent-specific hyperbaric chamber (YC3200J-X, Yantai Haote Oxygen Corporation, Yantai, Shandong, China) following established methodologies [18]. Briefly, mice were acclimatized in the chamber and exposed to 100% oxygen at 2.5 ATA daily. Chamber pressure was incrementally elevated to 2.5 ATA over 15 minutes, maintained for 60 minutes, then gradually normalized to ambient pressure over 15 minutes. Control cohorts (WT and untreated ApoE-/- mice) underwent identical chamber conditions with compressed air (1 ATA) for 90 minutes to account for procedural variables.
A novel object recognition assay was performed as described [19] using a 50
Contextual fear conditioning was conducted per established protocols [20]. During training, mice were placed in a conditioning chamber for a three minute baseline period followed by three two second, 0.8-mA foot shocks delivered at one minute intervals. Subjects were returned to home cages 30 seconds post-stimulation. Memory retention was assessed one hour later via a three minute re-exposure to the unmodified chamber with freezing behavior quantification. A 24-hour post-training extinction test replicated this procedure without shocks, with freezing duration recorded using automated tracking software.
After termination of the experiment, cohort-matched mice were euthanized (gradual exposure to CO2 (30% chamber volume displacement per minute) in a sealed chamber, followed by cervical dislocation to ensure death) following final body mass measurements. Blood samples were collected via retro-orbital venipuncture into anticoagulant-treated tubes. Cerebral tissues were divided for preservation: one hemisphere underwent fixation in 4% paraformaldehyde for histomorphometric analysis, while contralateral hemispheres were snap-frozen in liquid nitrogen and stored at –80 °C for molecular assays.
Collected blood samples were centrifuged at 3500
Serum concentrations of nitric oxide (NO) and malondialdehyde (MDA), alongside superoxide dismutase (SOD) enzymatic activity, were determined using species-specific Enzyme-Linked Immunosorbent Assay (ELISA) kits (A012‑1‑2 for NO, A003‑1‑2 for MDA, and A001‑3‑2 for SOD, Jiancheng Bioengineering Institute) following manufacturer protocols. Absorbance measurements were normalized to internal standards.
Total RNA was isolated from frozen cortical tissues using the RNeasy Mini Kit (R701, Vazyme Biotech, Nanjing, Jiangsu, China), with genomic DNA removal via on-column DNase digestion. cDNA synthesis employed 1 µg RNA template processed with the HiScript III Reverse Transcriptase system (R223, Vazyme Biotech). Quantitative PCR amplification was performed using the AceQ SYBR Green Master Mix (Q131, Vazyme Biotech) on a QuantStudio 6 Flex system (4485697, Thermo Fisher Scientific, Woodlands, Singapore). Relative gene expression was normalized to Gapdh and calculated via the 2-ΔΔCt method. The sequences of the primers are listed in Table 1.
| Gene symbol | Primer Sequence (5′→3′) |
|---|---|
| absent in melanoma 2 (AIM2) | F: GTCCTCAAGCTAAGCCTCAGA |
| R: CACCGTGACAACAAGTGGAT | |
| glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F: CAAAAGGGTCATCATCTCC |
| R: CCCCAGCATCAAAGGTG | |
| interleukin-1 beta (IL-1 | F: TCATTGTGGCTGTGGAGAAG |
| R: AGGCCACAGGTATTTTGTCG | |
| interleukin-6 (IL-6) | F: ATCCAGTTGCCTTCTTGGGACTGA |
| R: TAAGCCTCCGACTTGTGAAGTGGT | |
| inducible nitric oxide synthase (iNOS) | F: CCAAGCCCTCACCTACTTCC |
| R: CTCTGAGGGCTGACACAAGG | |
| nucleotide-binding oligomerization domain-like receptor 1 (NLRP1) | F: GGTGGTGTGAAGATGTTGTGT |
| R: TCCATGTTCATCGTAGGGACC | |
| nucleotide-binding oligomerization domain-like receptor 3 (NLRP3) | F: ATCAACAGGCGAGACCTCTG |
| R: GTCCTCCTGGCATACCATAGA | |
| NLR Family CARD Domain Containing 4 (NLRC4) | F: TCAAAGGCGACTGGAAAGAAG |
| R: CGCCACTCCTTGCAGAAAC | |
| tumor necrosis factor- | F: CATCTTCTCAAAATTCGAGTGACAA |
| R: TGGGAGTAGACAAGGTACAACCC |
Tissue sections underwent sequential xylene immersion for deparaffinization followed by graded ethanol rehydration (100%, 95%, 70%, E111992, Aladdin Chemistry Co., Ltd., Shanghai, China). After distilled water rinsing, slides were stained with 1% toluidine blue (pH 4.5, T492282, Aladdin Chemistry Co., Ltd.) for 10 minutes at 25 °C. Sections were dehydrated through ascending ethanol series, cleared in xylene, and mounted with synthetic resin for bright-field microscopy (FV3000, Olympus, Tokyo, Japan). Cell layer thickness quantification were performed in three matched layer (cornu ammonis area (CA)1, CA3, dentate gyrus (DG)) per animal by ImageJ software (v1.53t, Research Services Branch, National Institute of Mental Health, Bethesda, MD, USA) with 10 equidistant sampling points per section.
Antigen retrieval and staining protocols followed established methodology [21, 22]. Sections were probed with rabbit polyclonal anti-Amyloid-
The concentration of IL-6 and TNF-
All datasets are expressed as mean
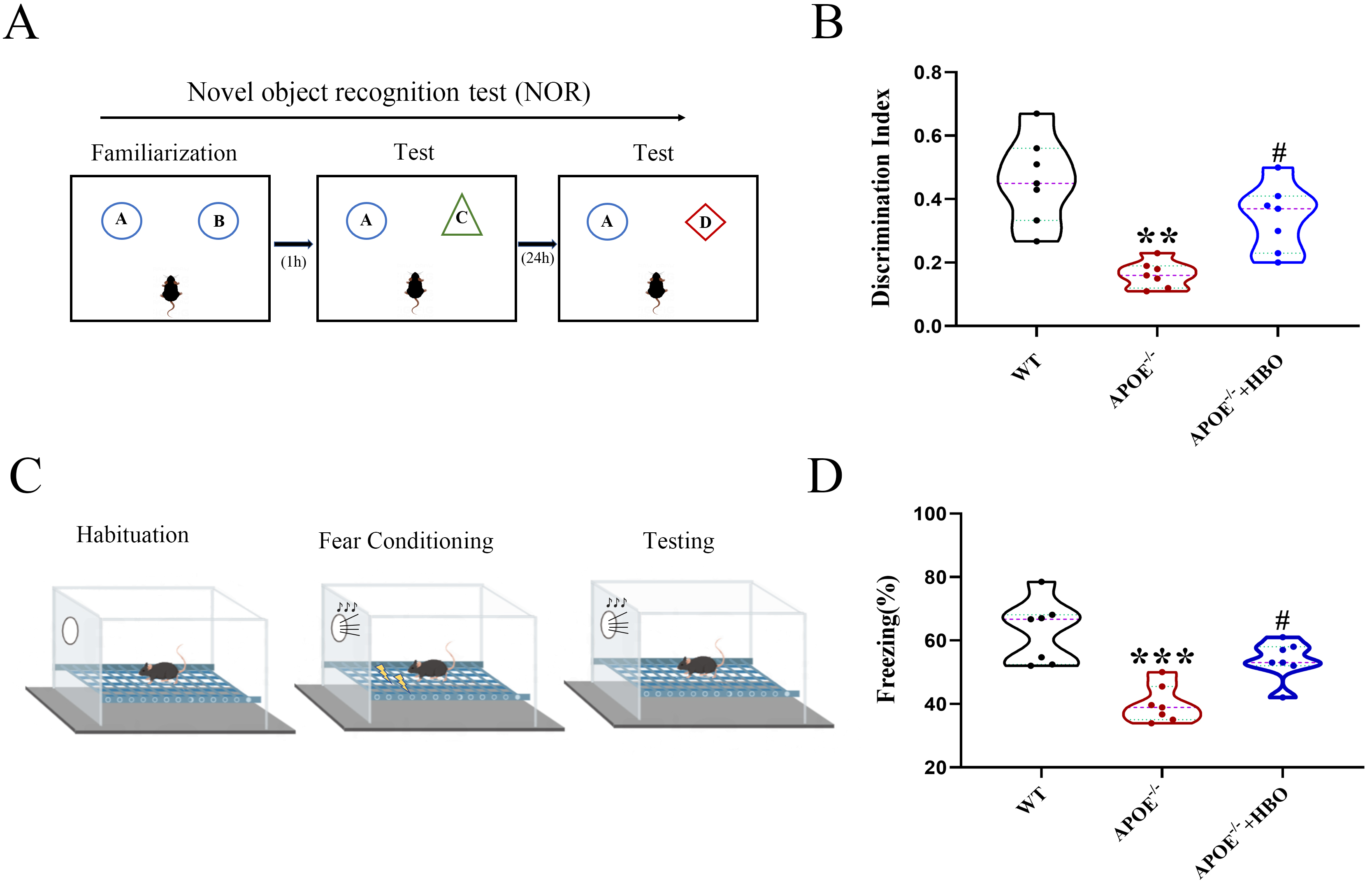
The overall experimental procedure is illustrated in Fig. 1. To evaluate the effect of HBO treatment on cognitive function, both novel object recognition and fear conditioning assays were performed. In the novel object recognition experiment, the discrimination indices of mice in the WT, ApoE-/- and ApoE-/-+HBO groups were 0.45
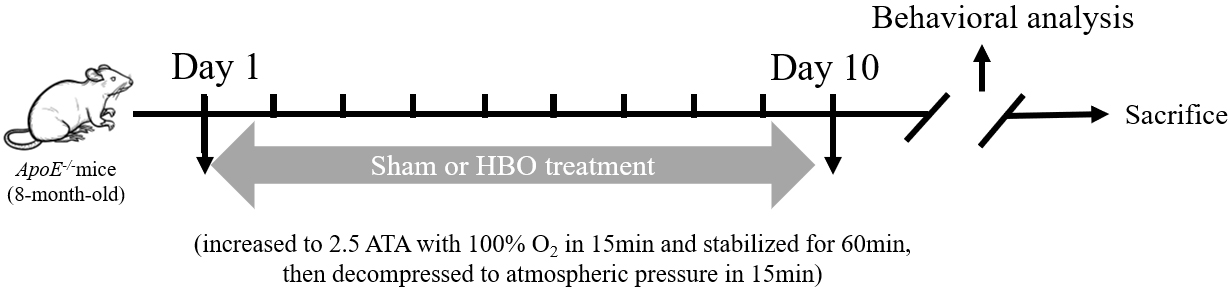
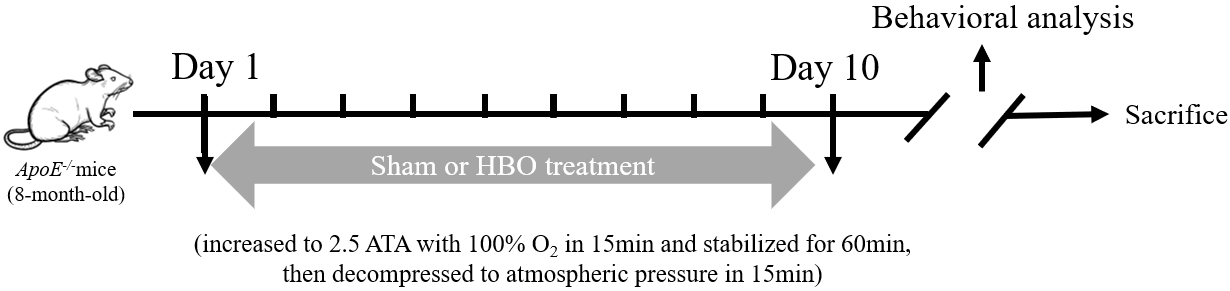 Fig. 1.
Fig. 1. Schematic illustration of the overall experimental procedure. A series of behavioral tests were performed after hyperbaric oxygen (HBO) treatment to assess cognitive function, including novel object recognition experiment and fear conditioning test. ATA, absolute atmospheric pressure; APOE-/-, apolipoprotein E-deficient.
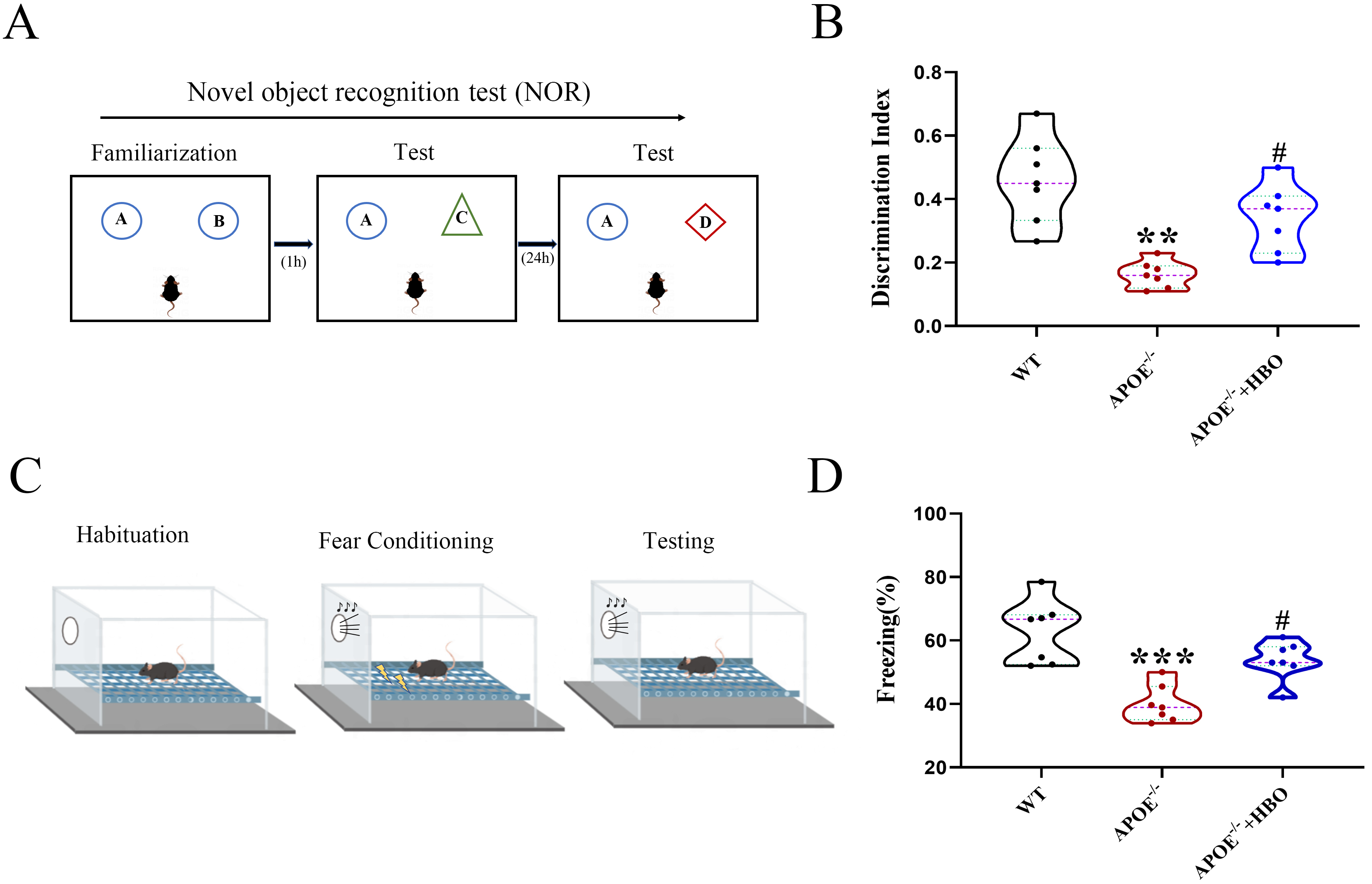 Fig. 2.
Fig. 2. Effect of HBO treatment on cognitive function and behaviors of mice. NOR experiment (A) and Fear conditioning test (C) were performed to assess the mice behavior. (B) Discrimination index of each group. (D) The freezing response. All data represent mean
When compared with WT mice, ApoE-/- mice exhibited a phenotype of abnormal lipid metabolism, characterized by elevated levels of TC, TG, LDL-C and decreased levels of HDL-C, but after the HBO treatment, the body weights, TC, TG, and LDL-C level were significantly reduced (one-way ANOVA: p
| Metabolic parameters | Groups | ||
| WT | APOE-/- | APOE-/-+HBO | |
| Body weight (g) | 32.63 | 35.32 | 32.42 |
| TC (mmol/L) | 2.79 | 4.32 | 2.23 |
| TG (mmol/L) | 0.97 | 3.56 | 1.7 |
| LDL-C (mmol/L) | 0.52 | 10.88 | 6.33 |
| HDL-C (mmol/L) | 2.73 | 0.72 | 0.67 |
Values represent mean
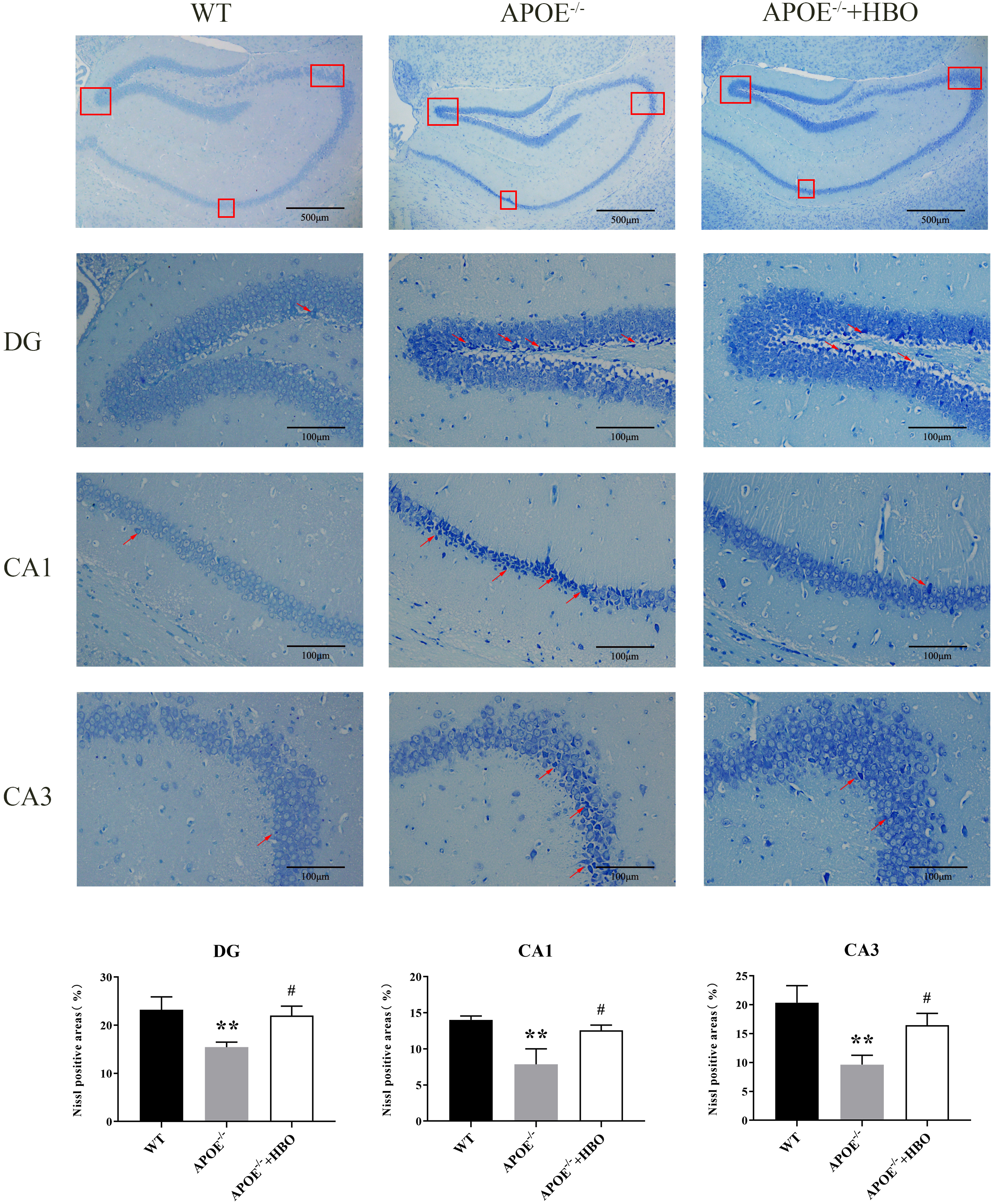
Changes in hippocampal structure are associated with memory impairment and cognitive dysfunction [22, 23, 24]. Pyramidal cells in the hippocampal region of the WT control mice had regular and orderly arrangements, full structures, uniform chromatin distribution, round and sizable nuclei, and transparent cytoplasm and nucleoli, as demonstrated by Nissl staining. Conversely, neurons in the hippocampus of mice in the ApoE-/- group’s were organized loosely and erratically, with some of them exhibiting noticeable shrinkage in addition to having darkly pigmented, condensed nuclei (Fig. 3). When compared with the WT group, the thickness of the cell layer in the CA3 area of the ApoE-/- mice decreased significantly. As shown in Fig. 3, HBO treatment partially reversed the neuronal morphological alterations seen in the ApoE-/- group, in particular the increased thickness of the cell layer in the CA3 area. Similar trends were observed in the other subregions CA1 and DG as well.
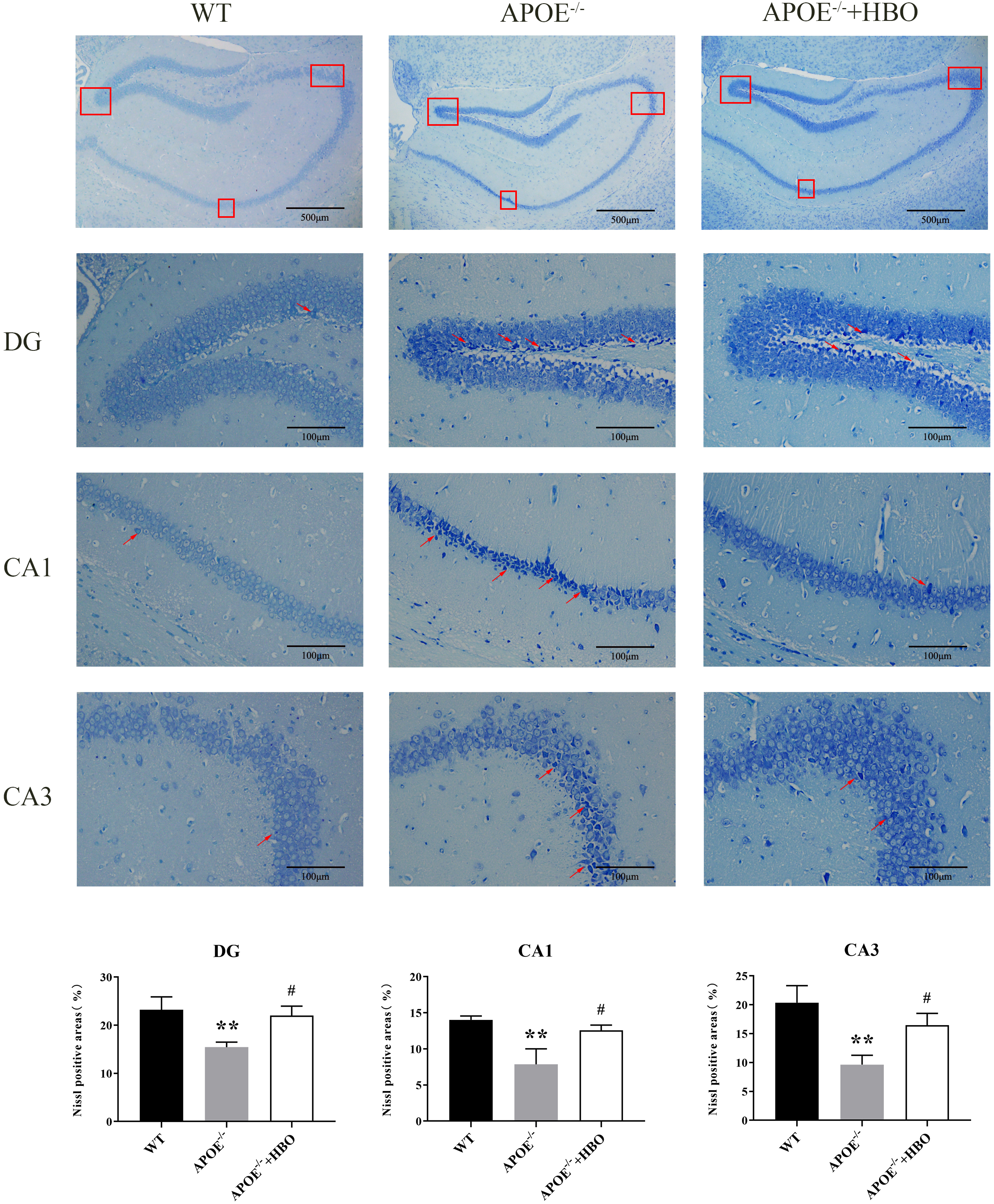 Fig. 3.
Fig. 3. Effect of HBO treatment on hippocampal damage. Hippocampal morphology with Nissl staining. The scale bar = 500 µm (the top panels), 100 µm (the middle panels). n = 4 in each group. The top of the image is the representative immunoblots and the bottom of the image is the quantification for analysis. The red boxes indicates different regions of the hippocampus that have been selected for analysis. In neuroanatomy, the hippocampus is often divided into subregions (e.g., CA1, CA3, or dentate gyrus), and the box highlights these areas. The red arrow shows the Nissl body. The changes in Nissl bodies can signal apoptosis or neurodegeneration. The arrow directs attention to these features. ** p
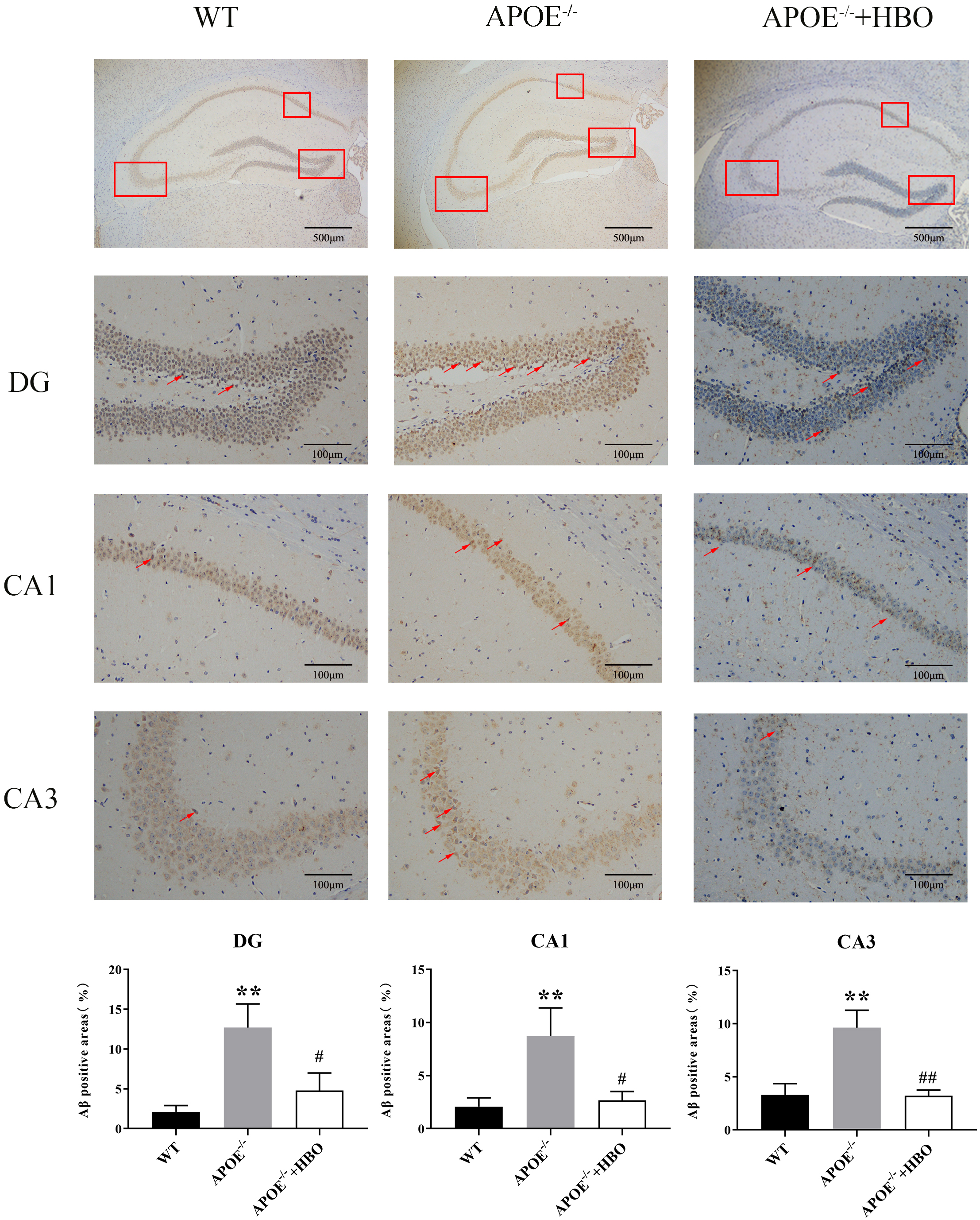
As one of the main components of neuronal cell membranes, lipids are intimately linked to the balance between normal metabolism and abnormal accumulation of A
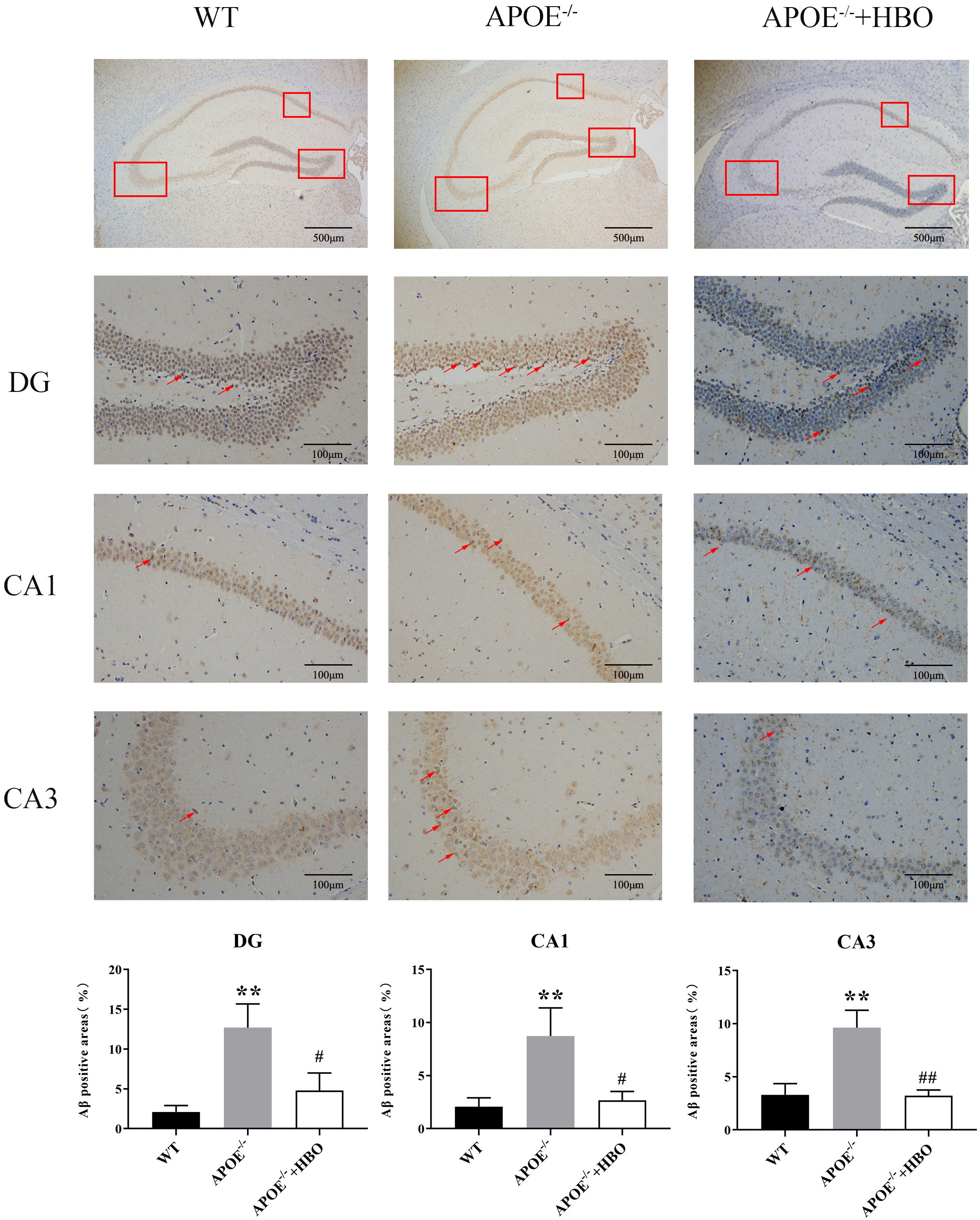 Fig. 4.
Fig. 4. Effect of HBO treatment on amyloid plaque burden in the hippocampus level of A
To determine changes in oxidative damage after HBO treatment in ApoE-/- mice, SOD activities, MDA, and NO concentrations were measured (Table 3). As compared with the WT group, ApoE-/- mice displayed significantly lower SOD activities (38% of WT), and elevated MDA concentration. Interestingly, the HBO treatment reversed the alterations of SOD and MDA in ApoE-/- mice (one-way ANOVA: p
| Biomarkers of oxidative stress | Groups | ||
| WT | APOE-/- | APOE-/-+HBO | |
| SOD activity (U/mL) | 11.0 | 4.2 | 12.4 |
| MDAconcentration (nmol/mL) | 32.1 | 43.5 | 14.5 |
| NO concentration (µmol/L) | 0.008 | 0.072 | 0.067 |
Values represent mean
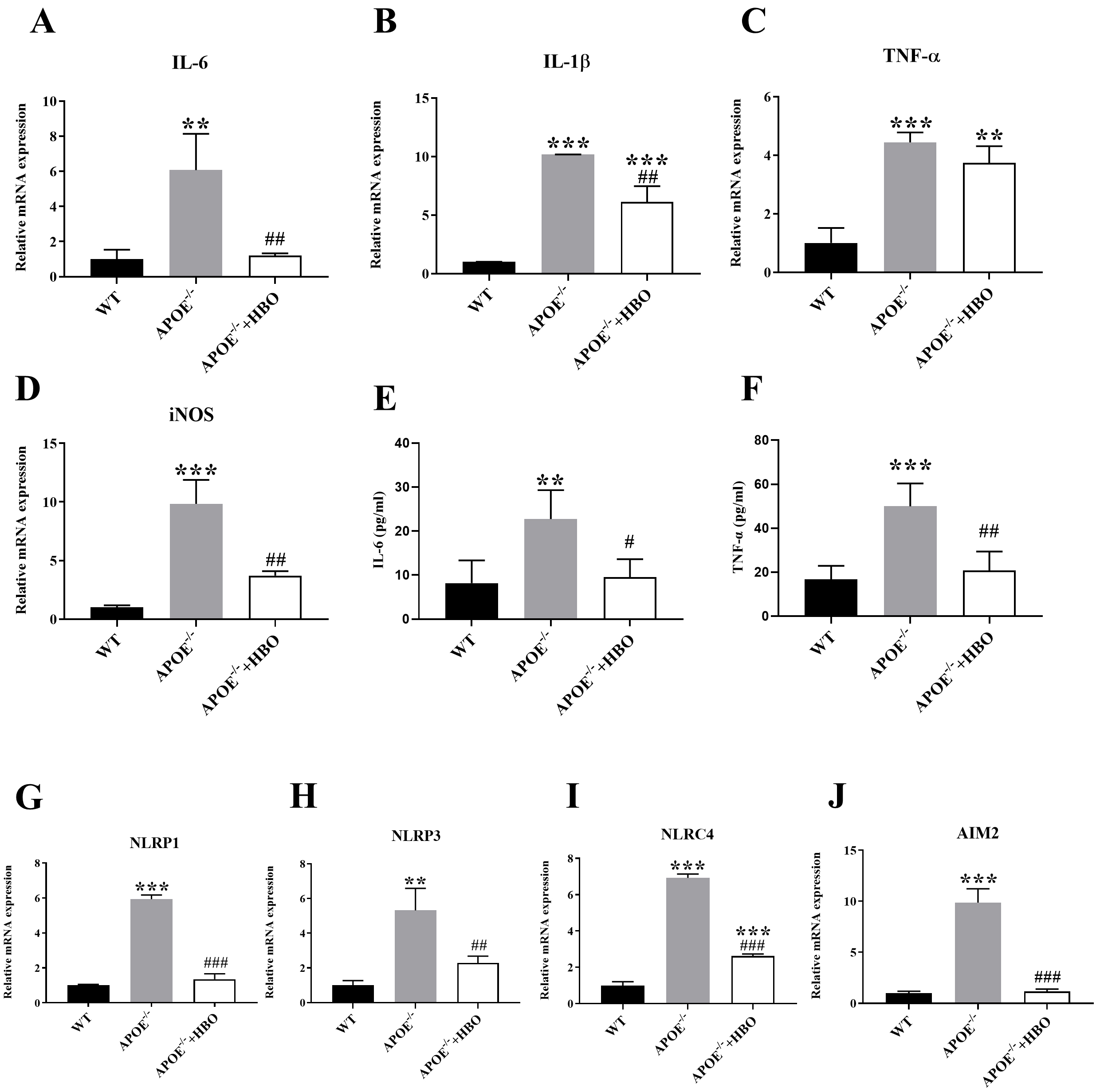
The mRNA expression of inflammatory factors such as IL-1
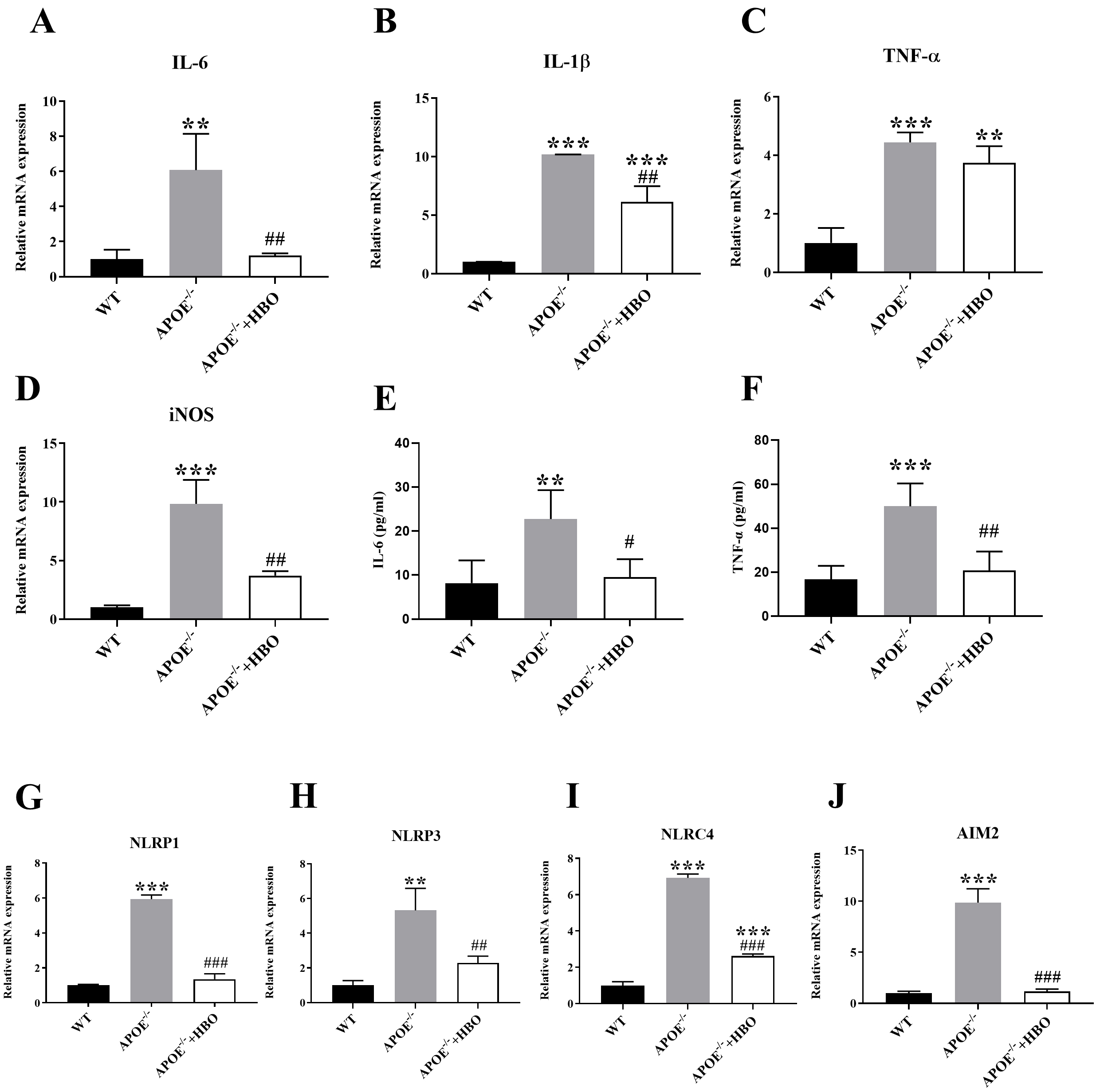 Fig. 5.
Fig. 5. Effect of HBO treatment on systemic inflammatory response. (A–D) Quantitative mRNA expressions of inflammation cytokine, IL-1
Dyslipidemia and obesity are the main risk factors for coronary heart disease, hypertension, and stroke, posing a serious threat to human health. As a result, the treatment of dyslipidemia has attracted widespread attention. HBO treatment has been approved by the FDA for many disorders [28, 31], and recently its potential benefits in treating other pathological diseases has also been studied, including traumatic brain injury, spinal cord injury, and stroke [32, 33, 34, 35]. In this study, the effects of HBO treatment on cognitive function, lipid metabolism, amyloid deposition, neuroinflammation, and other pathologies were investigated in an ApoE-/- model. Results revealed that HBO treatment improves cognitive function, and lipid metabolism levels, reduces neuronal damage and A
Consistent with previous results [36], the experiment reported here showed that ApoE-/- mice exhibit increase body weight and elevated serum levels of TC, LDL-C and TG. These findings confirm that ApoE deletion enhances lipoprotein degradation and indicate that the model used here was successful. Results showed that HBO treatment significantly reduced TG, TC, HDL-C and body weight levels in ApoE-/- mice, which is consistent with previous findings [37]. These changes in lipid profile suggest that HBO treatment may significantly improve lipid metabolism and effectively reduce the risk of dyslipidemia. Further research is needed to clarify which lipoproteins play a key role in the process.
Given that lipids are a significant component of the cell bilayer membrane, play a crucial role in both the normal physiological function of neurons and the structural development of the brain [38], dysregulated lipid homeostasis has been related to the pathological progression of various neurodegenerative diseases [39, 40]. Increasingly, studies have focused on the relationship between hyperlipidemia and neuropathy [40, 41]. For example, a Spanish cohort study found that patients with familial hypercholesterolemia had a significantly higher incidence of mild cognitive impairment than those without that condition [42], while a 21 year follow-up cohort study in Finland showed that middle-aged individuals with higher serum TC have a higher risk of developing dementia or AD in the future [43]. Hypercholesterolemia has been shown to accelerate the accumulation of A
Oxidative stress and inflammatory response are crucial in the development of dyslipidemia and related diseases, such as neurological diseases [47]. Under normal conditions, the body has certain levels of antioxidant enzymes that remove excess oxygen radicals in a timely manner and maintain homeostasis. SOD, the main antioxidant enzyme, contributes to a large accumulation of oxygen radicals when its activity decreases. As another significant indicator of antioxidant capacity, MDA serves as an indirect indicator of the extent of tissue peroxidation damage and the rate and degree of lipid peroxidation inside the body [48]. Chen et al. [49] documented that in a mouse aging model induced by D-galactose, HBO prevents cognitive impairments by reducing oxidative damage and suppressing inflammatory responses. In the present study, we show that HBO treatment can significantly improve oxidative damage by reducing the serum levels of MDA and increasing SOD activities in ApoE-/- mice. This indicates that HBO treatment improves dyslipidemia and associated problems may be through the suppression of oxygen-free radicals.
Lipid deposition promotes inflammation. The sustained excessive release of pro-inflammatory factors, such as TNF-
Following decades of clinical validation, HBO has solidified its role as a versatile and safe intervention across diverse medical disciplines. In recent years, its application has expanded into neuroscience, where it has shown therapeutic potential as a neuromodulation strategy for neurological and psychiatric disorders. Although preclinical and clinical studies have confirmed its neuroprotective benefits, the molecular mechanisms underlying these effects are not yet fully understood [8, 9, 10]. Our future investigation will employ RNA sequencing to map transcriptomic signatures to uncover conserved regulatory pathways and candidate molecular targets for cognitive enhancement. A limitation of our study is that we did not administer HBO to different types of animals in various forms, nor to a small group of humans with specific risk factors. As a result, we were unable to fully separate the effects of HBO on cognition from potential confounding factors. Throughout the treatment process, the medical staff should closely monitor the patient’s vital signs in order to respond promptly to any potential emergencies.
Given the urgency of dementia prevention, particularly among middle-aged individuals with genetic risk factors (e.g., APOE4 carriers), a family history of dementia, dyslipidaemia, or prodromal cognitive decline, we emphasize the importance of bidirectional translational research. Careful consideration is essential when translating these findings into clinical practice, and identifying the optimal treatment regimen will be a challenging task. Insights derived from preclinical models must undergo rigorous validation through stratified clinical trials to refine patient selection and treatment protocols [13, 54]. Furthermore, we need to further explore whether it is appropriate to prescribe an individualized treatment regimen for each patient.
Evidence indicates that HBO treatment is evolving from an adjunctive emergency intervention into a promising protective pharmacological strategy. While current research demonstrates HBO’s capacity to reduce oxidative stress and inflammatory responses, it is crucial to emphasize that causality has not yet been directly established. As this study primarily used mouse models, further clinical validation of these findings remains essential. This limitation may partially compromise the comprehensiveness of the reported outcomes. Continued investigation into HBO’s molecular mechanisms and novel therapeutic applications will advance its integration into clinical practice. To effectively translate HBO’s benefits into clinical settings (akin to “preventive strategies”), it is imperative to carefully assess patient-specific risk factors, comorbidities, and potential drug interactions that impact HBO-related protection.
The data in this study are available from the corresponding author upon request.
GYD and YXL conducted the majority of the experimental procedures. LXW and XC contributed to conceptualize and supervise the study. HJL was responsible for data collection and compilation. CQ analyzed the data and performed a statistical analysis. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University approved all animal procedures used in this study (Ethic Approval Number: 2008067). The animal procedures were performed in strict accordance with the guideline of the Institutional Animal Care and Use Committee of Nanjing Medical University.
Not applicable.
This research was funded by Changzhou Sci &Tech Program (CJ20230073), Young Talent Development Plan of Changzhou Health Commission (CZQM2020063), Natural Science Foundation of Jiangsu Province (BK20241858), Basic Research Foundation of Zhenjiang (JC2024024) and Medical Research Project of Jiangsu Provincial Health Commission (M2022071).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.