1 Laboratorio de Neurobiología, Instituto de Neurociencias, Instituto de Investigación Sanitaria San Carlos (IdISSC), Hospital Clínico San Carlos, 28040 Madrid, Spain
2 Área de Fisiología, Departamento de Ciencias Médicas, Facultad de Medicina de Ciudad Real, UCLM, 13071 Ciudad Real, Spain
3 Servicio de Neurología, Instituto de Neurociencias, Instituto de Investigación Sanitaria San Carlos (IdISSC), Hospital Clínico San Carlos, Universidad Complutense de Madrid, 28040 Madrid, Spain
Abstract
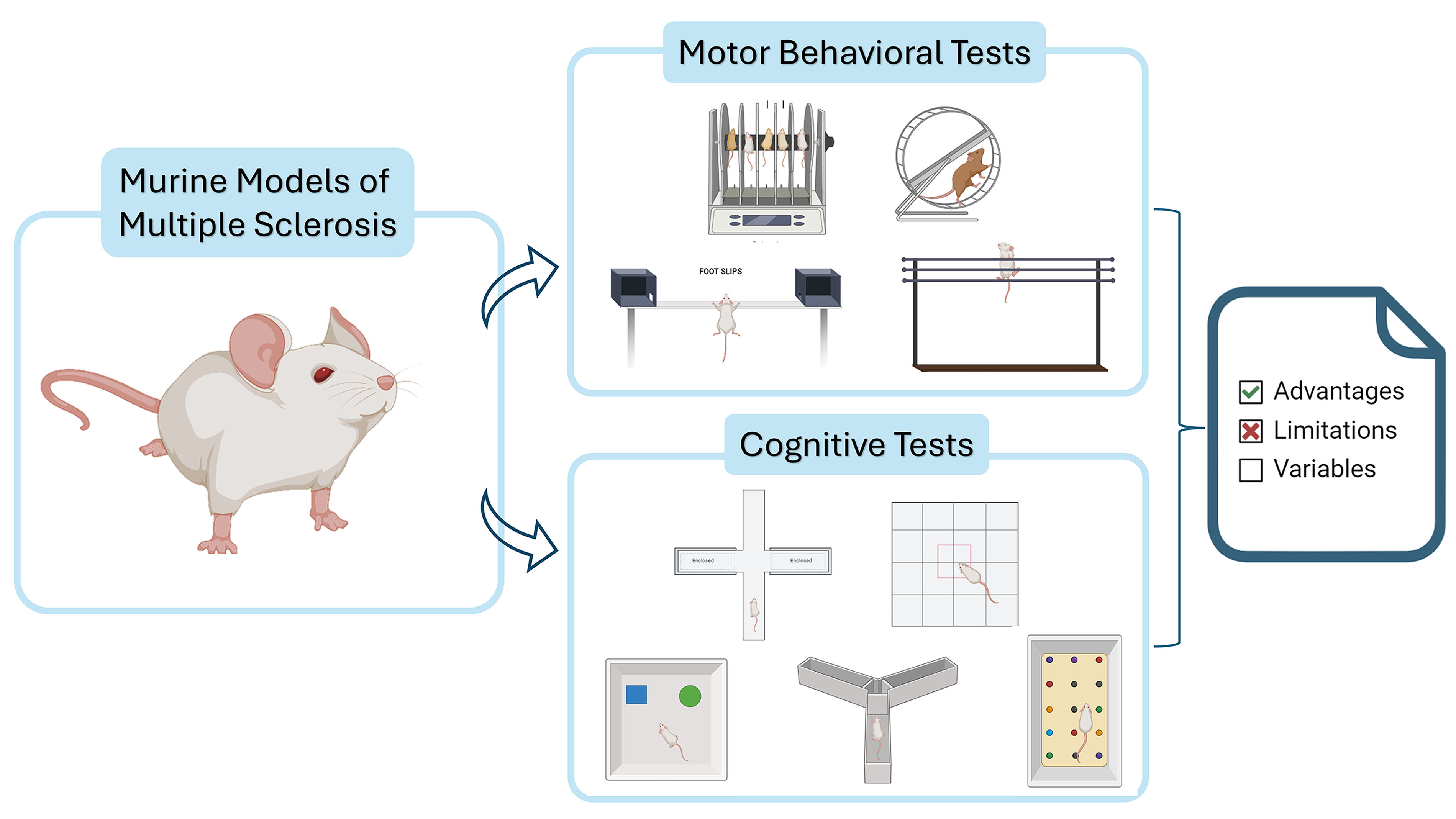
Multiple sclerosis (MS) is a neurodegenerative disorder characterized by progressive motor and cognitive impairments, affecting millions worldwide. It significantly reduces patients’ quality of life and imposes a burden on health systems. Despite advances in understanding MS, there is no cure, highlighting the need for effective therapeutic strategies. Preclinical animal models are critical for gaining insights into MS pathophysiology and treatments. However, these models fail to fully replicate the complexity of human MS, making it essential to choose appropriate models and behavioral tests to evaluate their efficacy.
This review examines various motor and cognitive behavioral tests used in preclinical MS models, discussing their strengths and limitations. The goal is to guide researchers in selecting the most appropriate tests for their models, while providing insights into how these tests are performed and analyzed.
We reviewed motor and cognitive behavioral tests used in MS models, detailing test procedures and evaluating their advantages and disadvantages.
This review offers a comprehensive overview that aids researchers in choosing the most suitable tests for their studies, improving the accuracy and reliability of preclinical MS research.
Understanding the strengths and limitations of these tests is crucial for making informed decisions, leading to better experimental designs and, ultimately, more effective therapeutic interventions for MS.
Graphical Abstract
Keywords
- experimental models
- behavioural test
- locomotor test
- multiple sclerosis
- mice
Multiple sclerosis (MS) is a complex autoimmune disease that primarily affects the central nervous system (CNS), leading to progressive demyelination and neurodegeneration. This pathological process results in a wide range of motor, sensory, and cognitive impairments, as MS affects key neural pathways, including the corticospinal tracts, cortex, subcortical structures, cerebellum, and corpus callosum. The distribution and composition of lesions can vary significantly both between and within individual patients, contributing to the diverse clinical manifestations of MS. Common symptoms include motor coordination difficulties, balance issues, ataxia, diplopia, sensory disturbances, cognitive dysfunction, depression, fatigue, spasticity, optic neuritis, bladder dysfunction, constipation, anxiety, pain, sleep disorders, and other ancillary or paroxysmal symptoms [1]. Understanding these clinical implications is essential for developing effective therapeutic strategies and guides the need for reliable preclinical models to evaluate potential treatments.
The precise etiology of MS remains elusive, with a multifactorial origin that includes genetic predisposition, environmental influences, dietary habits, and infectious agents contributing to disease onset and progression [2]. Despite significant advances in research, no single animal model fully captures the complexity of MS. Current models, such as the cuprizone and experimental autoimmune encephalomyelitis (EAE) models, primarily focus on specific aspects like demyelination or inflammation and do not entirely replicate the intricate nature of the disease in humans [3]. Refining studies using animal models could serve as a key strategy to enhance the translation of research findings to clinical stages [4].
Behavioral and motor tests are widely used in preclinical MS research to assess disease progression and the efficacy of therapeutic interventions. However, the generalizability of these tests across different rodent models and their relevance to other neurodegenerative diseases require further investigation. Results often vary depending on the specific MS model used, as different models tend to mimic distinct aspects or forms of the disease. For instance, the cuprizone model may exhibit more pronounced cerebellar deficits compared to the EAE model, which can influence performance outcomes on motor tests. Understanding these nuances is essential for selecting the most appropriate tests to ensure accurate evaluation. By improving the accuracy and relevance of the tests performed over animal models, researchers can increase the likelihood that therapeutic outcomes observed in preclinical trials will translate successfully to human patients.
This review provides a comprehensive overview of the behavioral, motor, and cognitive tests commonly employed in MS research, highlighting their strengths, limitations, and applicability to different experimental models. By deepening our understanding of how these tests translate across models and other neurodegenerative conditions, researchers can make more informed decisions, ultimately enhancing the rigor and translational value of their studies.
When conducting behavioral tests in rodents, it’s essential to maintain consistent and controlled conditions to ensure reliable and valid results. The following considerations should be taken into account [5]:
Controlled Testing Environment: The testing environment should be strictly regulated, paying close attention to noise levels, ambient temperature, lighting, and odors. When multiple experimenters are present, they should avoid verbal communication and move quietly to avoid startling the mice.
Consistency in Experimental Conditions: The conditions of the mice in the experimental and control groups should match based on age, sex, body weight, and motor function status. Mice with abnormalities should be excluded to avoid confounding the results.
Genetic Background: The genetic background of the rodents plays a crucial role in behavioral testing. Strain-specific behavioral phenotypes can significantly influence outcomes, so it is important to ensure that control and disease-model mice come from the same genetic background. This is especially vital in mutant or disease models like those used in MS research, where genetic variability can introduce unwanted bias.
Housing Conditions: The housing environment, including the number of cage mates, cage enrichment, and lighting schedules, can greatly influence behavior. These factors should be consistent across experimental groups to avoid introducing variability that could affect behavioral test outcomes [6, 7].
Test Battery Order: When using a battery of behavioral tests, the order in which tests are performed is important. Certain tests can affect the results of subsequent assessments (e.g., stress from one test may alter behavior in the next), so careful planning and resting periods between tests should be incorporated.
Rest and Recovery: Animals should be allowed adequate rest between tests to recover energy and mitigate the effects of previous tasks, which is especially important for physically demanding tests. This helps obtain more accurate measurements of their performance.
Cleaning Between Trials: The experimental apparatus should be thoroughly cleaned after each trial with a 70% ethanol solution to remove any droppings or olfactory cues that may influence the behavior of subsequent subjects.
Acclimation: Mice should be acclimated to the testing environment before the start of the tests to reduce fear and discomfort, aiding in more accurate behavioral assessments.
Blinded Data Analysis: To reduce experimenter bias, data analysis should be performed with the experimenters blinded to the group assignments of the mice whenever possible.
Protocol Adjustments: While standard protocols are essential, some flexibility is needed to adapt to the specific conditions of the laboratory and the available equipment. Any modifications should be documented and validated to maintain the integrity of the study.
Experimental Rigor: A rigorous experimental design should be maintained to avoid exaggerating efficacy and ensure translational success. Protocols should be optimized, standardized, and strictly controlled to enhance the reliability and reproducibility of the findings.
Motor impairment is the most studied parameter in experimental models of MS, primarily due to lesions in the cerebellum, spinal cord, and association cortex. However, it’s challenging to find a behavioral test that purely measures motor deficits and distinguishes them from the cognitive and psychological symptoms present in these models. Generally, as the complexity of the test task increases, so does its ability to detect subtle motor changes [8].
The most commonly used tests to assess motor function include the rotarod test, various types and protocols involving complex running wheels, and the balance beam or walking test. Less frequently used or not yet widely described in studies focused on rodent models of MS are the horizontal bar test, forelimb grip strength test, vertical pole test, and geotaxis test [8].
RotaRod is a simple test to evaluate motor function and coordination in rodents. Motor coordination or fatigue can be assessed by measuring the time the animal remains on a rotating rod before falling off. It is a widely used device described in animal experimentation that has seen improvements since its invention. In the latest models, the rod can spin constantly, accelerate, sway (forward and backward), or move through complex ramps of acceleration/deceleration [9, 10].
- Latency to Fall: Time in seconds until the mouse falls off the rotating rod at a set speed. This measures initial motor coordination and balance. The faster the cylinder rotates, the faster the mice will fall. Mice can be tested at a set speed for different trials or at a gradually accelerated speed.
- Fall Speed: Time in seconds for the mouse to reach the padded base once it starts falling. Impaired mice exhibit slower fall speeds compared to controls.
- Number of Rotations: Count of rotations the mouse completes with the cylinder before falling off. Increased rotations suggest compensatory motor strategies or decreased ability to disengage from the rotating surface [14].
- Ease of Use: The rotarod test is straightforward to perform and analyze, making it accessible for assessing motor deficits in MS models [15].
- High Throughput: Multiple mice can undergo testing simultaneously, increasing efficiency in data collection.
- Not reliable and not sensitive to detect subtle motor alterations.
- Mouse behaviour: Mice may refuse to balance on the cylinder by choosing to fall or cling to the cylinder and rotate with it.
- Results can be influenced by the mouse weight, heavier mice tend to fall easier.
Offers the ability to detect subtle deficits in motor skills and balance than other motor tests. The test evaluates an animal’s ability to walk on a long, narrow beam.
- A 6- or 12-mm wide beam that is 100 or 120 cm in length.
- A 60-watts lamp at the starting point as a stimulator to encourage the mice to start walking across the beam.
- A black box with nesting materials at the endpoint to encourage completion.
- A padded nylon hammock is placed beneath the beam to ensure gentle mice falls.
- A camera for recording trials and analysing performance [18] (Fig. 2).
The beam can be inclined to increase complexity and sensitivity of the test to facilitate detecting subtle motor deficits. It can be inclined downwards to encourage the mice to move forward [19, 20].
- Walking Time: The time taken for the mouse to traverse an 80 cm section of the beam. Motor-impaired mice typically require more time [19, 21].
- Number of footslips: Count of footslips, with notes on whether fore- or hindlimbs were involved [19, 21].
- Reliable and more sensitive to detect motor coordination deficits than other motor tests (e.g., rotarod test).
- Confounding factors includes motivation of the mice to cross the beam. Overtraining may lead to boredom, while anxiety can affect their ability to complete the task [16].
Motor behavior is mediated by a complex neural network that originates in the cortex and ends in the skeletal muscles, involving neuronal tracts and connection pathways of the first and second motor neuron. Various methods exist to determine gait abnormalities in small rodents, including the rotarod test paradigm. The Motor Skill Sequence (MOSS) is designed to detect latent deficits in motor performance. In a first step, we habituate mice to training wheels composed of regularly spaced crossbars until achieving maximum performance functionality.
- MOSS cage equipped with a 38-rung training wheel to allow initial spontaneous running behaviour assessment.
- MOSS cage equipped with a 16-rung complex running wheel for advanced motor skill evaluation.
- Activity Monitoring System Software for tracking and recording motor activity (Fig. 3).
 Fig. 3.
Fig. 3. Motor Skill Sequence (MOSS). The figure was created by BioRender (https://www.biorender.com/).
- Cumulative Distance Travelled: Measured in minutes, calculated based on the number of wheel revolutions and the distance covered per revolution.
- Maximum Running Velocity: Recorded in revolutions per minute (rpm).
- Number of Individual Runs: The total count of distinct running bouts.
- Maximum Run Duration: The longest continuous running period.
- The test is highly sensitive and reliable for detecting latent motor deficits, since it requires complex bilateral motor coordination.
- Voluntary wheel running can test fatigue at the same time, which is a frequent symptom in MS patients.
This test is based on the fact that rodents ability to grip is inversely proportional to the bar diameter.
While the Horizontal Bar Test and the Rotarod Test both assess motor coordination, they do so in different ways. The Rotarod Test adds an additional dimension of difficulty by incorporating speed and acceleration, which challenge the animal’s ability to balance dynamically as the rod rotates. This is a critical difference because dynamic balance and motor coordination are crucial for evaluating more subtle motor impairments, particularly in early-stage disease models. In contrast, the Horizontal Bar Test focuses solely on the animal’s static grip strength and does not assess the animal’s ability to respond to changing conditions like increasing speed, as seen in the Rotarod Test [25].
- 38 cm long Bar: Typically 3 bars are used of a 2 mm, 4 mm and 6 mm width.
- Supporting Frame: A sturdy frame to securely hold the bar at a fixed height, usually around 50 cm [25, 26, 27] (Fig. 4).
- Hang Time: The duration (in seconds) that the rodent can hang from the bar before falling. The scoring system is as follows: Falling between 1–5 sec = 1, Falling between 6–10 sec = 2, Falling between 11–20 sec = 3, Falling between 21–30 sec = 4, Falling after 30 sec = 5, Placing one forepaw on a bar support without falling = 5 [25].
- The test is sensitive to detect neuromuscular deficits and changes in motor coordination.
- The test is reproducible and easily standardized. It yields reliable, consistent results across different trials.
- Easy to set and conduct.
- Cost-Effective. The test requires inexpensive materials and is straightforward to perform in most laboratory settings [26, 27].
- Lack of Dynamic Components: The Horizontal Bar Test primarily measures static motor abilities and does not challenge the animal’s ability to maintain balance under changing conditions. This absence of a dynamic component limits its ability to mimic more complex motor tasks.
- Results can be influenced by the mouse weight, heavier mice tend to fall easier.
The Forelimb Grip Strength Test measures the muscle strength of rodents by having them grip a bar while an experimenter gently pulls their tail. This test, which can be conducted horizontally or vertically, is praised for its ease of use and reliability, despite variability due to motivational factors and body mass differences [28].
- Grip Strength Meter or Monitoring Devise (Fig. 5).
 Fig. 5.
Fig. 5. Forelimb Grip Strength Test. The figure was created by BioRender (https://www.biorender.com/).
This test can also be conducted vertically. This modification increases validity, reliability and lowers variability. It is theorized that mice are more motivated to grip the bar in the vertical position due to a greater fear of falling. Additionally, the vertical test better utilizes the force of gravity and the pull of the mouse’s tail by the experimenter, resulting in more accurate and less variable recorded results. In this position, the total forces involved are more effectively transmitted to the transducer [29].
- Forelimb strength as recorded by the apparatus.
- Easy and quick to perform.
- No need of animal training.
- Mouse motivational factors to grip the bar may increase variability in results.
- Differences in animal body mass can alter the results. This can be predicted if animal muscle force is proportional to body mass. However, a recent study has shown no correlation between body mass and the strength values recorded by the device [29]. Alterations in body mass due to age and/or other factors are not always linked to a proportional change in muscle weight but rather to a certain body composition [30].
The Weight Test evaluates the forelimb strength of rodents using a series of progressively heavier weights attached to a wire mesh ball. Mice are tested on their ability to hold each weight for three seconds, with rest periods in between attempts, to determine their maximum strength and endurance.
- A Ball of fine wire mesh that will serve as an easy gripping object for mice.
- Chain links of increasing weight that can be connected to the ball of fine wire, ranging from 20 g to 98 g (Fig. 6).
- Time holding each weight: Duration (in seconds) that the mouse holds each weight.
- Maximum reached held weight: The heaviest weight (in grams) that the mouse can hold [31].
- Simple and inexpensive material required.
- No training is required.
The Inverted Screen Test gauges rodents’ overall muscle strength, coordination, and fatigue by timing how long they can cling to an inverted wire mesh screen. This quick, cost-effective method provides a measure of motor function.
- A 43 cm square of wire mesh composed by 12 mm squares of 1 mm diameter wire, bordered by a 4 cm wooden frame.
- A padded bench to ensure gentle falls (Fig. 7).
- Latency to Fall: The duration until the animal falls off the screen is scored as follows: Score 1: 1–10 s, Score 2: 11–25 s, Score 3: 26–60 s and Score 4: +60 s.
- Fast and easy to perform.
- Requires simple and inexpensive materials.
- Low sensitivity in detecting subtle motor deficits.
- Motivation and anxiety of the animals can influence the results [31].
The Wire Hang Test measures grip strength, endurance, and coordination in rodents by timing how long they can cling to a wire suspended above the ground. While easy to perform and requiring minimal equipment, the test’s results can vary due to the different ways animals can hang on the wire and the need for training to reduce individual variation.
- A wire suspended 50–60 cm above the ground.
- Adhesive tape (for optional modifications) (Fig. 8).
- The test is relatively easy to perform with low equipment requirements.
The final reach and fall scores and measured for all animals tested. A survival curve can also be created using the timestamps for each instance of either the fall or reach score changing.
- Training is required to reduce individual variation and obtain consistent results.
- The different ways animals can hang on the wire may affect their performance, introducing variability.
The Cylinder Test evaluates the sensorimotor function of the forelimbs and detects unilateral motor impairments by observing the use of forepaw paws during rearing. Rodents are placed in a cylinder, and their forelimb activity is recorded and analyzed, leveraging natural exploratory behavior for straightforward and minimally invasive assessment.
- A cylinder: For rats: 20 cm diameter, 30 cm height. For mice: 15 cm diameter, 40 cm height.
- A mirror under the table to reflect the image of the cylinder.
- A camera for recording (Fig. 9).
- Ratio of left and right forepaw use. In cases of unilateral lesions, there is typically a decreased use of the contralateral forelimb paw during rearing.
- Easy and fast to perform.
- Based on natural exploratory behaviour, requiring no animal training.
- Minimal equipment required [34, 35].
- Not useful for models with global brain injury [33].
- Data analysis can be complex and time-consuming.
- Unconditional behaviors of mice, like remaining still or exploring the table’s edge, can lead to a low count of forepaw placements [5].
The Footprint Test assesses gait and motor coordination by analyzing the footprint patterns of rodents as they walk along a runway coated with non-toxic paint. This non-invasive method provides detailed information on stride length, base width, and gait symmetry, although it requires natural walking behavior and can involve time-consuming manual analysis.
- Runway: A straight, narrow path with a darkened shelter at one end to encourage the rodent to walk through it.
- Non-toxic Paint: Different colours for hind paws and fore paws.
- Paper or Absorbent Surface: Placed on the floor of the runway to capture the footprints (Fig. 10).
- Stride length: Distance between successive placements of the same paw.
- Front-base width: Distance between the left and right front paw prints.
- Hind-base width: Distance between the left and right hind paw prints.
- Gait Symmetry: Comparison of the left and right paw prints.
- Non-invasive and easy to perform.
- Provides detailed information about gait and motor coordination.
- Can be used repeatedly to monitor changes over time.
- Requires rodents to walk naturally, which may not always occur.
- Variability in individual rodent behavior can affect the consistency of results.
- Manual analysis of footprint patterns can introduce variability and human error.
- Manual analysis of footprints can be time-consuming.
- Inability to Capture Dynamic Parameters.
In recent years, automated systems have been developed to provide more detailed, accurate, and objective assessments of rodent gait patterns. These systems, such as CatWalk™, DigiGait™ and TreadScan™ use high-resolution video and specialized software to track the animal’s movements as it walks across a transparent or specialized platform [36].
CatWalk™: This system uses a glass walkway illuminated from below, with a high-speed camera capturing the footprints in real time. It measures not only static parameters like stride length and base width but also dynamic parameters such as swing duration, stance duration, and interlimb coordination, providing a more comprehensive analysis of gait [37].
DigiGait™: This system records the animal’s gait while walking on a motorized treadmill. The software generates a digital profile of each limb’s movement, offering detailed analysis of stride, step frequency, and paw placement. DigiGait™ is particularly useful for detecting subtle gait abnormalities that are difficult to observe with traditional methods [38, 39].
TreadScan™: Another automated system that uses treadmill-based gait analysis, TreadScan™ captures gait dynamics, including limb velocity, angle, and interlimb coordination. This method offers a highly sensitive assessment of gait irregularities and motor deficits in disease models like MS [40, 41, 42].
The Pole Test assesses rodent locomotion, motor coordination, and basal ganglia-related deficits, including bradykinesia. Mice are placed on a vertical pole to measure their time to turn and descend. This straightforward and cost-effective test effectively detects motor impairments but requires prior training and can be influenced by the rodent’s weight, motivation, and anxiety.
- A vertical pole. Typically, 50–60 cm in length and 1–2 cm in diameter.
- A padded base platform to catch the rodent if it falls.
- Camera for recording the test to analyse the rodent’s movements (Fig. 11).
- Latency to Turn (Tturn): Time taken to turn from the head-up to the head-down position.
- Time to Descend (TD): Time taken to descend from the top to the bottom of the pole [44].
- Total Time: Sum of the turn time and descent time.
- Falls: Frequency and timing of falls during the test [46].
- Sensitive to Motor Deficits. Effectively detects motor coordination and balance impairments.
- Simple and Inexpensive. Requires minimal equipment and is easy to set up.
- Validity is questioned in MS mouse model. Scarce studies are published.
- Heavier rodents may not perform well in this test.
- Animal training is required.
- Motivational and anxiety factors may alter results.
Demyelination and inflammation can lead to functional alterations not only in the motor domain but also in the cognitive aspect. The immune response targeting myelin observed in multiple sclerosis models, and the myelin loss in demyelinating models, cause a clear loss of the myelin sheath covering the axon. This leaves the axon exposed and vulnerable to degenerative processes, and consequently, neuronal degeneration, which can lead to cognitive impairments. Below are the main cognitive tests useful in these experimental models.
This is one of the most frequently test used to detect cognitive dysfunction in mice involved in MS experimental models. The test is based on the rodent’s innate tendency to explore novel environments, which requires intact short term and spatial memory.
- A Y-shaped maze with three arms of equal length, typically 30–50 cm each, oriented 120° from each other. Arms are labelled (e.g., A, B and C). The entry of each arm includes a divider or door that can be removed or placed according to the protocol being performed.
- Optional: Reward or food that will be placed at the end of some arms according to the protocol being performed.
- A camera to record the task (Fig. 12).
This test can be carried out following two different protocols.
- Protocol 1:
Three arms remain open. The mouse is placed at a starting arm and is allowed to explore all three arms freely for 8 minutes without being previously familiarized with the maze. The behaviour of the mouse is recorded. A mouse with no lesions in the hippocampus and prefrontal cortex is expected to alternate between all three arms spontaneously and inspect all of them.
- Protocol 2:
This protocol includes two steps:
First, during training the mouse is placed in a starting arm. One of the entries of the three arms is blocked allowing the mouse to move freely between the other two arms for 5 minutes. One of the arms may include a reward to increase motivation during the exploration time. Afterwards, the mouse is returned to its cage and remains there for e.g., 2 hours (Inter Trial Interval, ITI). The task will be more challenging as the ITI increases since it will require greater memory skills. Meanwhile the maze is cleaned and prepared for the second step.
Second, during testing the mouse is placed again in the starting arm but this time is allowed to move freely between all three arms for 5 minutes and none is blocked. If spatial and short memory have no deficits, the mouse will remember which arm wasn’t entered yet and will be more prone to explore the novel arm (the arm that was blocked on the first step).
- Number of Arm Entries: An arm entry is defined when all four limbs of the mouse are within the arm. Indicates the level of activity and exploration.
- Time Spent in Each Arm: Assesses preference or aversion, which can reflect anxiety or other behavioural changes.
- Spontaneous Alternation: The percentage of consecutive entries into all three arms without repetitions. A lower alternation rate may indicate working memory deficits. Alternation is defined by the following formula: % Alternation = Number of alternations/(Total number of arm entries –2)
- The test is straightforward and easy to perform, requiring minimal training for the rodents.
- Basic material needed.
- The test is non-invasive since it leverages natural exploratory behaviour.
- Data analysis is time consuming.
- Mouse behaviour can be confounding and play a role in the results.
- The test is sensitive to environmental external factors such as lighting and noise, which need to be strictly controlled.
- Primarily assesses working memory and spatial learning, and may not capture other cognitive impairments associated with MS [5].
The Novel Object Recognition (NOR) test evaluates visual non-spatial short-term recognition memory, primarily engaging the hippocampus. It involves a systematic procedure with an open field arena and distinct objects to gauge a rodent’s ability to recognize novel stimuli compared to familiar ones.
- An open field arena, which is typically a square or circular enclosure. Some protocols specify the arena to measure 60
- Objects for the rodents to explore, usually two identical objects for the familiarization phase and a novel object for the test phase. The novel object should be similar in aspect and size but have different texture and shade to allow differentiation by the mice. It is not recommended to use objects of different colours due to mice limited ability to distinguish between colours [52, 53].
- A camera for recording purposes (Fig. 13).
 Fig. 13.
Fig. 13. Novel Object Recognition Test. The figure was created by BioRender (https://www.biorender.com/).
(1) The test is conducted in three steps: habituation, familiarization and testing.
(2) Habituation: The mouse is placed individually in the open field box without any objects and is allowed to explore the arena freely for 10 minutes. The mouse is returned to its cage.
(3) Familiarization: The day after, two identical objects are placed in the open field arena and the mouse is allowed to explore the area for 5–10 minutes. The mouse is returned to its cage. The time spent exploring each object is recorded.
Testing: After 4 hours, one of the objects is replaced by the novel item and the mouse is permitted to explore the arena for 5–10 minutes. Healthy rodents tend to notice new objects and inspect them for a longer period.
- Exploration Time: The total time spent exploring both objects.
- Discrimination Index: (Time exploring the novel object – Time exploring the familiar object)/(Total time exploring both objects).
- Preference or recognition index: Time exploring the novel object/Total time exploring both objects. This provides a normalized measure of memory performance. A preference for the novel object indicates intact recognition memory [54].
Positive exploratory behaviour is defined by the times the nose or the forepaws of the mouse contact the object or by the times the nose of the mouse is up to 0.5 cm far from the object and is directed towards it. Behaviours such as sitting, standing, leaning on the object, or turning around are not considered an exploration.
Scoring can also be used as follows: the nose touch = 1 point; two paws on object = 2 points; four all paws on the top of object = 3 points. Points are tallied and touch to familiar versus unfamiliar objects are compared [55, 56].
- Basic material required.
- Sensitive to cognitive impairments.
- Data analysis is time consuming.
- Primarily assesses short-term recognition memory and may not fully capture other cognitive domains affected by MS.
- Rodents’ exploratory behaviour can be influenced by factors such as anxiety, motivation, and environment, which need to be carefully controlled.
The Open Field Test evaluates locomotor activity, exploratory behavior, and anxiety-like responses in rodents by utilizing their natural aversion to open, brightly lit environments. It involves observing the mouse’s behavior in a large, enclosed arena, providing insights into their motor functions and emotional state.
- An Open Field Area. A large, enclosed space that can be circular or square. It must be sufficiently large for the mouse to feel exposed and with high walls to prevent escape.
-Recording camera [58].
-Software for data analysis (Fig. 14).
Motor activity assessment:
- Horizontal Activity (Ambulation): Measured by the total distance travelled in cm [59].
- Vertical Activity (Rearing): Frequency of the mouse standing on its hind legs. This indicates exploratory behaviour and motor function.
Anxiety and Emotionality assessment:
- Zone Entries and Time Spent in Zones: Time spent in the centre versus outer zones ratio can be calculated. Healthy mice tend to enter the inner zones more frequently than anxious mice [8, 60].
- Thigmotaxis (Wall-Hugging): Tendency to remain close to the walls calculated as: (Total time spent close to the walls)/(Total test time)
- Latency: Time taken to initiate movement. A shorter latency period is linked to higher exploratory behaviour and less anxiety [61].
- Number of defecations. Increased number of defecations indicates higher emotionality [57, 62].
- Easy to set up and carry out.
- The test can analyse several parameters at the same time, which increases its efficiency. These parameters include motor activity, anxiety and emotionality [36].
- Motor assessment can be confounded by anxiety or other psychological factors and vice versa. Psychological parameters registered that are concluded by tasks that rely on the mobility of the mouse can be confounded by locomotor deficits of the mouse [57, 61].
- Sensitive to external environmental factors such as variations on lighting and noise [62].
- High variability due to the availability of multiple protocols and different setup designs make it difficult to compare studies.
- Limited free available softwares for data analysis.
- Data analysis is time consuming.
The Elevated Plus Maze (EPM) Test assesses anxiety-like behaviors in rodents by exploiting their aversion to open spaces and heights alongside their curiosity for novel environments. By comparing their exploration of open versus enclosed arms, researchers can infer levels of anxiety based on the rodent’s preference for safe, enclosed spaces over open, elevated areas.
- An apparatus with four arms (50 cm long
- A recording camera.
- Computer software for data analysis (Fig. 15).
- Total distance travelled.
- Time spent in each type of arm, closed vs open: Reduced time in open arms and increased time in closed arms suggest heightened anxiety.
- Number of Arm Entries: More entries into open arms suggest lower anxiety [65].
- Easy to perform.
- High validity and reliably results.
- The test is sensitive to previous experiences of the mouse. In case mice are tested during the same day with different methods, the EPM should be performed first.
- Time spent on the central platform varies among trials and can significantly alter results. This time is difficult to interpret and reduces the time spent in open and enclosed arms.
- The exploratory behaviour and time spent in open arms decrease significantly after the first trial, known as the one-trial tolerance phenomenon [66].
- Unpredictable mouse behaviour can introduce confounding factors.
- Data analysis is time consuming.
The Elevated Zero Maze (EZM) evaluates anxiety-like behaviors in rodents by using a circular apparatus with both open and enclosed sections. Unlike the Elevated Plus Maze, the EZM’s continuous circular design avoids central platform ambiguities and does not exhibit the one-trial tolerance phenomenon, offering a clearer measure of anxiety through time spent and entries into open versus enclosed areas.
- An annular (circular) apparatus elevated above the floor, divided into four equal quadrants with two open (without walls) and two enclosed (with high walls) sections.
- A recording camera (Fig. 16).
- Time Spent in Open vs. Enclosed Sections: Increased time in open sections suggests lower anxiety, while more time in enclosed sections indicates higher anxiety.
- Number of Entries into Each Section: More entries into open sections suggest reduced anxiety.
- Total Distance Travelled: Can indicate overall activity levels.
- The circular design eliminates the ambiguity of time spent in a central platform, unlike the EPM.
- The one-trial tolerance phenomenon is not described in the EZM [66].
- Similar to the EPM.
The Marble Burying Test leverages the natural digging and burying behaviours of rodents to assess alterations in exploratory and anxiety-related behaviours. It is well-documented that mice with hippocampal lesions, such as those induced by cuprizone demyelination, perform poorly in this test. This poor performance is attributed to cognitive deficits, motivational issues, and increased anxiety, all of which are functions mediated by the hippocampus [68].
- A cage filled with a 5 cm deep layer of wood chip bedding, lightly tamped to create a flat, even surface.
- 9 to 25 marbles, arranged according to the protocol used, placed evenly in the cage following a regular pattern, about 4 cm apart from each other [69] (Fig. 17).
- Number of Marbles Buried: This is a direct measure of digging behavior and is recorded using the following scoring system: Not buried: 0, Partially buried (up to 2/3 buried): 0.5, Buried (2/3 their depth or more): 1 [70].
- Simple and cost-effective setup.
- Non-invasive method to assess anxiety and obsessive-compulsive behaviours.
- Interpretation of results may be complicated by other variables affecting digging behaviour, such as general activity levels or motor function deficits.
A critical challenge in preclinical MS research is the lack of standardized protocols across laboratories. Variability in animal handling, test design, apparatuses, and environmental conditions can lead to inconsistent results, undermining reproducibility, especially when small sample sizes are used. Differences in methodology, even subtle ones, can significantly affect outcomes in behavioral and motor tests.
To improve consistency, it is essential to harmonize testing protocols across research groups [71]. Standardized guidelines for commonly used motor and cognitive tests—such as the Rotarod, Open Field, and Balance Beam—should include detailed instructions for animal handling, apparatus specifications, and data collection methods. Inter-laboratory test validation and external quality control measures could further ensure the reliability of findings across studies.
One model for these efforts is the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a large-scale collaborative project aimed at improving clinical trials in Alzheimer’s disease (AD). Since its inception in 2006, ADNI has focused on standardizing data collection and sharing clinical, neuroimaging, cognitive, and biofluid samples to enhance the understanding of disease progression. The initiative’s rigorous standardization of methods has led to significant advancements in clinical trial efficiency, including improved subject selection, detection of treatment effects, and the development of biomarkers [72].
Learning from ADNI’s successful approach to harmonizing methodologies, MS research could benefit from similar strategies to enhance reproducibility and the translational relevance of its findings. By adopting standardized protocols and collaborative data-sharing practices, MS research could significantly improve the comparability of preclinical studies, ultimately accelerating the development of effective therapeutic interventions.
While traditional motor and cognitive tests remain essential in MS research, the adoption of emerging technologies like artificial intelligence (AI) and automated video-tracking systems can address many of the reproducibility issues. Unlike traditional manual scoring, which can be subjective, automated systems such as EthoVision® and DeepLabCut use advanced algorithms to precisely track rodent movements, identifying subtle motor impairments that may otherwise go undetected [73, 74].
These tools also enhance the objectivity of cognitive assessments by providing standardized data on behaviors like anxiety and spatial memory [75]. Incorporating AI-based platforms not only improves the sensitivity of these tests but also helps minimize inter-laboratory variability, making results more reproducible [76]. As these technologies become more widely adopted, they have the potential to significantly enhance the translational relevance of preclinical MS research, complementing traditional methods and overcoming challenges related to reproducibility.
Selecting appropriate models and behavioral/motor tests is crucial in neurological research, particularly when studying complex diseases like MS. The choice of test can significantly influence the sensitivity, reliability, and overall validity of the findings.
In the cuprizone model, which induces demyelination similar to that seen in MS, the selection of motor function tests is particularly critical due to varying sensitivity and reliability across different tests. For instance, the rotarod test, though widely used, has been shown to be less effective at detecting subtle motor deficits resulting from cuprizone exposure. This test typically measures motor coordination and balance by recording the time an animal can stay on a rotating rod. While it is reliable for assessing more pronounced motor impairments, it may not be sensitive enough to detect the early or subtle deficits that occur in the cuprizone model [8].
In contrast, more complex tests such as the walking beam and complex wheel are better suited for identifying these subtle deficits [8]. The walking beam test, which requires animals to walk along a narrow beam, challenges their balance and coordination, making it more sensitive to minor impairments. Similarly, the complex wheel test, which involves navigating a wheel with irregular rungs, is particularly effective at identifying early motor deficits in the cuprizone model. This test not only provides reproducible results but also offers a more nuanced view of motor function. However, it is important to note that the complex wheel test may also reflect cognitive impairments, as cuprizone-induced demyelination and gliosis can affect both motor and cognitive functions. This overlap complicates the interpretation of results, as it becomes challenging to distinguish whether observed deficits are primarily motor-related or cognitive in nature.
On the other hand, in the EAE model, which causes lesions mainly in the white matter of the spinal cord, the rotarod has been recognized as a reliable and reproducible tool for measuring motor function. In this context, the rotarod’s ability to correlate with the clinical score and the extent of inflammatory lesions makes it a valuable test for assessing disease progression [77, 78]. The rotarod’s effectiveness in the EAE model highlights the importance of context when selecting behavioral tests. What may be less effective in one model (e.g., cuprizone) could be highly valuable in another (e.g., EAE), underscoring the need for model-specific considerations.
The selection of appropriate models and tests is not merely a procedural decision but a critical aspect of experimental design that can influence the outcomes and interpretations of neurological research. Researchers must carefully consider the strengths and limitations of each test within the context of their specific model to ensure that they accurately capture the desired aspects of disease pathology and progression. This careful selection process is essential for generating meaningful and reliable data, ultimately advancing our understanding of complex neurological conditions like MS.
Rodent models offer valuable insights into the pathophysiology of MS and are widely used for evaluating potential therapies. However, translating preclinical findings from rodent models to clinical outcomes in humans remains a significant challenge due to species-specific differences and the complexity of MS. MS in humans is highly variable, with multiple forms including relapsing-remitting, secondary progressive, and primary progressive MS, each presenting distinct motor, cognitive, and sensory deficits. While rodent models, such as the cuprizone and EAE models, replicate certain aspects of MS (e.g., demyelination or inflammation), they typically do not encompass the full clinical spectrum observed in human patients [79].
For example, motor tests like the Rotarod or the Walking Beam are effective at detecting motor coordination and balance deficits in rodents, which are relevant to MS symptoms such as ataxia and balance problems in humans. However, these tests may not fully capture the more complex motor dysfunctions seen in different MS types, such as spasticity, tremors, and muscle weakness, which are harder to mimic in rodent models. Additionally, rodent models are limited in their ability to reproduce the variability of MS subtypes, as many models primarily focus on acute or relapsing forms of the disease, leaving progressive forms less understood.
Despite these limitations, preclinical research using rodent models has significantly advanced our understanding of MS pathology. Study has shown that demyelination in the cerebellum and corticospinal tracts correlates with motor deficits—findings that align with clinical observations in MS patients [80]. Although the variability of symptoms in MS and its different forms complicate the direct translation of preclinical findings to clinical stages, there have been some notable success cases. The development of fingolimod, the first oral disease-modifying treatment for MS, can be seen as a key milestone. Various rat and mouse models of experimental EAE were instrumental in determining fingolimod’s therapeutic efficacy at different stages of the disease. Moreover, the creation of Sphingosine-1-phosphate receptor 1 (S1P1R) knock-out mice enabled a deeper exploration of the mechanisms through which fingolimod promotes remyelination and activates repair processes, findings that have been observed in both animal models and MS patients [81].
Other instance, several therapies, such as glatiramer acetate (GA: Copaxone) and natalizumab (Tysabri), were tested first in the mouse model of EAE and then went on to clinical trials. The EAE model has provided significant insights into immune-mediated processes in MS, leading to the development of these therapies that target key mechanisms involved in the disease. However, not all findings in rodent models directly translate to clinical success, underscoring the need for careful interpretation of preclinical data and its applicability to human disease [82].
Moving forward, the development of more refined models that replicate progressive MS or other specific subtypes could improve the relevance of preclinical results to clinical outcomes. These models would help bridge the gap between rodent research and clinical applications, ultimately enhancing the translational value of findings in MS research.
Although behavioral and motor tests are indispensable tools in MS research, they have several limitations. A key issue is the lack of standardization across laboratories, which can lead to variability in results depending on the specific protocols, handling, and test apparatus used [83]. This lack of consistency poses challenges for comparing studies and achieving reproducibility across different research groups.
Furthermore, many of the tests currently employed in rodent models primarily assess gross motor impairments and cognitive functions, potentially overlooking subtle MS symptoms such as fatigue, sensory disturbances, or fine motor deficits, which are prevalent in human patients but difficult to model in rodents. This can limit the translational value of preclinical testing when applied to clinical MS. Moreover, rodent models often reproduce only certain aspects or stages of MS, such as inflammation or demyelination, and may not accurately reflect the complex, multi-faceted nature of the disease in humans, particularly in terms of progressive MS forms [84].
Addressing these limitations will require the development of more sophisticated testing methods that better align with human MS symptoms and a deeper exploration of how different rodent models can be used to study various MS subtypes. New technologies, such as automated tracking systems and AI-based behavioral analysis, could offer more detailed and objective assessments, enhancing the reliability and translational potential of these tests in MS research.
Behavioral and motor testing in experimental models of MS plays a crucial role in preclinical research for several reasons:
While these tests are indispensable in advancing MS research, addressing gaps in standardization and reproducibility remains crucial. Future efforts should focus on developing standardized testing protocols and incorporating advanced technologies like artificial intelligence (AI) to enhance data consistency and sensitivity. Learning from successful standardization initiatives in other fields could greatly benefit MS research and improve the translation of preclinical findings into clinical applications.
In summary, behavioral and motor tests are essential tools that support the evaluation of new treatments, understanding of disease mechanisms and development of biomarkers in MS research. Streamlining these methods and focusing on clinical relevance will be key to advancing more effective and personalized therapies for MS patients.
Lead researchers, UGP and JMG; study design, UGP, JAMG and JMG; manuscript drafting, all authors; critical review of the manuscript, all authors; collect and sort references and design and draw the figures: OMFK, DDOH, BSC, MSBM, SFM, MGM, TLG, FSB. All authors have read and agreed to the published version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We also thank BioRender (https://www.biorender.com/) for the use of their materials in the preparation of this paper. https://BioRender.com/l98d262 and https://BioRender.com/l68j869.
This research was funded by the Spanish Ministry of Science and Innovation, grant number AES, ISCIII PI21/00242.
The authors declare no conflict of interest. Ulises Gomez-Pinedo is serving as one of the Editorial Board members of this journal. We declare that Ulises Gomez-Pinedo had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Bettina Platt.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.































