1 Department of Anesthesiology, The Second Affiliated Hospital, Zhejiang University, School of Medicine, 310009 Hangzhou, Zhejiang, China
2 Department of Anesthesia, Lishui Maternal and Child Health Center, 323000 Lishui, Zhejiang, China
3 Department of Anesthesia, Hangzhou Plastic Surgery Hospital, 310000 Hangzhou, Zhejiang, China
Abstract
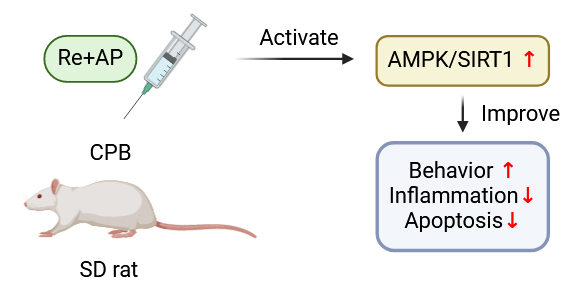
The effects of remimazolam (Re) in combination with andrographolide (AP) on learning, memory, and motor abilities in rats following cardiopulmonary bypass (CPB) surgery were studied.
We hypothesized that the combination of Re and AP could improve postoperative cognitive dysfunction (POCD) in rats after CPB by modulating nervous system inflammation. Cognitive function was assessed using the Morris Water Maze test, and the concentrations of tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) in serum were measured by enzyme-linked immunosorbent assay (ELISA). Apoptosis was evaluated using western blotting and the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining assay.
The results indicated that both Re and AP independently improved cognitive function in rats after CPB and inhibited the secretion of inflammatory factors and apoptosis in hippocampal tissues. Combined administration of Re and AP enhanced the alleviation of POCD compared with monotherapy. The adenosine monophosphate-activated protein kinase/silent information regulator of transcription 1 (AMPK/SIRT1) signaling pathway was activated by the combination of Re and AP.
Collectively, the combination of Re and AP treatment significantly improves POCD in rats after CPB through activation of the AMPK/SIRT1 signaling pathway.
Graphical Abstract
Keywords
- remimazolam
- andrographolide
- postoperative cognitive dys-function
- cardiopulmonary bypass
- AMPK
- SIRT1
Postoperative cognitive dysfunction (POCD) refers to mild cognitive impairments that occur following surgery, primarily manifesting as deficits in memory, attention, and information processing capacity [1]. Reports indicate that the incidence of POCD is positively associated with advanced age [2, 3] and is linked to neuropsychiatric conditions such as Alzheimer’s disease and dementia [4]. Understanding the pathogenesis of POCD is of paramount importance for developing effective interventions. Remimazolam (Re) is an ultrashort-acting benzodiazepine sedative known for its high clearance rate, short duration of action, rapid hemodynamic recovery, and minimal respiratory depression [5]. Its mechanism of action involves inducing hyperpolarization of the neuronal membrane and inhibiting neuronal activity, leading to sedation [6]. Compared to propofol, Re exhibits lesser cardiovascular effects, greater hemodynamic stability, and faster postoperative recovery [7]. These properties suggest that Re may have a neuroprotective effect, potentially mitigating the cognitive impairments associated with POCD.
Andrographolide (AP), the principal active constituent isolated from the Andrographis paniculata, possesses diverse pharmacological properties, including antibacterial, anti-inflammatory, antiviral, hypoglycemic, and immunomodulatory activities [8, 9]. In recent years, the protective effects of AP have been reported in various neurological disorders [10, 11]. Specifically, AP can effectively suppress the expression of pro-inflammatory cytokines, thereby attenuating inflammatory responses [12, 13]. However, the application of AP in the prevention and treatment of POCD has not yet been explored.
Adenosine monophosphate-activated protein kinase (AMPK) is a ubiquitous serine/threonine protein kinase that plays a critical role in regulating cellular energy metabolism [14]. It has been demonstrated that decreased AMPK levels in the brain tissue of rats with cognitive decline [15, 16]. Activation of the AMPK signaling pathway was found to improve cognitive function in Alzheimer’s disease [17]. Silent information regulator of transcription 1 (SIRT1) is an important downstream target of AMPK and plays a crucial role in age-related neurodegenerative diseases [18]. Activation of the AMPK/SIRT1 pathway in Alzheimer’s disease improves behavioral outcomes, reduces oxidative stress, and diminishes neuroinflammation [19]. Therefore, we hypothesized that the combination of Re and AP may improve POCD in rats after cardiopulmonary bypass (CPB) via modulation of the AMPK/SIRT1 pathway. Behavioral changes and inflammatory factor level were used to evaluate the therapeutic effects of Re and AP. Our experimental results aim to provide a theoretical basis for the clinical treatment of POCD.
Healthy male Sprague-Dawley (SD) rats (weight: 400–450 g) were used in this study. The study was apporved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University (No. 2024399). Rats with pre-existing cognitive dysfunction or those unable to perform swimming tasks were excluded from the Morris Water Maze (MWM) navigation test. A total of 60 rats were randomly divided into six groups (Fig. 1, created using BioRender):
Sham operation group (Sham),
POCD rat model established using CPB,
CPB rats treated with Re (Re group), Re tosilate was purchased from HengRui Medicine Co., Ltd. (#H20190034; Lianyungang, Jiangsu, China).
CPB rats treated with AP (AP group), AP was purchased from Sigma-Aldrich (#5508-58-7; St. Louis, MO, USA).
CPB rats treated with Re and AP (Re+AP group),
CPB rats treated with Re, AP, and the AMPK inhibitor BML-275 (Re+AP+BML). BML-275 was purchased from Selleck (#S7840; Houston, TX, USA).
For drug administration, a single intraperitoneal injection was given 30 minutes prior to surgery. The dose of Re was 2.5 mg/kg for the Re group [20], and the dose of AP was 1.0 mg/kg for the AP group [21, 22].
 Fig. 1.
Fig. 1. The experimental design flow chart (created using BioRender). CPB, cardiopulmonary bypass; AP, andrographolide; Re, remimazolam; SD, Sprague-Dawley. The figure was created by BioRender (https://www.biorender.com/).
Rats were initially sedated within a transparent container using 2.5% sevoflurane (national medical products administration approval number: H20173007, Hengrui, Shanghai, China) inhalation until loss of consciousness. Subsequently, anesthesia was induced via intraperitoneal injection of 2% pentobarbital sodium (30 mg/kg; Sigma-Aldrich, St. Louis, MO, USA). Following tracheal intubation, the rats were connected to a ventilator (Ugo Basile 7025 Rodent Ventilator, Comerio, Italy) for mechanical ventilation at a respiratory rate of 70 breaths/min, tidal volume of 2 mL/100 g body weight, and an inspiratory-to-expiratory (I:E) ratio of 1.00:1.25. The airway peak pressure was maintained between 0.5 and 1.5 kPa, and end-tidal carbon dioxide (PETCO2) levels were kept within the range of 35–40 mmHg (1 mmHg = 0.133 kPa). A 24-gauge (G) needle was inserted into the right femoral vein to establish venous access, through which 6% hydroxyethyl starch was administered. A 22-G needle was inserted into the tail artery to monitor arterial pressure and facilitate blood gas analysis. Additional vascular access was obtained by inserting a 22-G needle into the left femoral artery and an 18-G needle into the right jugular vein. The CPB circuit was assembled using a silicone pump tube, a constant flow peristaltic pump (ST600-2J; Baoding Lange Constant Flow Pump Co., Ltd., Shanghai, China), a membrane oxygenator for animal experiments (national instrument registration 20143451839; Kewei Medical Equipment Co., Ltd., Guangdong, China), and an automated blood warmer (AM-301-4B0; Belmont BUDDY PLUS, Billerica, MA, USA). After 60 minutes of CPB, the flow was discontinued, and mechanical ventilation was reinitiated. Heparin was neutralized with protamine, and resuscitative measures including fluid replacement and rewarming were undertaken to maintain hemodynamic stability. The arteriovenous cannulae were removed, and the vessels were ligated. Incisions were then meticulously sutured. Upon recovery, spontaneous breathing was re-established, and the tracheal cannula was removed following suctioning of secretions.
The MWM test was performed three days after CPB surgery to evaluate cognitive abnormalities and memory and learning capabilities in rats. The MWM is a black circular pool divided into four virtual quadrants, with four equidistant entry points. The quadrant containing the submerged platform is designated as the target quadrant.
In the positioning navigation experiment, rats were released into the water from each of the four quadrants over five consecutive days. The time spent searching for the hidden platform within one minute (the escape latency) was written. If the platform was not found within 60 seconds, the trial was terminated, and the escape latency was recorded as one minute. This procedure was repeated four times daily.
On day 6, rats were placed into the water facing the pool wall from each of the four quadrants. The swimming distance, the time spent in the target quadrant, and the number of times the rats crossed the former location of the platform within 60 seconds, were written. The training trajectories and movement data were captured and analyzed using the SUPER MAZE Small Animal Behavior Recording and Analysis System (V2.1, Shanghai Xinsoft Information Technology Co., Ltd., Shanghai, China).
The Grip Test was performed to evaluate motor deficits following the MWM test [23]. Rats were held by the tail so that their forelimbs could touch a wooden pole attached to two vertical supports approximately 40 cm in height. Performance was scored as follows:
Score 0: Unable to grip and falls down.
Score 1: Grips with one or two front limbs or hind limbs.
Score 2: Hangs with one or two front limbs and attempts to climb up.
Score 3: Hangs with one or two front limbs and with one or two hind limbs.
Score 4: Hangs with both front limbs and hind limbs and uses the tail for holding.
Score 5: Hangs with both front limbs and hind limbs, uses the tail for holding, and reaches the vertical supports.
Neurological deficits were assessed according to previously established criteria [24]. The scoring criteria were as follows:
0 points: No signs of neurological deficits.
1 point: Left upper limb flexion after lifting tail, not fully straightened.
2 points: Rotate to the left and move in circles.
3 points: Climb to the left.
4 points: Unconscious movements and signs of disturbance of consciousness.
Additionally, the Bederson score (0–5) was used to evaluate neurological deficits [25]:
1 point: Forelimb curvature.
2 points: Forelimb flexion, reduced resistance to lateral thrusts.
3 points: Circling.
4 points: Rotate around the caudal axis of the skull.
5 points: No spontaneous movement.
Following the behavioral experiments, rats were euthanized with an overdose of pentobarbital sodium (160 mg/kg). Frozen sections of hippocampal tissue were prepared, fixed in 4% paraformaldehyde, and subsequently immersed in an ice bath containing PBS for 2 minutes. After rinsing, 50 µL of terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL, C1086, Beyotime, Shanghai, China) detection solution was added, and the tissues were incubated in the dark at 37 °C for 60 minutes. Following 4′,6-diamidino-2-phenylindole (DAPI, C1002, Beyotime, Shanghai, China) staining and mounting with 50% glycerol, apoptotic cells were visualized under a fluorescence microscope (Nikon Ti2-U, Tokyo, Japan) by observing green fluorescence.
Venous blood samples were collected and centrifuged. The supernatant was isolated and stored at –80 °C. The concentrations of serum tumor necrosis factor alpha (TNF-
Hippocampal tissues were homogenized, and centrifuged at 10,000
All data were compared using Student’s t-test or one-way ANOVA, and shown as the mean
Firstly, the rat POCD model was established. The cognitive abilities, memory, and learning capabilities of all rats were evaluated. The escape latency time of rats in the CPB group was approximately twice as long as that in the sham group (Fig. 2a); the time spent in the target quadrant (Fig. 2b) and the number of platform crossings (Fig. 2c) were also approximately half of those in the sham group. Additionally, the grip test score was significantly reduced in the CPB group compared to the sham group (Fig. 2d). These findings indicate that POCD was successfully induced in rats following CPB. The classical neurological deficit scores of rats in the sham operation group were 0, whereas those in the CPB group were greater than 3 (Fig. 2e). Similarly, the Bederson score, another indicator of neurological deficit, averaged 4 in the CPB group, while the sham group had a score of 0 (Fig. 2f). Both Re and AP significantly decreased the escape latency, neurological deficit score, and Bederson score in rats after CPB, and significantly improved cognitive function indicators (Fig. 2a–f). Furthermore, the combination of Re and AP enhanced the effects of either Re or AP alone in improving cognitive abnormalities and memory and learning capabilities in rats in the CPB group (Fig. 2a–f). Moreover, compared with monotherapy, the combination of Re and AP significantly enhanced the recovery ability of various indicators including blood pressure, blood glucose, heart rate and respiratory rate in rats in CPB group (Table 1).
 Fig. 2.
Fig. 2. The cognitive abnormality and memory and learning capability of rats were evaluated. The (a) trajectory maps, escape latency time, (b) target quadrant time, and (c) number of platform crossings were obtained by the MWM Test. (d) The grip test score, (e) neurological deficit scores, and (f) Bederson score were recorded as well. Rats were divided into five groups: rats after sham surgery of CPB, rats after CPB surgery, rats after CPB surgery and were treated with 2.5 mg/kg Re, rats after CPB surgery and were treated with 1.0 mg/kg AP, and rats after CPB surgery and were treated with 2.5 mg/kg Re along with 1.0 mg/kg AP. **p
| sham | CPB | Re | AP | Re+AP | |
| Blood pressure (mmHg) | 102 | 78.4 | 90.8 | 88.5 | 101.2 |
| Blood glucose (mg/dL) | 101.9 | 78.7 | 91.7 | 89.2 | 97.2 |
| Heart rate (bpm) | 408.9 | 301.6 | 334.9 | 332.6 | 393.5 |
| Respiratory rate (bpm) | 84.4 | 69.4 | 74.5 | 73.8 | 83.7 |
***p
#p
&&&p
Next, pro-inflammatory factors in the serum of each group were measured. As hypothesized, the levels of TNF-
 Fig. 3.
Fig. 3. Combination of Re and AP inhibits inflammatory factor secretion and apoptosis of hippocampal tissue of rats after CPB. The concentration of (a) TNF-
Additionally, the protein expression of SIRT1 and p-AMPK in the hippocampal tissue of CPB rats were significantly reduced. Pretreatment with Re and AP alone prominently increased the protein levels of SIRT1 and p-AMPK. The combination of Re and AP pretreatment further enhanced the protein expression of SIRT1 and p-AMPK compared to individual treatments (Fig. 4a). Subsequently, the AMPK inhibitor BML-275 significantly reversed the effects of Re and AP on regulating the proteins in the AMPK/SIRT1 signaling pathway (Fig. 4b). Moreover, BML-275 significantly attenuated the beneficial effects of the combination treatment of Re and AP. Specifically, BML-275 increased the escape latency of rats treated with Re and AP after CPB (Fig. 5a), reduced the time spent in the target quadrant (Fig. 5b), decreased the number of platform crossings (Fig. 5c), and lowered the grip test score (Fig. 5d). Additionally, the neurological deficit score (Fig. 5e) and the Bederson score (Fig. 5f) were also increased. Furthermore, the levels of TNF-
 Fig. 4.
Fig. 4. The western blot images along with analysis of proteins of AMPK/SIRT1 pathway in hippocampal tissues (a) before and (b) after BML interference. ***p
 Fig. 5.
Fig. 5. The cognitive abnormality and memory and learning capability of rats were evaluated. The (a) trajectory maps, escape latency time, (b) target quadrant time, and (c) number of platform crossings were obtained by the MWM Test. (d) The grip test score, (e) neurological deficit scores, and (f) Bederson score were recorded as well. Rats were divided into four groups: rats after sham surgery of CPB, rats after CPB surgery, rats after CPB surgery and were treated with 2.5 mg/kg Re along with 1.0 mg/kg AP, and rats after CPB surgery and were treated with 2.5 mg/kg Re, 1.0 mg/kg AP, and 0.2 mg/kg BML-275. **p
 Fig. 6.
Fig. 6. Activation of AMPK/SIRT1 signaling pathway induced by Re and AP improves the cognitive function following CPB in rats. The concentration of (a) TNF-
In this study, we explored the regulatory effects and mechanisms of the combination of Re and AP on cognitive abnormalities and memory and learning capabilities in rats subjected to CPB. Our data suggested that the combination of Re and AP improved cognitive function following CPB in rats, inhibited the secretion of inflammatory factors, and reduced apoptosis in hippocampal tissues. The combination therapy enhanced the effects of monotherapy by significantly ameliorating POCD in rats after CPB via the activation of the AMPK/SIRT1 signaling pathway. Cognitive function decline is a significant and long-lasting issue in patients undergoing CPB heart surgery. Newman et al. [26] reported that the incidence of POCD in elderly patients was 50–70% at 1 week after CPB surgery, 30–50% after 6 weeks, and the incidence remained high at 20–40% even 6 months post-surgery. In the present study, our data indicated that cognitive abnormalities and memory and learning capabilities were significantly reduced in rats after CPB surgery.
Re possesses a short half-life, leading to rapid onset and recovery. Importantly, its minimal impact on the cardiovascular system and reduced injection pain make it a favorable choice [27]. A recent study has shown that patients undergoing bronchoscopy under intravenous Re anesthesia did not exhibit POCD [28]. Additionally, research by the Liao team has reported that Re may help decrease the incidence of early POCD in old patients following radical gastrectomy [29]. Our data support these findings, indicating that Re can improve POCD induced by CPB surgery in animal models. AP was found to exhibit antibacterial, anti-inflammatory, and antiviral properties [30]. A recent study has also demonstrated that AP reduces the levels of proteins related to the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-
To investigate the underlying mechanism, we focused on the role of the AMPK/SIRT1 signaling. POCD shares molecular pathways with neuropsychiatric diseases such as dementia, depression, and Alzheimer’s disease [2]. Understanding the neuronal protective mechanisms is therefore crucial. The SIRT1 gene locates on chromosome 10 and exhibits diverse subcellular localization, sensing changes in energy levels within cells [32]. SIRT1 is widely distributed throughout the human body and plays a key role in metabolic health by deacetylating target proteins in various tissues [33, 34, 35]. In the central nervous system, SIRT1 is primarily expressed in neurons, particularly in the hippocampus, cortex, cerebellum, and hypothalamus, where it exerts critical functions [36]. Enhanced SIRT1 activity in mammals has been shown to confer protection against metabolic disorders, neurodegeneration, and inflammation, making it an attractive therapeutic target [32, 37, 38]. AMPK acts as a metabolic sensor of cellular ATP levels and a key regulator of energy homeostasis [14]. SIRT1 plays a multifaceted role in inflammatory responses and immunity. Research has shown that AMPK activation can increase intracellular NAD+ concentrations, thereby activating SIRT1 expression [39, 40]. In recent years, the AMPK/SIRT1 signaling pathway has been implicated in neurodegeneration. For example, resveratrol protects against alcohol-induced neurodegeneration via the AMPK/SIRT1 pathway in rats and humans [41], and tryptophan metabolites have been found to improve neurodegeneration through the AMPK/SIRT1 pathway during aging [42]. Our study suggested that the AMPK/SIRT1 signaling pathway can be activated in POCD and that this pathway can be modulated by the combination of Re and AP.
However, there are limitations to the current study. The sample size of animals should be expanded. Other potential targets and signaling pathways related to neuroinflammation and neuroprotection, such as nucleotide-binding oligomerization domainlike-receptor protein 3 (NLRP3) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), which have been reported in POCD [3, 43], should be further investigated. Whether the combination treatment can regulate these signals to alleviate POCD warrants further exploration. Re works by acting on gamma-aminobutyric acid (GABA) receptors and is rapidly converted to an inactive metabolite by tissue esterase [44], whether the enhancement of GABA is related to the inhibition of AMPK/SIRT1 signaling pathway should also be further stuided.
In summary, the combination treatment of Re and AP significantly improves POCD in rats after CPB via the activation of the AMPK/SIRT1 signaling pathway. These findings suggest a potential novel therapeutic approach for preventing POCD in clinical settings.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
KX conceived the study; CC conducted the experiments; LL analyzed the data; CC was a major contributor in writing the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was approved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University (No. 2024399). All experiments were performed in accordance with relevant guidelines and regulations.
Not applicable.
This syudy was supported by Zhejiang Province Traditional Chinese Medicine Science and Technology Program Project (Application Study of Remimazolam Combined with AP on Cognitive Function after Extracorporeal Circulation in Rats via AMPK/SIRT1 Signaling Pathway, 2024ZL096).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/JIN25665.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






