1 Department of Veterinary Anatomy, College of Veterinary Medicine and BK21 FOUR Program, Chonnam National University, 61186 Gwangju, Republic of Korea
2 Department of Nephrology, Washington University in St. Louis School of Medicine, St. Louis, MO 63110, USA
Each significant scientific advancement reveals new levels of complexity in the brain, widely considered the most complex organ in the body. The hippocampus, a crucial structure within the medial temporal lobe, is one of the most fascinating areas of the brain. The hippocampus is essential for cognitive function, memory retention, and emotional regulation. Investigating the pathophysiology, including functional changes, of various neurological disorders—such as neurodegeneration and neuroinflammation—is vital for advancing treatment strategies and therapeutic interventions [1, 2]. The hippocampus demonstrates significant neurogenesis and long-lasting plasticity. Therefore, understanding its underlying mechanisms is critical for developing therapies for related behavioral disorders. The hippocampus is often the focus of discussions on neuroplasticity and adult neurogenesis owing to its continuous and dynamic generation of new neurons. Newborn neurons integrate into adult brain circuits, enhancing neuroplasticity by strengthening and refining the information transmitted by older neurons [2]. This unique capacity positions the hippocampus at the core of discussions on changes in neuroplasticity and neurogenesis within the adult brain [2]. While extensive research has highlighted the involvement of the hippocampus in cognitive function and emotional regulation, further investigation is necessary to understand its lesser-known functions. Furthermore, advancements in our understanding of hippocampus neuroplasticity and neurogenesis indicate that this knowledge could be vital for developing novel therapeutic strategies for various neurological disorders.
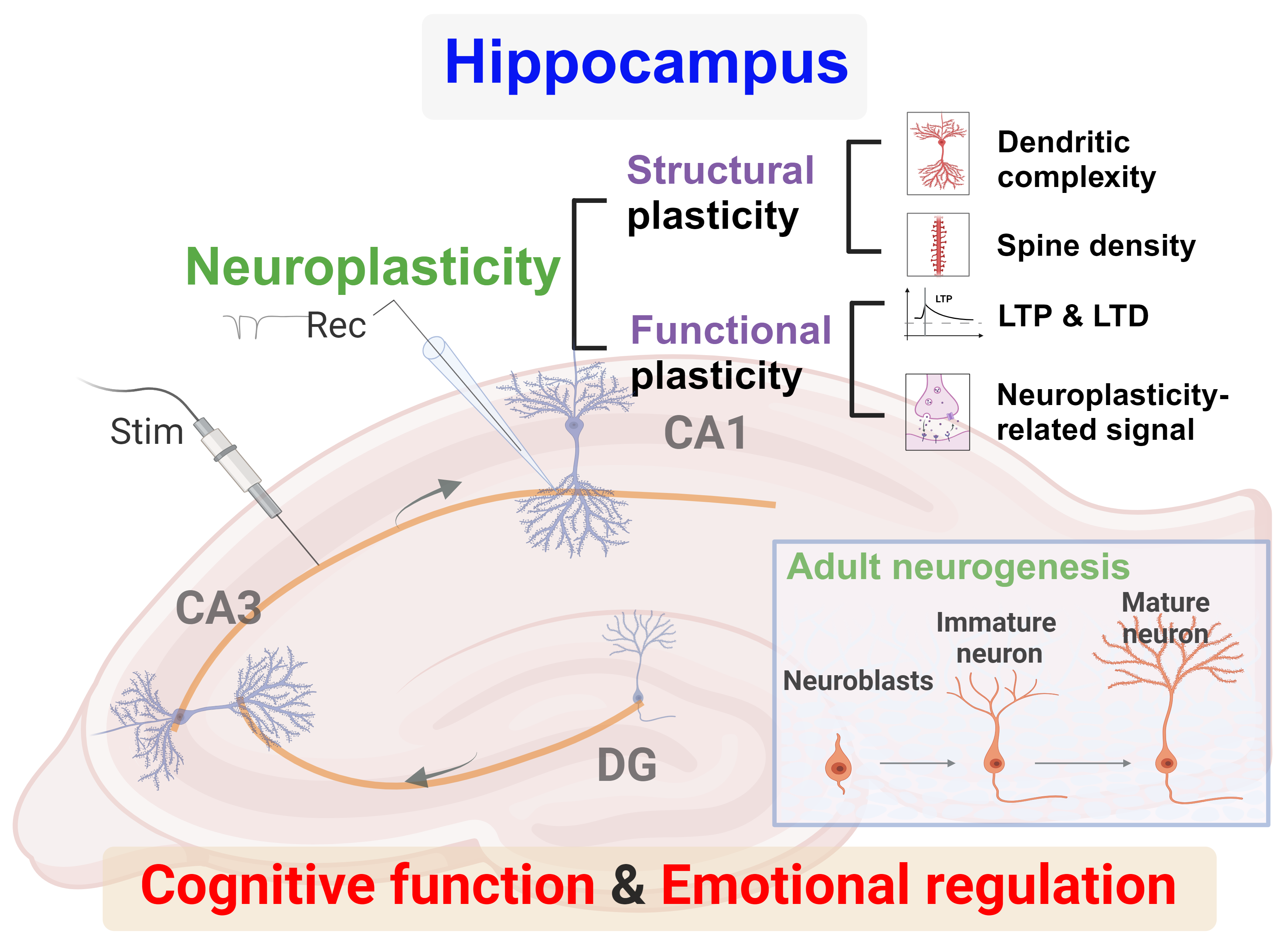
Neuroplasticity refers to the ability of the nervous system to adjust in response to stimuli from both external and internal environments [3]. This process involves several physiological mechanisms, such as the generation, survival, migration, and integration of new neurons; the growth of neurites; the formation and modification of mature synapses; and the development and remodeling of neural networks. Neuroplasticity can be classified into structural plasticity and functional (synaptic) plasticity (Fig. 1). Structural plasticity refers to changes in the synaptic region, including the retraction or extension of the synapses due to the remodeling of axons, dendrites, and dendritic spines. Functional plasticity involves the rearrangement of synaptic components, receptor distribution, neurotransmission regulation, and modulation of synaptic efficacy. These mechanisms are significantly affected by factors such as stress hormones, neurotransmitters, and environmental cues. Both physiological and pathological conditions primarily associate neuroplasticity modulation in the hippocampus with learning, memory, and emotional regulation [4, 5]. Recent epidemiological and experimental studies have aimed to elucidate hippocampal adaptability in neurological disorders to better understand the molecular pathways associated with changes in hippocampus function [6, 7]. The precise correlation between hippocampal neuroplasticity and dysfunction in hippocampus-related neurological disorders remains only partially understood, highlighting the need for further research.
 Fig. 1.
Fig. 1.
Overview of neuroplasticity and adult neurogenesis in the hippocampus. Created using BioRender.com (https://www.biorender.com/). Abbreviations: CA, cornu ammonis; DG, dentate gyrus; LTD, long-term depression; LTP, long-term potentiation; Rec, recording; Stim, stimulation.
Neurogenesis refers to the significant process of generating new neurons in the brain [8]. Its role in physiological and pathological conditions has been the focus of extensive research. Adult neurogenesis, a critical component of brain neuroplasticity, occurs continuously in the hippocampus throughout life (Fig. 1). Various environmental and cell-intrinsic factors tightly regulate hippocampal neurogenesis, allowing the brain to adapt to environmental changes [9]. Several neurological disorders impair hippocampal neurogenesis, highlighting the central role of the hippocampus in neuroplasticity research [1, 10]. Hippocampal neurogenesis is closely associated with memory formation and emotional regulation, accompanied by changes in neuroplasticity. Dysregulated adult hippocampal neurogenesis contributes to the etiology of certain neurological disorders. Therefore, understanding the mechanisms of hippocampal neurogenesis and its role in neuroplasticity is essential for developing therapeutic strategies for these disorders.
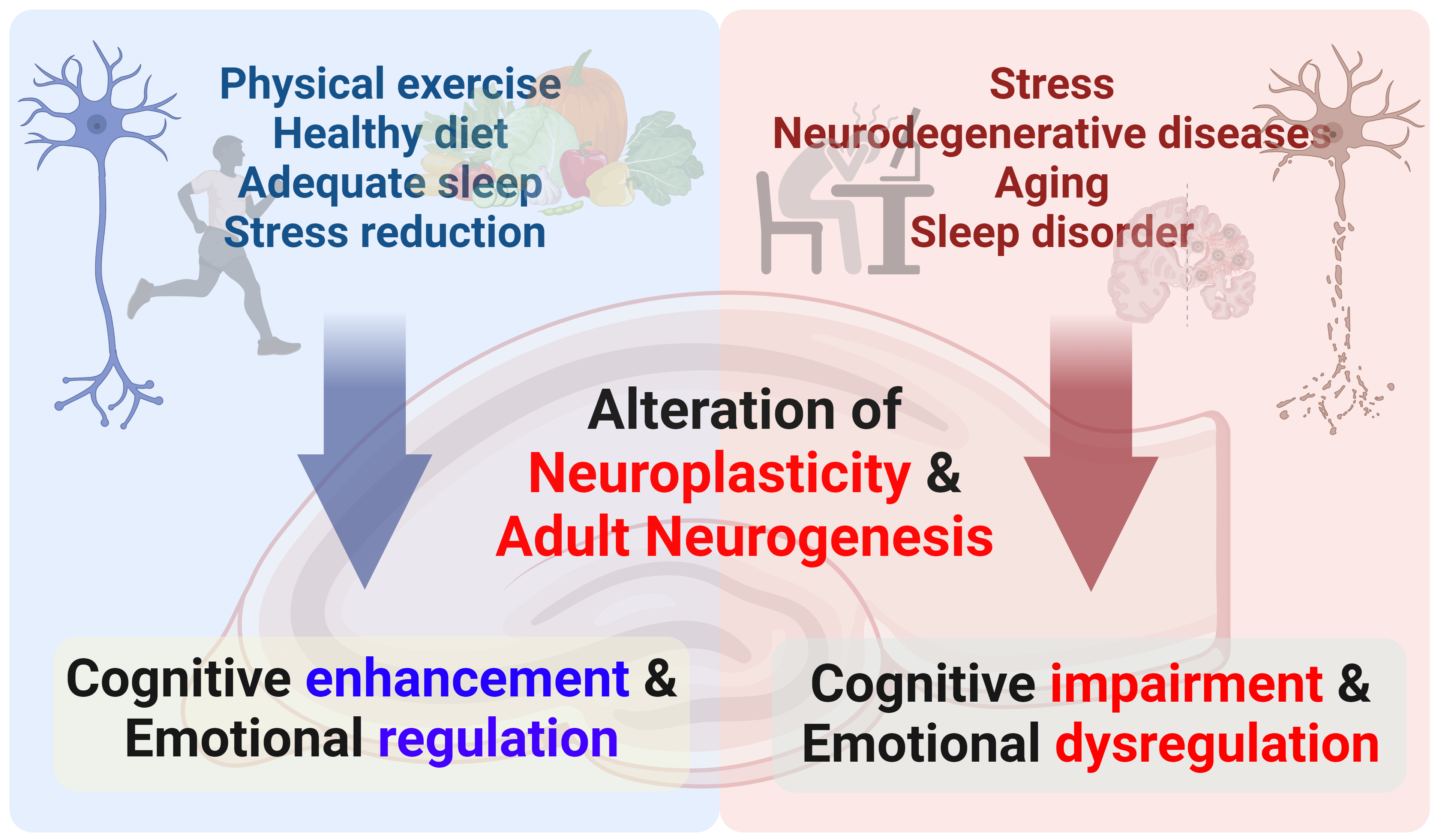
Fig. 2 illustrates the various factors regulating hippocampal neuroplasticity and neurogenesis, which subsequently influence hippocampal function. While stress and neurodegeneration can impair these processes, factors such as physical exercise and adequate sleep can facilitate them [5, 11, 12, 13, 14]. Exercise, particularly, has a significant effect on hippocampal neuroplasticity and neurogenesis. Regular physical activity enhances the synthesis of brain-derived neurotrophic factor, an essential neurotrophin that promotes neuronal survival and the development of new synapses and neurons. Extensive research has demonstrated that physical activity enhances neurogenesis in the hippocampus, which subsequently strengthens synaptic connection and improves performance on cognitive tasks that rely on the hippocampus, such as memory and spatial learning [13, 14]. These findings support the idea that regular physical activity may mitigate the emotional and cognitive decline associated with aging and neurodegenerative diseases. However, a deeper understanding of the mechanisms underlying neuroplasticity and neurogenesis in the hippocampus requires further research. Advancing targeted therapeutics could facilitate brain health and aid in the prevention or mitigation of cognitive and emotional decline.
 Fig. 2.
Fig. 2.
Schematic illustration of hippocampal neuroplasticity and neurogenesis and their effect on hippocampal function. Created using BioRender.com (https://www.biorender.com/).
Collectively, the hippocampus has been a primary focus in research on neuroplasticity and adult neurogenesis, particularly in relation to changes observed in neurological conditions. Extensive research has aimed to clarify the underlying mechanisms behind these changes, addressing existing knowledge gaps, with several review articles contributing to the ongoing discourse [1, 4, 11, 12, 15]. Recent advancements involving both original research and review articles have significantly enhanced our understanding of the molecular mechanisms underlying these processes. Behavioral interventions have demonstrated the ability to modulate hippocampal neuroplasticity and neurogenesis to varying degrees, highlighting the complexity of these pathways. Given the intricacies involved, future studies should examine the effects of various physiological and pathological conditions on hippocampal neuroplasticity and neurogenesis. Specifically, investigating the underlying mechanisms through which various behavioral cues and neurological factors influence these functions is crucial. This research will help clarify the varied responses observed in the hippocampus, offering insight into the potential roles of hippocampal neuroplasticity and neurogenesis in both normal brain function and disease conditions.
HJ, PDEWM, and CM conducted literature research and authored the paper. CM were involved in paper topic selection. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Agriculture and Food Convergence Technologies Program for Research Manpower Development Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (RS-2024-00398561).
The authors declare no conflict of interest. Poornima D. E. Weerasinghe-Mudiyanselage and Changjong Moon are serving as the Guest editors of this journal. Changjong Moon is serving as one of the Editorial Board members of this journal. We declare that Poornima D. E. Weerasinghe-Mudiyanselage and Changjong Moon had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Bettina Platt.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.