1. Introduction
Among age-related neurodegenerative disorders, Parkinson’s disease (PD) stands out as the second-highest occurring disorder. The distinctive clinicopathological features of PD involve the gradual and specific loss of dopaminergic (DA-ergic) neurons in the midbrain and the substantia nigra pars compacta (SNpc) regions of the brain. Additionally, a notable characteristic is the pathological buildup of -synuclein (-syn) aggregates [1]. Consequently, the dorsal striatum and other target areas have a dopamine deficiency, which causes chief motor dysregulations such as bradykinesia, stiffness, and tremors. Furthermore, non-motor manifestations, like depression and sleep disturbances in PD patients, are also evident [1].
Numerous studies have revealed that the brains of PD patients and animal models with synucleinopathies contain an abnormal accumulation of P-Ser129--syn, a pathologically active phosphorylated form of -syn [2, 3, 4, 5]. In healthy brains, -syn undergoes minimal phosphorylation, while there are dramatically elevated P-Ser129--syn levels during PD pathology in brains afflicted with Lewy pathology [2, 6]. This suggests a potential correlation between this post-translational modification and heightened deposition of -syn, coinciding with Lewy body formation and the beginning of the neurodegenerative process. Current strategies, including treatments inhibiting -syn’s aberrant aggregation, have generated significant interest as potential interventions that could potentially slow or arrest the advancement of the pathological condition.
The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model is widely used due to its ability to replicate several key features of PD, including DA-ergic neuron degeneration and motor deficits. This model is well-characterized and allows for the study of neurodegenerative processes and potential therapeutic interventions. While specific -synucleinopathy models, such as transgenic mice expressing human -syn, provide insights into the role of -syn aggregation in PD, the MPTP model remains valuable for its simplicity, reproducibility, and relevance to DA-ergic system degeneration [7, 8]. We selected the MPTP model for its established utility in investigating neurodegeneration and evaluating neuroprotective strategies, which are critical aspects of PD research.
Oxidative stress mediates vital contributions in PD pathogenesis while activating glycogen synthase kinase-3 beta (GSK-3). Increased GSK-3 leads to -syn toxicity and the dysregulation of the Wnt/-catenin signaling mechanism. The canonical Wnt/-catenin signaling process, essential for embryonic developmental and tissue homeostasis processes, also controls neuronal function in the central nervous system (CNS) [9]. This pathway is an intriguing candidate that appears to be dysregulated in PD and has been linked to several neuropathologies [10]. Research suggests activating this pathway could restore neurogenesis and enhance brain self-repair capacity [11], making it a promising therapeutic candidate for countering neurodegenerative conditions. Furthermore, the pathogenesis of PD is associated with the activity of GSK-3, a key regulator of the Wnt/-catenin signaling pathway. GSK-3 restricts -catenin’s cytoplasmic stabilization and, hence, nuclear migration, effectively preventing the conventional Wnt pathway activation [12]. GSK-3 inhibition has been shown to promote -catenin’s stabilization, thus promoting Wnt signaling-mediated neuroprotective benefits in PD models [13]. Elevated expression of GSK-3 in the nigral neurons of post-mortem PD brains and its aberrant phosphorylation at the Tyrosine 216 (Tyr216) is also evident in the striatum of individuals with PD [14, 15]. GSK-3 activity is strictly controlled through the negative regulation occurring via GSK-3 Serine-9 phosphorylation at the N-terminal region [16]. Pharmacological agents inhibiting GSK-3activation may mitigate neurodegeneration in PD [17].
While neuronal autophagy is widely recognized as a defensive and beneficial mechanism for the physiological functioning of the nervous system, its paradoxical involvement in neuronal cell death has become increasingly evident. Numerous studies employing environmental toxins, hereditary PD-risk genes, and postmortem PD brain samples have illuminated the contributions of autophagy in instigating PD-associated DA-ergic neuronal loss [18, 19]. Particularly noteworthy is the identification of autophagic-regulated loss of nigral neurons in PD patients [20]. Oxidative stress induced by MPTP or dopamine toxicity has been demonstrated to amplify the formation of autophagic vacuoles, intensifying autophagic activity and subsequently leading to neuronal cell death [21, 22]. This dual role of autophagy, serving both neuroprotection and contributing to neurodegeneration, underscores its multifaceted involvement within the intricate framework of neuronal processes.
The drug targeting strategies in PD treatment have limited efficacies and are often associated with side effects. Phytochemicals present alternative, more efficacious, and safer treatment strategies against PD pathology. Owing to its diverse neuropharmacological characteristics, ranging from anti-oxidative to anti-inflammatory, the phenolic aldehyde vanillin exhibits promise against the pathophysiology of PD. The aim of this study was to determine if vanillin inactivates GSK-3, thus potentiating the Wnt/-catenin signaling in MPTP-induced nigrostriatal degeneration while protecting the DA-ergic system. By examining critical pathways implicated in PD pathogenesis, such as increased -syn expression, autophagic-neuronal loss, and Wnt/-catenin/GSK-3 activity, we seek to understand the underlying mechanism of therapeutic potential of Vanillin in PD.
2. Materials and Methods
2.1 Ethical Statement Regarding Animal Usage
All animal handling and experimental procedures in this study followed standard techniques and protocols. The experiments involving C57BL/6 mice were conducted in accordance with the protocols approved by the Institutional Animal Ethics Committee (IAEC) of Jawaharlal Nehru University (JNU), New Delhi (approval code: 10/GO/ReBi/99/CPCSEA/March 10, 1999). Every effort was made to minimize animal suffering and ensure their well-being throughout the study. We have followed ARRIVE guidelines 2.0.
2.2 Experimental Animals and Dietary Regimen
65 animals Male Mice (C57BL/6) were used in the present study. Male C57BL/6 mice, weighing 25–30 grams and aged 2.5–3 months were raised and housed under controlled laboratory conditions. The mice were provided free access to a standard mouse pellet diet and filtered water ad libitum throughout the study. The housing environment maintained a temperature of 21 2 °C, a humidity level of 55%, and followed a continuous 12-hour light/dark cycle. All animal-related procedures were conducted in compliance with the regulations of Central Laboratory Animal Resource (CLAR) at Jawaharlal Nehru University (JNU), New Delhi, India. To ensure proper adaptation, the mice underwent a one-week acclimatization period prior to the commencement of the experimental phase. Furthermore, before the start of the experiment, the mice were appropriately trained on all behavioural equipment to ensure optimal performance and accurate results.
2.3 Experimental Design and Treatment Groups
Adult male C57BL/6 mice were randomly assigned to five groups:
Control Group: Received normal saline (i.p.) as a vehicle.
MPTP Group: Received intraperitoneal (i.p.) injections of MPTP (79F3538, Sigma-Aldrich, St. Louis, MO, USA) (20 mg/kg) once a day for 5 consecutive days [23].
Vanillin Group: Vanillin (BCBV5242, Sigma-Aldrich, St. Louis, MO, USA) (60 mg/kg body weight, p.o.) was administered daily for 21 days, dissolved in normal saline.
MPTP+Vanillin Group: Mice were subjected to MPTP once every 24 hours for five consecutive days via intraperitoneal administration, followed by daily oral administration of Vanillin for 21 days at a dose of 60 mg/kg body weight.
MPTP+L-DOPA (Levodopa/3,4-Dihydroxy-L-phenylalanine) Group: Mice were subjected to MPTP once every 24 hours for five consecutive days via intraperitoneal administration, followed by daily oral administration of L-DOPA (PHR1217, Sigma-Aldrich, St. Louis, MO, USA) for 21 days at a dose of 5 mg/kg body weight.
The assignment of mice to the respective groups was randomized to minimize bias and ensure a balanced distribution of characteristics among the experimental cohorts.
2.4 Evaluation of Motor Behaviour
2.4.1 Forced Swim Test (FST)
The Forced Swim Test (FST) was conducted using a transparent glass cylinder with dimensions of 25 cm in height and 13 cm in diameter [24]. The cylinder was filled with water to a depth of approximately 18 cm, maintained at a temperature of 22 °C. Each mouse was individually placed in the cylinder, and the swimming behaviour was systematically video recorded and analyzed by an unbiased observer unaware of the experimental groups. The behaviour was recorded for a duration of 6 minutes. The immobility time (defined as the duration during which an animal spends floating with minimal movements to keep its head above the surface of the water) of the mouse during the final 4 minutes of the test was manually recorded.
2.4.2 Cylindrical Test
Animals were placed in a glass cylinder that was 30 cm high and 20 cm inside [25]. The mice were left undisturbed in the cylinder for a period of 5 minutes, during which their behaviour was recorded. An observer who was unaware of the experimental group counted the instances in which each forepaw was utilized to start weight-shifting motions, such as landing and wall contact.
2.4.3 Wire Hanging Test
The test is based on the concept that neuronal degeneration in the basal ganglia circuits leads to motor impairments [26]. The hanging wire test is used to assess muscle strength and prehensile reflex, evaluating the animal’s ability to grasp a stretched horizontal wire with its forepaws and sustain suspension. Mice were put on a horizontal rod measuring 5 5 mm area, 35 cm long, and positioned between two poles of 50 cm height. The suspension time, i.e., duration until mouse drops, was recorded during the test. This parameter is a reliable indicator for detecting neuromuscular abnormalities associated with motor strength.
2.5 Western Blot
Protein expression was determined as per our previous procedure [27]. 50 µg of proteins were fractionated by Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred onto a Polyvinylidene fluoride (PVDF) membrane (18289A10, Bio-Rad, Boston, MA, USA). The blots were blocked and incubated with the primary antibodies at 4 °C overnight. The next day, the blots were incubated with Horseradish peroxidase (HRP)-tagged anti-rabbit secondary antibodies (1:2000; catalogue number 7074S, CST, Danvers, MA, USA) for 2 hours at room temperature. The blots were then developed on an X-ray film in the darkroom using a luminol-based enhanced chemiluminescence (ECL) substrate. The blots were then scanned, and density was measured using ImageJ 1.8.0 software (LOCI, University of Wisconsin, Madison, WI, USA). The expression of the proteins was normalized against the expression of housekeeping protein -actin. The list of primary antibodies used for immunoblotting is given in Table 1. The original figures of Western blot can be found in the Supplementary Materials.
Table 1. List of antibodies used in Western blotting. | Antibody | Host | Product details | Dilution |
| -catenin | Rabbit | CST, Danvers, MA, USA, Cat # 6B3 | 1:1000 |
| Glycogen synthase kinase-3 beta (GSK-3) | Rabbit | CST, Danvers, MA, USA, Cat # 27C10 | 1:1000 |
| P-Ser9-GSK-3 | Rabbit | Affinity Biosciences, Cincinnati, OH, USA, Cat # AF2016 | 1:250 |
| Wnt-3a | Rabbit | GeneTex, Irvine, CA, USA, Cat #GTX64367 | 1:500 |
| P-Ser129--synuclein (-syn) | Rabbit | Affinity Biosciences, Cincinnati, OH, USA, Cat # AF3285 | 1:1000 |
| Microtubule-associated protein 1A/1B-light chain 3 (LC3) | Rabbit | Invitrogen, Rockford, IL, USA, Cat # PA1-16931 | 1:1000 |
| p62/Sequestosome (SQSTM1) | Rabbit | Invitrogen, Rockford, IL, USA, Cat # PA5-20839 | 1:1000 |
| -actin | Rabbit | Abcam, Waltham, MA, USA, Cat # ab8227 | 1:10,000 |
2.6 Immunostaining
Mice were anaesthetized with thiopentone sodium (173277, Neon Laboratories Ltd., Mumbai, Maharashtra, India) (150 mg/kg body weight, i.p.). After that, the mice were transcardially perfused with normal saline, followed by a 4% paraformaldehyde (PFA) (00547007-1, Thermo Fisher Scientific, Mumbai, Maharashtra, India) dissolved in phosphate-buffered saline (PBS). Tissues were cut into 20 µm thick slices with a cryotome, mounted on 1% gelatin-coated glass slides, and immunostained. Sections were blocked in a humid chamber for 1 h with 5% bovine serum albumin (BSA) (3534981, SRL Pvt. Ltd., Taloja, Maharashtra, India) and then incubated with primary antibodies overnight at 4 ℃. The next day, tissue sections were rinsed with PBS and incubated with a secondary antibody at room temperature for 2 h. After that, they were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Batch No 034M4031V, Sigma-Aldrich, Burlington, MA, USA) for 30 min. For immunostaining, tissue sections were mounted with fluorescent mounting media and visualised under a confocal microscope (Eclipse Ti-2, Nikon, Melville, NY, USA). The list of antibodies used for immunostaining is given in Table 2.
Table 2. List of antibodies used in Immunostaining. | Antibody | Host | Product details | Dilution |
| P-Ser9-GSK-3 | Rabbit | Affinity Biosciences, Cincinnati, OH, USA, Cat # AF2016 | 1:500 |
| P-Ser129--syn | Rabbit | Affinity Biosciences, Cincinnati, OH, USA, Cat # AF3285 | 1:500 |
| LC3 | Rabbit | Invitrogen, Rockford, IL, USA, Cat # PA1-16931 | 1:1000 |
2.7 Statistical Analysis
Throughout the experiment, an observer blinded to the study design and treatment condition performed all behavioural scoring, examination of the histopathological characteristics, and cell counting. GraphPad Prism software version 7 was used to conduct the statistical analysis (GraphPad Software, Inc., San Diego, CA, USA). All the data is presented as a mean standard error of the mean (SEM). To assess the significant differences between the control, MPTP, Vanillin, MPTP + Vanillin and MPTP+L-DOPA groups, a one-way analysis of variance (ANOVA) was used, followed by a Tukey test that assumes the data has a normal distribution. *p 0.05, **p 0.01, and ***p 0.001 were used to signify significant differences when compared to the control, and #p 0.05, ##p 0.01, and ###p 0.001 when compared to the MPTP groups.
3. Results
3.1 Vanillin Ameliorates MPTP-Induced Motor Impairments
To determine the potential neuroprotective effects of Vanillin on motor balance and coordination in the MPTP-induced PD model, we performed the FST, wire hanging, and cylindrical test at the end of the dosing (Fig. 1). Our FST results (Fig. 1B,C; F = 213.3) show that the MPTP-intoxicated mice showed more immobility time (p 0.001) compared to the control mice. On the other hand, Vanillin (p 0.001), MPTP+Vanillin (p 0.05), and MPTP+L-DOPA (p 0.01) treated mice showed less immobility compared to the MPTP-intoxicated mice. Wire hanging test results (Fig. 1D,E; F = 44.29) reflected a similar trend. MPTP-intoxication resulted in a considerably decreased latency to fall (p 0.001) compared to the control mice, whereas Vanillin (p 0.001), MPTP+Vanillin (p 0.01), and MPTP+L-DOPA (p 0.05) groups showed an increased latency to fall compared to the MPTP-treated mice. Similarly, cylinder test results (Fig. 1F,G; F = 48.05) showed that mice treated with MPTP were more static and exhibited lesser (p 0.01) rearing compared to the control mice. In contrast, Vanillin (p 0.05), MPTP+Vanillin (p 0.05), and MPTP+L-DOPA (p 0.05) treated mice were less static and showed more rearing compared to the mice subjected to MPTP injections. These results indicate Vanillin attenuated MPTP-induced motor dysfunction in MPTP-lesioned mice.
 Fig. 1.
Fig. 1. Effect of Vanillin on behavioral characteristics in mice with MPTP-induced PD. (A) Schematic representation of MPTP injection, Vanillin treatment, and timeline of the study. (B) Image representation for FST. (C) Graphical representation of immobility time (sec.) in mice of all the groups. (D) Image representation for wire hanging test. (E) Graphical representation of the latency to fall in sec. in mice of all the groups. (F) Image representation for cylinder test. (G) Graphical representation of the numbers of rearing between different groups. Results are presented as mean SEM. Statistical difference is shown by **p 0.01 and ***p 0.001 when compared to the control mice and #p 0.05; ##p 0.01 and ###p 0.001 when compared to the MPTP treated mice. MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; FST, Forced Swim Test; SEM, standard error of the mean; L-DOPA, Levodopa/3,4-Dihydroxy-L-phenylalanine; PD, Parkinson’s disease.
3.2 Vanillin Decreases the Pathological P-Ser129--Syn Protein Expression
The dysregulations in -syn are critical in PD pathogenesis. More specifically, -syn aggregation is linked to neuronal dysfunction and degeneration in PD. -syn undergoes several post-translational modifications. Phosphorylation of the serine 129 residue has been recognized as a crucial pathological marker for PD. The expression of the pathological form of -syn, which is phosphorylated, leading to its aggregation in DA-ergic neurons, was assessed using western blotting and immunohistochemical staining in the present study (Fig. 2). The western blot results revealed the higher expression of the P-Ser129--syn in the MPTP-intoxicated PD mice group in contrast to the vehicle control group (Fig. 2A,B; F = 7.919, p 0.01) whereas Vanillin (p 0.05), MPTP+Vanillin (p 0.05) and MPTP+L-DOPA (p 0.05) treated mice showed lower expression of the P-Ser129--syn compared to MPTP-treated mice group. Furthermore, immunohistochemical staining of P-Ser129--syn also showed increased immunoreactivity in MPTP-induced PD mice compared to the vehicle control group (F = 8.634; Fig. 2C,D, p 0.01). Whereas in the Vanillin (p 0.01), MPTP+Vanillin (p 0.05) and MPTP+L-DOPA (p 0.01) treated group, the immunoreactivity of P-Ser129--syn was found to be significantly reduced in comparison with the MPTP-treated group. These findings demonstrate that Vanillin treatment significantly alleviated the expression of the P-Ser129--syn, indicating that Vanillin mitigated the aberrant P-Ser129--syn protein expressions in MPTP-treated mice.
 Fig. 2.
Fig. 2. Effect of MPTP administration and Vanillin treatment on the P-Ser129--syn expression in the SNpc of mice brain. (A) The role of MPTP and Vanillin administration on the P-Ser129--syn levels in the substantia nigra pars compacta (SNpc) of the mouse brain (B) Bar graphs depict the comparative expression of P-Ser129--syn (Fold change) in SNpc of the mice of all the groups. (C) Representative images showing P-Ser129--syn immunoreactivity in the SNpc of the mice of all the groups (Scale bar: 20 µm). For visualization, the secondary antibody Goat anti-rabbit immunoglobulin G (IgG) (Alexa fluor® 488) was used, followed by counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). (D) Bar graphs depict the comparative expression of P-Ser129--syn immunoreactivity between the groups. Values are given in the form of mean SEM (n = 3), **p 0.01 vs. controls and #p 0.05, ##p 0.01 vs. MPTP group. -syn, -synuclein; SNpc, substantia nigra pars compacta.
3.3 Vanillin Hinders GSK-3 Activity and Facilitates P-Ser9-GSK-3 Phosphorylation
We investigated the expression of GSK-3 and P-Ser9-GSK-3 in the SNpc region of mice. Our western blot findings indicated that MPTP-administered mice had significantly elevated GSK-3 protein expression compared with the control group (Fig. 3A,B; F = 9.37, p 0.05). Vanillin (p 0.01), MPTP+Vanillin (p 0.05) and MPTP+L-DOPA (p 0.01) treated mice showed significantly decreased GSK-3 protein expression compared to the MPTP-treated group. The enzymatic activity of GSK-3 is modulated by phosphorylation. Phosphorylation at serine-9 significantly reduces the enzymatic activity of GSK-3. The results from the western blot also showed a significant decrease in the protein expression of P-Ser9-GSK-3 (Fig. 3A,C, p 0.001) in MPTP-exposed mice in contrast with the control group. The protein expression level of P-Ser9-GSK-3 was substantially increased in Vanillin (p 0.05), MPTP+Vanillin (p 0.05) and MPTP+L-DOPA groups compared to the MPTP-intoxicated mice. Similarly, our immunofluorescence results also showed a significant decrease in the expression of P-Ser9-GSK-3 (Fig. 3D,E, p 0.001) in MPTP-intoxicated mice in contrast to the control group. The immunoreactivity of P-Ser9-GSK-3 was substantially increased in Vanillin (p 0.001), MPTP+Vanillin (p 0.05) and MPTP+L-DOPA (p 0.01) mice in comparison to the mice treated with MPTP. These results indicate that Vanillin treatment negated the GSK-3 activity and mediated P-Ser9-GSK-3 phosphorylation in MPTP-treated mice. Fig. 3D represents representative immunofluorescence images of P-Ser9-GSK-3.
 Fig. 3.
Fig. 3. Effect of MPTP administration and Vanillin treatment on the GSK-3 and P-Ser9-GSK-3 expression in the mice SNpc. (A) The representative western blot images of GSK-3 and P-Ser9-GSK-3 protein expression in the mice of all the groups. (B,C) Bar graphs depict the relative GSK-3 and P-Ser9-GSK-3 protein expression (Fold change) in the SNpc region of mice of all the groups. (D) Representative confocal image of P-Ser9-GSK-3 expression between the mice of all the groups (Scale bar: 20 µm). For visualization, the secondary antibody Goat anti-rabbit IgG (Alexa fluor® 488) was used followed by counterstaining with DAPI. (E) Bar graphs depict the relative fluorescence intensity of P-Ser9-GSK-3 expression in SNpc of mice of all groups. Values are given as mean SEM (n = 3), ns, non-significant, *p 0.05, ***p 0.001 vs. control and #p 0.05, ##p 0.01 and ###p 0.001 vs. MPTP group.
3.4 Vanillin Potentiates the Wnt/-Catenin Pathway in MPTP-Intoxicated Mice
Aberrations in the Wnt/-catenin signaling pathway have been demonstrated to have a vital role in the pathophysiology of PD. In this study, we assessed the expression of Wnt-3a and -catenin in the SNpc area of mice. Our western blot results revealed that MPTP-lesioned mice showed significantly decreased protein expression of Wnt-3a (Fig. 4A,B; F = 29.96, p 0.001) compared to the control group. Inversely, Vanillin (p 0.001), MPTP+Vanillin (p 0.01) and MPTP+L-DOPA (p 0.05) treated mice showed increased protein expression of Wnt-3a compared to the MPTP-lesioned mice. -catenin plays a vital role in the regulation of Wnt/-catenin signaling. The expression of -catenin was also significantly downregulated in MPTP-intoxicated mice compared to the control group (Fig. 4A,C; F = 10.98, p 0.001). The protein expression of -catenin was upregulated in Vanillin (p 0.001), MPTP+Vanillin (p 0.01) and MPTP+L-DOPA groups compared to the MPTP-intoxicated mice. These observations indicate that Vanillin treatment in MPTP-induced mice significantly upregulated the protein expression of Wnt-3a and -catenin. These data indicate that the neuroprotective effects of Vanillin in vivo appear to be mediated through the activation of the Wnt/-catenin signaling pathway.
 Fig. 4.
Fig. 4. Effect of MPTP intoxication and treatment with Vanillin on -Catenin & Wnt-3a protein expression levels. (A) Representative western blot images of -Catenin & Wnt-3a protein expression in the SNpc region of mice brain. (B,C) Bar graphs depict the comparative expression of -Catenin & Wnt-3a protein (Fold change) in SNpc of mice of all the groups. Data is expressed as mean SEM (n = 3), ns, non-significant, ***p 0.001 vs. controls and #p 0.05, ##p 0.01 and ###p 0.001 vs. MPTP groups.
3.5 Vanillin Decreases Autophagic-Cell Death by Modulating the LC3Π/Ι and p62 Protein Expression
The only known autophagy protein marker that specifically interacts with autophagosomes is light chain 3 (LC3-II). Upon exposure to reactive oxygen species (ROS), microtubule-associated protein 1A-LC3-I is converted to microtubule-associated protein 1B-LC3-II, which triggers autophagy. LC3-II indicates the presence of autophagosomes, which help clear the cell of accumulated proteins and defective organelles. We determined the effect of MPTP exposure and Vanillin treatment LC3-II expressions by performing western blot analysis and immunohistochemistry. Our immunofluorescence results show that MPTP treatment significantly increased LC3 expression and punctate distribution in the cytoplasm of DA-ergic neurons (Fig. 5A,B; F = 15.87, p 0.001). The number of LC3 puncta was significantly reduced in Vanillin (p 0.001), MPTP+Vanillin (p 0.05), and MPTP+L-DOPA (p 0.01) groups in contrast to the MPTP group. Similarly, our western blot result also shows that LC3-II/I ratio was significantly higher (Fig. 5C,D; F = 11.67, p 0.05) in the MPTP-treated group in contrast to the control group, suggesting increased autophagosome formation and thus increased autophagy in DA-ergic neurons. The LC3-II/I ratio was significantly reduced in Vanillin (p 0.01), MPTP+Vanillin (p 0.05), and MPTP+L-DOPA (p 0.05) groups compared to the mice treated with MPTP, indicating reduced autophagic-cell death in DA-ergic neurons. To validate our observation, we also monitored the expression of the p62 protein, which acts as a cargo protein autophagy substrate, so decreased p62 expression may be associated with increased autophagy. Our western blot findings indicate that the protein expression of p62 was markedly reduced in the MPTP group (Fig. 5E; F = 27.82, p 0.001) compared with the control. However, the expression of p62 was higher in Vanillin (p 0.01), MPTP+Vanillin (p 0.05), and MPTP+L-DOPA (p 0.001) treated mice than the MPTP exposed mice, indicating Vanillin treatment modulated the MPTP-induced aberrant p62 proteins expressions. These data indicate that LC3-II binds to p62 and selectively mediates the autophagy-dependent degeneration of DA-ergic neurons in the MPTP group, which was reversed when these mice were treated with Vanillin.
 Fig. 5.
Fig. 5. Effect of MPTP and Vanillin on the LC3 and p62 expression in the SNpc region of the mice brains. (A) The representative confocal images of LC3 expression in the mice of all the groups (Scale bar: 20 µm). (B) Bar graph shows the comparative expression of LC3 immunoreactivity in SNpc region of the mice of all the groups. (C) Representative western blot images of LC3 Π/Ι protein (Fold change) and p62 protein in the Control, MPTP, Vanillin, MPTP+Vanillin and MPTP+L-DOPA treated groups. (D,E) Bar graphs depict the comparative protein expression of LC3 Π/Ι and p62 protein (Fold change) in SNpc region of the mice in all the groups. Results are expressed as mean SEM (n = 3), *p 0.05, **p 0.01, ***p 0.001 and vs. controls and #p 0.05, ##p 0.01 and ###p 0.001 vs. MPTP treated mice.
4. Discussion
In the present study, we have determined the potential neuroprotective effects of Vanillin in PD by assessing its effect on motor balance and coordination in the MPTP-induced PD mice model. Combining results from all three behavioral tests—the FST, wire hanging, and cylindrical tests allows for a more comprehensive assessment of the animal’s motor function. In coherence with previous studies, we found that MPTP administration induces depressive behavior and impaired motor activity [28]. However, vanillin effectively reversed these behavioral and motor dysfunctions. A similar motor deficit alleviating effect of vanillin has been previously reported in a rotenone-induced rat model of PD [29]. PD, which involves the aberrant accumulation of -syn inside the SNpc, which causes the selective and gradual death of neurons [30]. The post-translational modification of -syn is a crucial factor in its aggregation [31]. Phosphorylated -syn, particularly P-Ser129--syn, is a key hallmark of PD and related synucleinopathies [31, 32]. Research has demonstrated that the administration of MPTP leads to elevated levels of P-Ser129--syn in mice SNpc [33]. Consistent with these, our immunofluorescence and western blot results also showed a significant increase in P-Ser129--syn expression in MPTP-intoxicated mice. In contrast, Vanillin treatment significantly ameliorated the P-Ser129--syn expression in MPTP-induced mice.
The Wnt/-catenin signaling is implicated in the pathogenesis of PD, regulating ventral midbrain precursor development, proliferation, and differentiation [34, 35, 36]. Dysregulated Wnt/-catenin signaling is reported to be involved in the development of PD [37, 38, 39]. This signaling is altered by GSK-3, an inhibitor of this crucial pathway, which phosphorylates and ubiquitinates -catenin. The inactivation of GSK-3 increases -catenin levels, potentially offering neuroprotective effects [40]. Reports suggest that activating the Wnt/-catenin may facilitate the differentiation and regeneration of DA-ergic neurons [41, 42]. This highlights the potential of targeting Wnt/-catenin signaling components, as well as GSK-3, which may be an effective therapeutic intervention for PD [34]. Blocking GSK-3 reduced -syn protein expression and, consequently, prevented cell death in PD disease models [14]. Neuroinflammation, involving activated astrocytes and microglia, contributes to -syn fibril formation [43]. GSK-3, an enzyme implicated in this process, exacerbates neuroinflammation when overactive. A recent study shows that inhibiting GSK-3 via Ser9 phosphorylation reduces astroglial and microglial reactivity, lowering pro-inflammatory cytokine levels and ROS, thereby offering neuroprotection [44]. These findings suggest that GSK-3 inhibitors could be a promising therapeutic approach to mitigate neuroinflammation and slow the progression of neurodegenerative diseases. Notably, in vivo, studies have also revealed that human GSK-3(S9A) mutated form exhibit elevated P-Ser129--syn and p-Tau in TH-positive DA-ergic neurons in mice as they age [17, 45]. These findings underscore the intricate interplay between GSK-3, -syn phosphorylation, and the subsequent neurodegenerative processes, shedding light on potential targets for therapeutic intervention. Phosphorylation regulates the activity GSK-3 activity. The enzymatic GSK-3 activity gets reduced following the phosphorylation at serine-9 [46]. The results from both our immunofluorescence and western blot analysis showed a decrease in the protein levels of P-Ser9-GSK-3 in MPTP-intoxicated mice. The MPTP-induced aberrant protein levels of P-Ser9-GSK-3 was markedly alleviated following the Vanillin treatment. Next, we assessed the protein levels of Wnt-3a, -catenin in the SNpc region of mice. Our results revealed that MPTP-lesioned mice had significantly reduced protein expression of Wnt-3a. Vanillin was significantly able to rescue this decreased Wnt-3a levels. -catenin protein levels were also considerably downregulated in MPTP-intoxicated mice. These reduced levels of -catenin upregulated following Vanillin treatment in MPTP-exposed mice. These findings imply that the neuroprotective properties imparted by Vanillin in vivo may be largely mediated by the activation of the Wnt/-catenin signaling, mainly by suppressing the GSK-3 activity.
Although neuronal autophagy is primarily recognized as a defensive mechanism, it can paradoxically contribute to neuronal cell death. Numerous studies have revealed the involvement of dysregulated autophagy in DA-ergic neuronal degeneration [47, 48]. Notably, PD patients showed autophagy in nigral neurons [49]. Studies have observed that MPTP administration elevates LC3-II expression and decreases p62 expression, indicating the activation of the autophagy-lysosomal pathway [50, 51]. Furthermore, a recent study indicates that induced autophagy can cause cell death in MPP+-exposed human neuroblastoma cells [52]. Our immunofluorescence findings demonstrate that increased LC3 immunoreactivity in the MPTP-induced mouse model is consistent with these observations. Vanillin treatment significantly lowered the LC3 immunoreactivity in the MPTP-administrated group. Reduced p62 expression and an elevated LC3-II/LC3-I ratio indicate increased autophagic activity. Our results also show that the expression of LC3/I protein was elevated and Vanillin treatment significantly reduced the ratio of LC3/I protein expression in MPTP-intoxicated mice. Additionally, p62 levels were decreased in the MPTP-administrated rats, which further shows the increased autophagic-cell death in MPTP-induced mice, and Vanillin administration markedly increased the p62 expressions. The effect of autophagy and autophagic cell death in dopaminergic neurodegeneration appears to be contingent upon the particular cellular setting and the initial causal cause. Improving the comprehension of autophagy stress [53] and the mechanisms governing autophagic-cell death holds promise for developing therapeutics to restore DA-ergic neuronal homeostasis in PD. Furthermore, additional physiologically relevant knowledge on the autophagic pathway’s activity status in PD patients is required to assess whether promoting or suppressing autophagy would be more beneficial in reducing the symptoms of the disease and delaying its progression. Future studies aimed to addressing the present dispute will be critical in translating these discoveries into viable therapies for PD.
In this study, we primarily focused on assessing the activity of GSK-3 and the activation of the Wnt/-catenin pathway in the context of MPTP-induced nigrostriatal degeneration, as these pathways are known to be involved in neuroprotection. While our study aimed to investigate the protective effect of Vanillin on the dopaminergic system by targeting GSK-3 activity and the Wnt/-catenin pathway, demonstrating the co-expression of related signaling molecules with dopaminergic neuron-specific markers would have provided stronger evidence. Thus, future studies should incorporate techniques such as immunofluorescence co-staining with specific markers for dopaminergic neurons to further validate our findings and enhance the understanding of Vanillin’s potential therapeutic effects on PD. Additionally, our study focused specifically on the SNpc region, and the mechanisms directly related to the Wnt/-catenin signaling pathway. However, we did not investigate this pathway in the striatum region, which indeed plays a crucial role in the dopaminergic system and the pathophysiology of PD in the striatum. Future studies should consider examining this pathway in the striatum to provide a more comprehensive understanding of PD and to identify additional therapeutic targets against PD.
5. Conclusions
In conclusion, our study highlights the intricate mechanisms involved in PD etiology and the potential therapeutic role of Vanillin. The aberrant accumulation of -syn in the SNpc contributes to neurodegeneration in PD. Vanillin treatment effectively mitigates the elevated levels of phosphorylated P-Ser129--syn. Moreover, our study of Wnt/-catenin signaling highlights the critical role it plays in PD pathogenesis and highlights the possibility of using GSK-3 as a target for neuroprotection and neurorestoration. GSK-3, implicated in both -syn phosphorylation and -catenin degradation, emerges as a critical player in PD pathophysiology. Vanillin treatment not only mitigates GSK-3 activity but also positively modulates Wnt/-catenin signaling, suggesting its neuroprotective effects. The dual role of autophagy in neuroprotection and neurodegeneration, revealing its involvement in DA-ergic neuronal degeneration in PD. Treatment with vanillin significantly reduces abnormal autophagic-cell death, suggesting a possible function for vanillin in reestablishing DA-ergic neuronal homeostasis. Thus, by inhibiting GSK-3 activity and reducing autophagic cell death in DA-ergic neurons, vanillin may stimulate the Wnt/-catenin signaling pathway, which in turn may explain its neuroprotective effect in our study Fig. 6. This study enhances our understanding of the complex interplay of molecular pathways in PD and underscores Vanillin’s promising therapeutic implications for the disease.
 Fig. 6.
Fig. 6. Possible mechanisms that mediate the neuroprotective action of Vanillin on MPTP-intoxicated PD mice model. DVL, Dishevelled; CK-1, casein kinase 1; APC, adenomatosis polyposis coli; Lrp, lipoprotein receptor-related protein; TrcP, transducin repeats-containing protein; Ub, ubiquitin; Axin, axis inhibition protein; DA-ergic, dopaminergic.
Availability of Data and Materials
All the data produced during the study are already included in the manuscript, and no further data is required to reproduce the results.
Author Contributions
ACM conceptualized, supervised the study and edited the final manuscript. LR performed the experiments and wrote the manuscript. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
All the experiments were carried out on C57BL/6 mice following standard guidelines and regulations of the Institutional Animal Ethics Committee (IAEC), Jawaharlal Nehru University (JNU), New Delhi (10/GO/ReBi/99/CPCSEA/March 10, 1999). All the Standard methods and protocols were followed for animal handling and experiments. All efforts were made to reduce animal suffering.
Acknowledgment
The authors would like to acknowledge the Central Instrumentation Facility (CIF), School of Life Sciences, Jawaharlal Nehru University, New Delhi, India.
Funding
This study was supported by the Department of Biotechnology (DBT), Govt. of India (BT/PR38493/TRM/120/465/2020), and (BT/PR47726/CMD/150/26/2023), Ministry of Science and Technology (Govt. of India).
Conflict of Interest
The authors declare no conflict of interest.
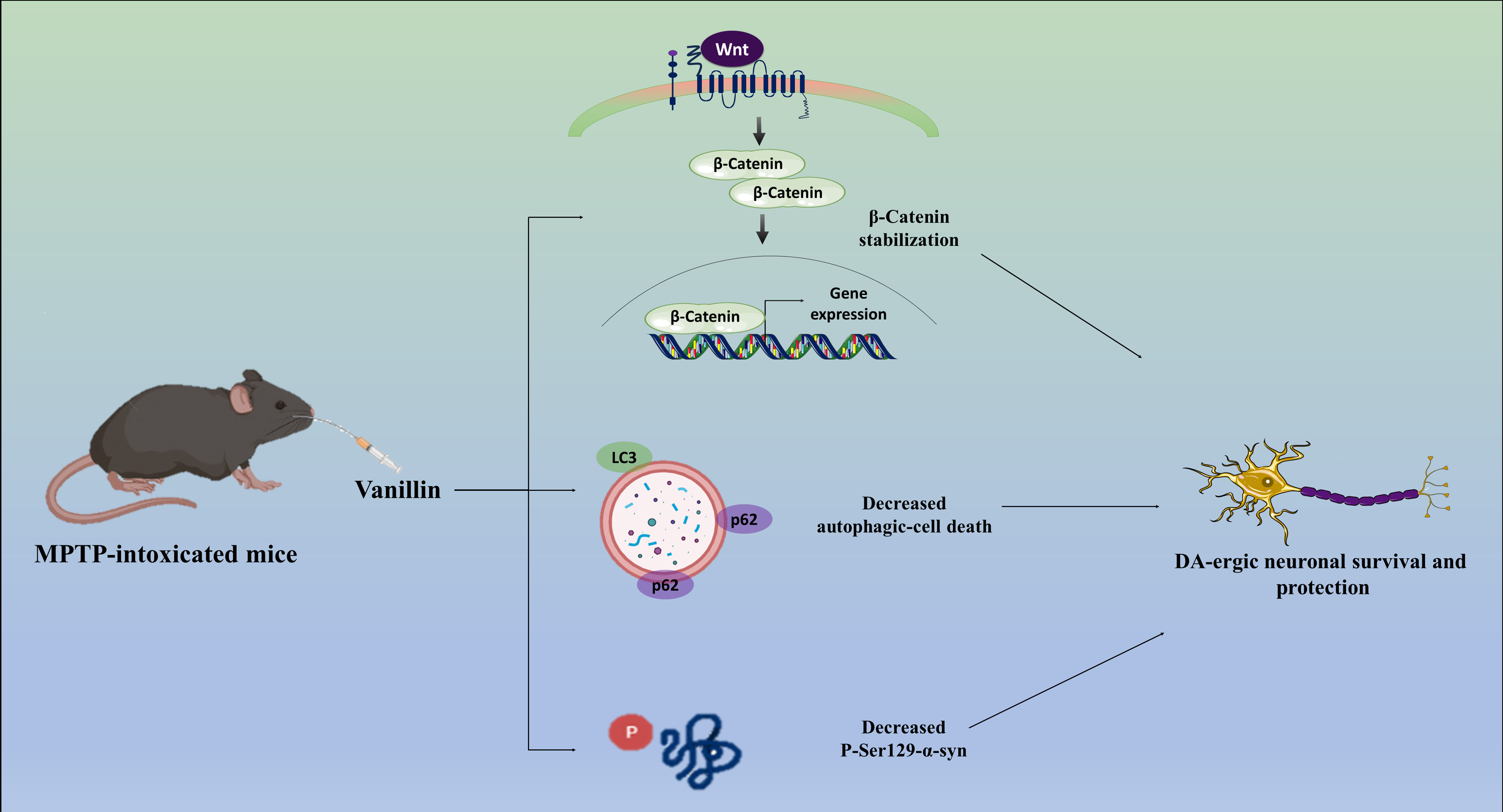
 Fig. 1.
Fig. 1.  Fig. 2.
Fig. 2.  Fig. 3.
Fig. 3.  Fig. 4.
Fig. 4.  Fig. 5.
Fig. 5.  Fig. 6.
Fig. 6. 





