1 Department of Spine Surgery, Shenzhen Pingle Orthopedic Hospital, Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine, 518118 Shenzhen, Guangdong, China
2 Department of The Third School of Clinical Medicine, Guangzhou University of Chinese Medicine, 510006 Guangzhou, Guangdong, China
†These authors contributed equally.
Abstract
Spinal cord injury (SCI) is a severe central nervous system disorder with no currently available effective treatment. Microglia are immune cells in the central nervous system that play crucial roles in the SCI occurrence, development, and recovery stages. They exhibit dynamic polarization over time and can switch between classical activation (M1) and alternative activation (M2) phenotypes to respond to environmental stimuli. The M1 phenotype is involved in initiating and sustaining inflammatory responses, while the M2 phenotype exerts anti-inflammatory effects and promotes tissue repair in damaged areas. Inhibiting M1 polarization and promoting M2 polarization have become hotspots in regulating neuroinflammation and treating SCI. This article provides a comprehensive review centered on modulating microglial polarization phenotypes for SCI treatment.
Keywords
- microglia
- polarization
- spinal cord injury
Spinal cord injury (SCI) is a severe disorder of the central nervous system (CNS), characterized by severe loss of sensory and motor function. It is often the result of falls from heights and traffic accidents [1, 2]. According to reports, SCI global incidence ranges from 3.6 to 195.4 per million people, imposing a significant burden on families and societies [3]. Over the past few decades, researchers have employed various strategies to reduce neural damage and restore neural function [4]. However, there is still no universally recognized effective method for treating SCI [5, 6]. The mechanism of SCI comprises two stages: primary injury and secondary injury [7]. Primary injury to the spinal cord occurs following trauma such as contusion, laceration, and compression, which directly results in cell death and instant loss of neural function. Secondary SCI involves various complex cellular and biochemical processes, including oxidative stress, inflammatory responses, cellular autophagy, and apoptosis [8]. Certain drugs are used to modulate these pathological processes and reduce or even reverse secondary SCI [9].
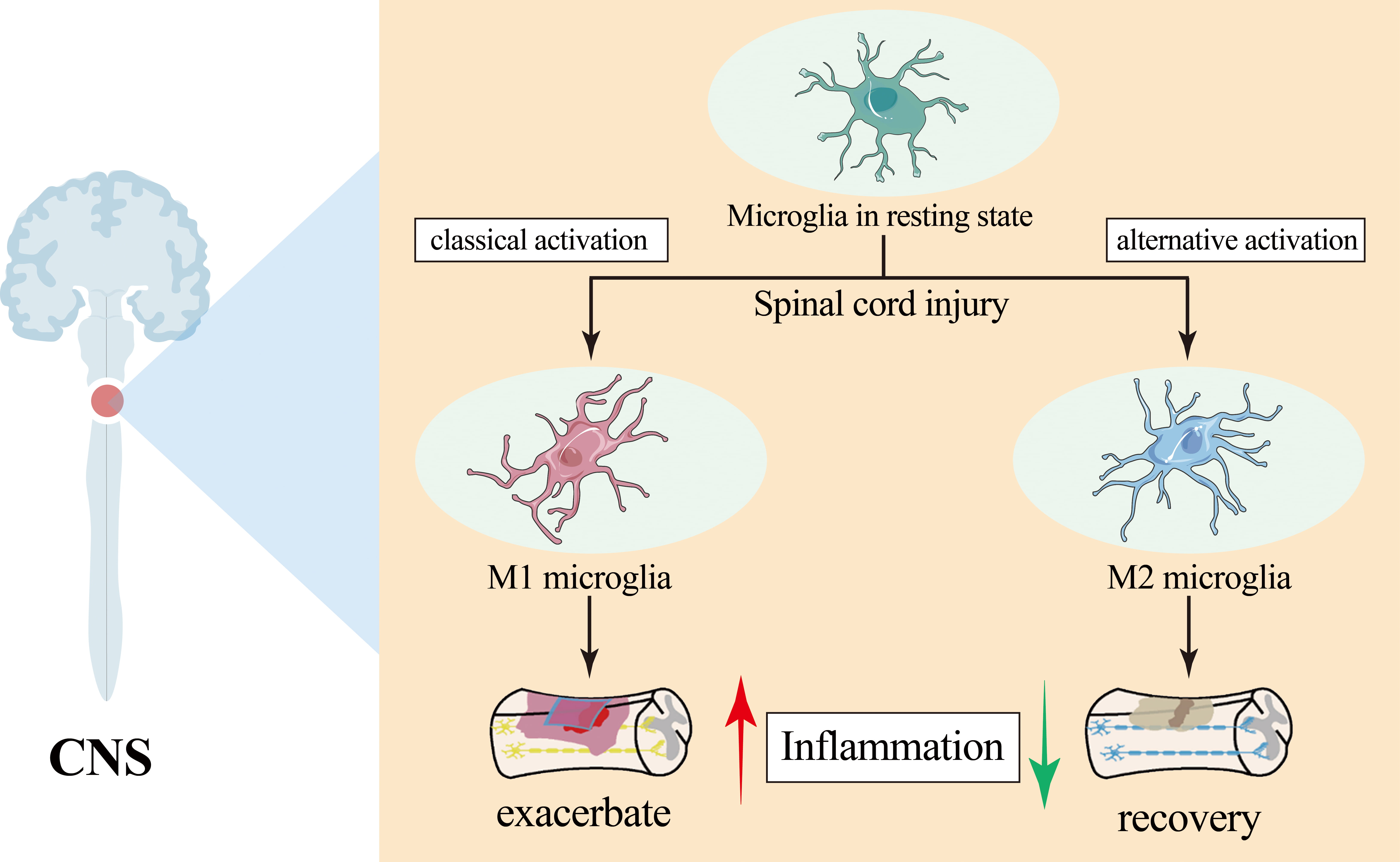
SCI-induced neuroinflammation involves the activation of microglia and the upregulation of pro-inflammatory cytokines, among other factors [10]. Microglia are one of the main resident cells of the spinal cord, and play a crucial role in regulating the development of neuroinflammation after SCI [11]. Microglia polarization is a microenvironment-dependent dynamic process, involved in the different stages of injury and its severity. An oversimplified and outdated view recognized that microglia polarization phenotypes are mainly classified into classical activation (M1) and alternative activation (M2). M1 microglia exacerbate neuroinflammation leading to cell death or functional impairment, which hinders SCI repair [12, 13]. On the contrary, the M2 phenotype suppresses inflammation by producing anti-inflammatory factors and promoting the recovery of nearby neural function. Numerous studies have demonstrated that enhancing the M2 polarization of microglia, while inhibiting M1 polarization, can improve the efficacy of SCI treatment [14, 15] (Fig. 1). Traditionally, activated M2 subtypes consist of M2a, M2b and M2c, with each having different functions in the CNS [16]. However, the classical all-or-nothing states are oversimplified and inconsistent with recently discovered phenotypes in the CNS [17]. It has been shown that microglia are not limited to a strict polarization of M1 or M2 but can display a range of intermediate phenotypes, with more subtle and dynamic functional responses that vary according to the specific environmental demands [18]. Despite this recognition, academics have yet to agree on a definition of microglial identity compared to other cell types, or on the number, dynamic nature, or definition of microglial states [19]. Although the classification of M1 and M2 is oversimplified, it helps improve our understanding of the functional state of microglia during injury progression and aids in exploring new therapeutic strategies. Thus, this classification still contributes to the understanding of microglial cell function in SCI. This article is a comprehensive review of SCI treatment methods that focus on modulating microglia polarization phenotypes.
 Fig. 1.
Fig. 1.
Polarization and effects of microglia after spinal cord injury. Microglia polarization phenotypes are mainly classified into classical activation (M1) and alternative activation (M2). M1 microglia exacerbate neuroinflammation and the M2 microglia suppresses inflammation. CNS, central nervous system.
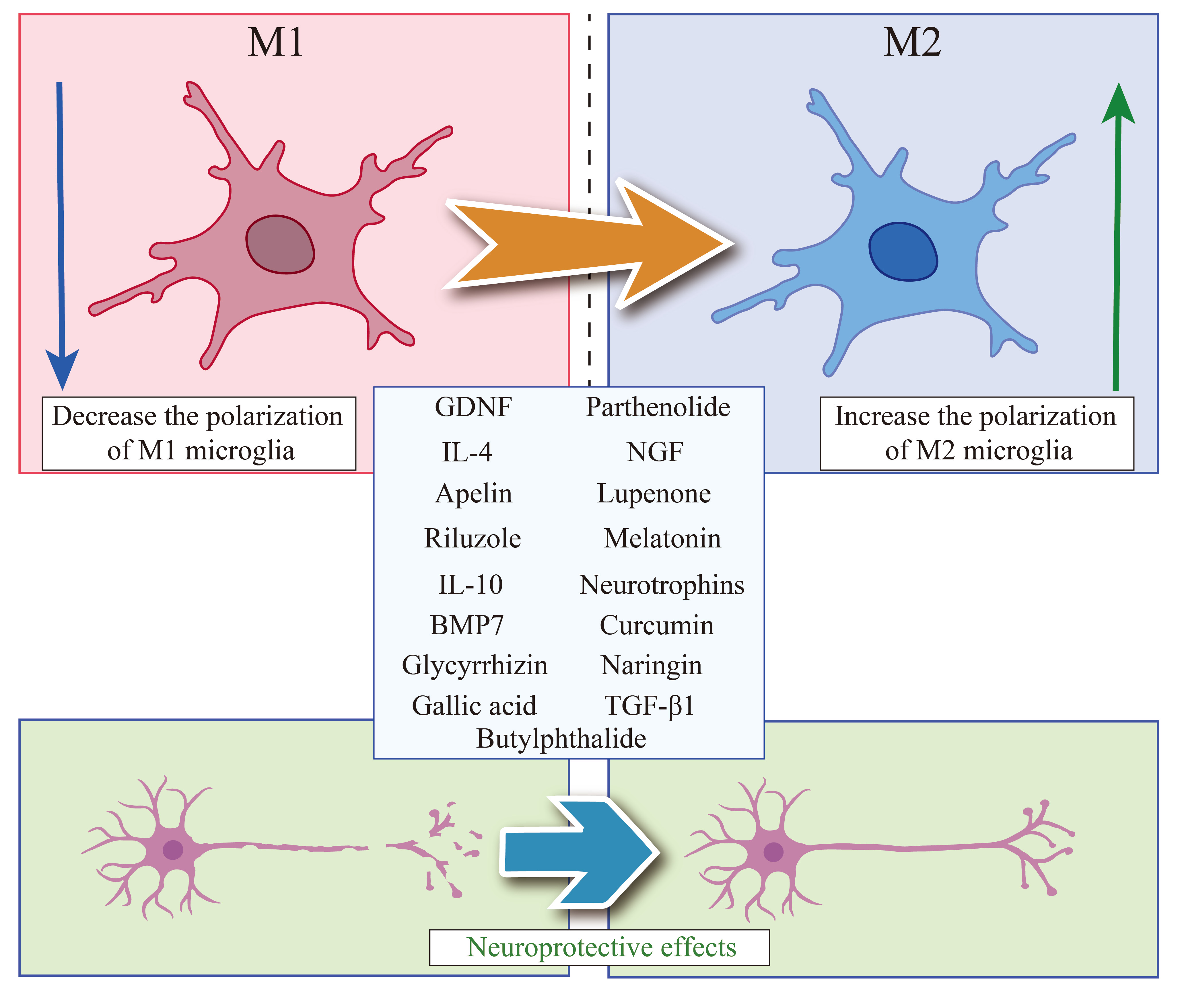
Cytokines are a class of low molecular weight proteins that play important roles in immune responses and in other physiological processes such as cell proliferation, differentiation, apoptosis, and inflammation [20]. They are primarily secreted by immune and stromal cells in response to various physiological and pathological processes [21]. Cytokines can be broadly categorized into several classes, including growth factors, interleukins (IL), interferons (IFN), tumor necrosis factors (TNF), and chemokines [22]. Imbalances in cytokines can lead to serious autoimmune diseases or inflammatory conditions, including rheumatoid arthritis and asthma [23]. Therapies targeting cytokines are effective in treating a variety of diseases [24]. Research suggests that cytokines can induce post-SCI recovery of motor function by mediating neurogenesis, neuroprotection, angiogenesis, and inflammatory responses [25] (Fig. 2).
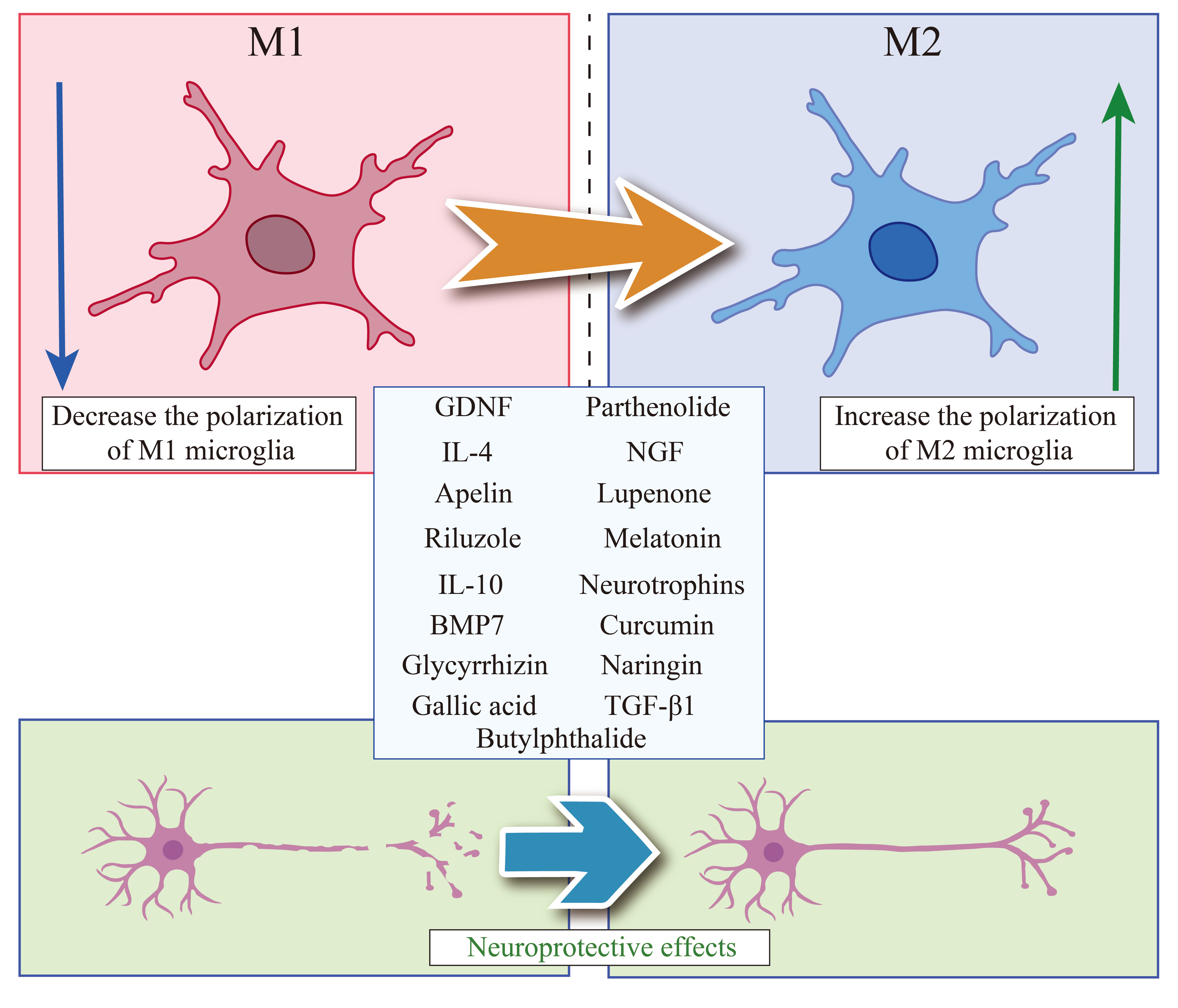 Fig. 2.
Fig. 2.
Molecules that can regulate the polarization phenotype of
microglia for SCI treatment. Summarized the current molecules that can inhibit
polarization of M1 microglia and promote polarization of M2 microglia, which can
play a neuroprotective role in the treatment of SCI. SCI, spinal cord injury;
GDNF, glial cell-derived neurotrophic factor; IL-4, interleukin 4; IL-10,
interleukin 10; BMP7, Bone morphogenetic protein 7; NGF, nerve growth factor;
TGF-
Transforming growth factor-
Bone morphogenetic protein 7 (BMP7) has been reported to exert neuroprotective effects in different models of neurological disorders [28]. Wei et al. [29] investigated whether the neuroprotective function of BMP7 is associated with modulating microglia polarization. The results demonstrated that BMP7 inhibits microglia activation and promotes their conversion into M2 phenotype. These events result in the reduction of inflammatory cytokines’ secretion into the microenvironment during SCI acute inflammatory phase, thereby promoting functional recovery.
IL-10 is an anti-inflammatory cytokine that can promote the recovery of neurological function after SCI [30]. Research suggests that IL-10 inhibits monocytes/macrophages inflammatory response, regulates the polarization of microglia and macrophages towards the M2 phenotype, and promotes neuronal survival [31].
IL-4 is a pleiotropic cytokine that plays a crucial role in regulating immune functions. It is considered the most potent polarizing cytokine for M2 microglia cells. IL-4 has shown beneficial activity in animal models of stroke, SCI, and multiple sclerosis [32]. Xu et al. [33] suggest that a 24-hour treatment with IL-4 induces M2 polarization of murine microglia, which alleviates neuroinflammation and neuronal apoptosis.
Neurotrophins are part of a unique family of polypeptide growth factors that regulate the proliferation, differentiation, survival, and death of neuronal and non-neuronal cells [34]. Many reports suggest that neurotrophins have neuroprotective functions and play a crucial role in neurological recovery after SCI [35]. In recent years, the most extensively studied neurotrophins are brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF). Komori et al. [36] suggest that BDNF may be associated with microglia M2 polarization. Ma et al. [37] developed nanoparticle scaffolds loaded with glial cell-derived neurotrophic factor (GDNF) for spinal cord repair. The results indicate that these nanoparticles can significantly promote microglia M2 polarization [37]. Moretti et al. [38] developed a method for treating SCI using a combination of multiple drugs. They found that NGF epidural injection reduces the activation of M1 microglia, thereby alleviating inflammation in the nervous system [38]. Akhmetzyanova et al. [39] found that the use of GDNF to reduce the phagocytic activity of microglia promoted the expression of a neuroprotective phenotype. Xie et al. [40] found that neuromuscular function improved in aged rats after treatment with GDNF, implying that GDNF has therapeutic potential for neuromuscular dysfunction in the elderly. These experimental results suggest that GDNF may be a promising therapeutic approach to promote nerve regeneration after SCI.
Neuropeptides are endogenous bioactive peptides present in various systems of the human body. They participate in neural regulation through information transmission in the nervous system and exhibit multiple effects as hormones and cytokines. Recently, melatonin and apelin have received widespread attention in the treatment of CNS diseases [41]. Melatonin is a hormone that plays a crucial role in regulating circadian rhythms. In the past, the role of melatonin was mainly attributed to its ability to promote the initiation and maintenance of sleep. However, a large body of research suggests that melatonin also regulates microglia polarization from M1 to M2 phenotypes [42, 43, 44]. Yan et al. [45] investigated melatonin potential mechanisms in treating SCI. The results indicated that melatonin activates the nuclear factor erythroid 2-related factor 2 (Nrf2)/Kelch-like epichlorohydrin (ECH)-associated protein 1 (Keap1) signaling pathway and promotes microglia polarization towards the M2 phenotype [45]. Apelin is an endogenous ligand that binds the G protein-coupled receptor angiotensin-like receptor 1. According to reports, apelin has therapeutic effects on CNS diseases, such as stroke and SCI [46]. Liu et al. [47] found that apelin promotes the proliferation and differentiation of neural stem cells (NSCs) into neurons. Meanwhile, it can also reduce the polarization of M1-type microglia and A1-type astrocytes, promoting motor function recovery.
Recently, other natural and synthetic small molecules have been reported to
improve impaired spinal cord neurological function by modulating microglia
polarization [48]. Lupenone is a natural small molecule extracted from plants,
such as bananas and danshen. It has been reported in numerous studies to have
excellent anti-inflammatory effects [49, 50]. Li et al. [51] evaluated
lupenone therapeutic effects in a mouse model of SCI and found that it prevents
SCI exacerbation by inhibiting inflammasome activation. Additionally, it enhances
the transformation of pro-inflammatory M1 microglia into anti-inflammatory M2
microglia. Curcumin is a natural small molecule extracted from the rhizomes of
the medicinal plant turmeric. It has been widely reported to possess a strong
anti-inflammatory activity by inhibiting the production of inflammatory cytokines
among others [52]. Curcumin inhibits astrocyte activation, reduces the release of
pro-inflammatory cytokines, promotes the production of anti-inflammatory
cytokines, and helps alleviate neuropathic pain. Moreover, it facilitates the
transition of microglial phenotype from M1 to M2 [53, 54]. Wu et al.
[55] found that curcumin inhibits the activation of the nuclear factor kappa B
(NF-
In general, molecules can quickly pass through cell membranes, be absorbed by the digestive system, cause less immune response in the body, and are easy to synthesize, store, transport, and standardize, making them a promising strategy for SCI treatment. In recent years, many molecules have been reported to treat SCI by regulating the polarization of microglia. However, due to the complex pathological state during SCI, these molecules have not yet been clinically applied. In the future, safer and effective molecules need to be discovered and synthesized, and validated through clinical experiments before they can be truly applied to the treatment of SCI.
The Food and Drug Administration (FDA) defines gene therapy as follows: “the
administration of genetic material to modify or manipulate the expression of a
gene product or to alter the biological properties of living cells for
therapeutic use [71]”. At present, gene therapy includes four major strategies:
inhibition of abnormal transcribed RNA using microRNA, degradation of abnormal
mRNA using RNA interference, decrease of mutant proteins, and DNA genome editing
with methods such as clustered regularly interspaced short palindromic repeats
[72]. Using viral or non-viral vectors to correct or modify host genes shows
promising prospects in SCI treatment. Guo et al. [73] found that the
expression of Specific protein 1 (Sp1) in microglia increases after SCI,
suggesting that Sp1 may be associated with microglia M1 polarization. In another
report, they transfected synthetic siRNA-Sp1 into microglia and confirmed that
its silencing inhibits microglia M1 polarization by exerting neuroprotective
effects and promoting functional recovery after SCI. 5-Hydroxytryptamine receptor
2B (Htr2b) is one of the receptors of serotonin (5-HT) and is generally thought
to promote inflammation. Chen et al. [74] applied shRNA lentivirus
targeting of Htr2b and the results showed that its knockdown inhibits microglia
M1 polarization and neuroinflammation after SCI. TNF-
The above studies suggest that regulating the polarization of microglia through gene therapy is a potent method for treating SCI. The safety of several viral vectors for gene therapy has been widely reported after decades of clinical studies. Although cell therapy for SCI has entered the clinical trial stage, research on gene therapy for SCI has not yet reached the clinical stage [77]. Improving the transfection efficiency of gene vectors, localizing and regulating gene delivery, and continuing preclinical research to confirm the safety and effectiveness of gene therapy are future directions.
In all biological systems, cells secrete exosomes in physiological and
pathological states. Exosomes are tiny vesicles encapsulated by membranes that
are essential in the process of communication between cells [78]. Exosomes can
influence the differentiation of neuroglial cells and regulate neuroinflammation.
This process triggers the release of cytokines and inflammatory mediators,
enhances resistance to cell apoptosis, and exhibits neuroprotective effects [79].
Luo et al. [80] found that Adipose-derived MSCs exosomes inhibit the
expression of inflammatory factors in the spinal cord tissues and M1 microglia,
promote M2 microglia, and activate the Nrf2/heme oxygenase-1 (HO-1) pathway.
Xue et al. [81] confirmed that injection of bone marrow mesenchymal stem
cell (BMSCs) exosomes suppresses microglia M1 polarization-mediated inflammation.
Ren et al. [82] evaluated the regulation of inflammation of Schwann
cell-derived exosomes and demonstrated that these exosomes attenuate the
inflammation by suppressing M1 polarization and stimulating M2 polarization.
Fan et al. [83] developed an exosomes-loaded electroconductive hydrogel
and showed its capacity to modulate microglia M2 polarization via the
NF-
Exosomes are crucial mediators of intercellular communication, widely present in body fluids, and play a role in various pathological processes in the body. Compared to cells, exosomes have a smaller nanoscale structure and good biocompatibility. They can pass the blood-brain barrier and hold promise as drug carriers [84]. However, several issues need addressing when using extracellular vesicles for clinically treating SCI, such as developing standardized methods for separating high-purity extracellular vesicles, identifying optimal sources of extracellular vesicles, and establishing protocols for storage and transportation. Further research is still needed to address these issues and pave the way for the clinical application of extracellular vesicles in the treatment of SCI.
The use of autologous or allogeneic cell transplantation to repair SCI tissue damage is a potential treatment [85]. Transplanting NSCs, BMSCs, and olfactory ensheathing cells (OECs) have been studied as potential SCI therapeutic methods [86, 87]. Transplanted NSCs reduced the number of infiltrated immune cells and biased microglia towards a regenerative M2 phenotype, suggesting a long-term impact on the functional recovery of SCI rats [88]. Guo et al. [89] demonstrated that transplanted olfactory OECs effectively enhance neural survival and axonal outgrowth. The therapeutic effect is mainly attributed to the anti-inflammatory activity of OECs which modulates the polarization of microglia from the M1 to M2 phenotype. Pang et al. [90] found that MSCs transplantation after SCI may induce a shift in the phenotype of microglia/macrophages from M1 to M2, providing an anti-inflammatory and reparative microenvironment for motor recovery.
Cell therapy is considered the most promising strategy for treating SCI. Cells transplanted into the region of SCI regulate inflammatory responses or promote axonal regeneration and nerve repair through multidirectional differentiation and secretion of cytokines or neurotrophic factors [91]. Despite promising progress in research related to cell therapy, many challenges remain. These include determining the optimal number of cell transplants, identifying the optimal time window, addressing concerns about tumorigenicity, and improving the low survival rate of transplanted cells. Therefore, cell therapy still requires substantial data support before it can be applied on a large scale in clinical settings.
In recent years, regenerative medicine based on biomaterial has rapidly developed, and biomedical materials-based strategies for SCI repair have also received widespread attention [92, 93]. Biomedical materials have promising prospects in regulating microglial polarization to repair spinal cord injuries [94]. They can be categorized as nanoparticles, gels, and scaffolds and their functions involve transmitting signaling molecules, and encapsulated cells [95].
Nanoparticles are the smallest carriers and are typically employed for transporting small molecule drugs. These nanoparticles extend the residence time of drugs in the lesion area, enhance the local effective concentration, and concurrently reduce the systemic impact of drugs on the body [96]. Gopalakrishnan et al. [97] developed nanoparticles incorporating carbohydrate antigens and showed that these nanoparticles activate resting human microglia and polarize them toward a putative M2 state. Zhou et al. [98] used gold nanoclusters loaded with berberine to reduce inflammation by inhibiting the activation of M1 phenotype microglia, which simultaneously inhibited neuronal apoptosis after SCI.
Hydrogel is a three-dimensional polymer material with a widely hydrophilic structure, capable of providing a suitable aqueous environment for cells and promoting cell proliferation [99]. A biocompatible hydrogel loaded with fat extract was used to treat a model of spinal cord contusion in mice. The composite promoted the polarization of macrophages from an inflammatory M1 phenotype to an anti-inflammatory M2 phenotype [100]. Yu et al. [101] developed a fibronectin hydrogel containing lycium barbarum oligosaccharide and nasal mucosa-derived MSCs for SCI restoration by leveraging the inflammatory licensing effect and microglia M2 polarization.
At present, some biomaterials used for the treatment of SCI have entered the clinical trial stage and have demonstrated evidence of safety and effectiveness [102]. Biomaterials can serve as carriers for targeted delivery and sustained release of drugs in SCI areas, mimicking the soft tissue microenvironment to effectively guide and support the repair process. They can also be combined with cell therapy to create a conducive environment for transplanted cells, promoting their proliferation and differentiation. Despite the promising prospects of combining biomaterials with other therapies for SCI treatment, there is still insufficient clinical research in this area, necessitating further studies to confirm its effectiveness.
Physiotherapy is a practical treatment for people with spinal cord injuries.
Acupuncture, current stimulation, and other treatments have been widely reported
to significantly improve motor function in SCI patients [103, 104]. Some physical
therapies have also been proven to alleviate neuroinflammation and secondary
injury after SCI [105]. Tan et al. [106] confirmed that transcranial
direct current stimulation reduces the proportion of microglia M1 phenotype and
increases the proportion of the M2 phenotype. Zhao et al. [107] found
that electroacupuncture improves blood brain barrier (BBB) scores, decreases the proportion of M1
macrophages, TNF-
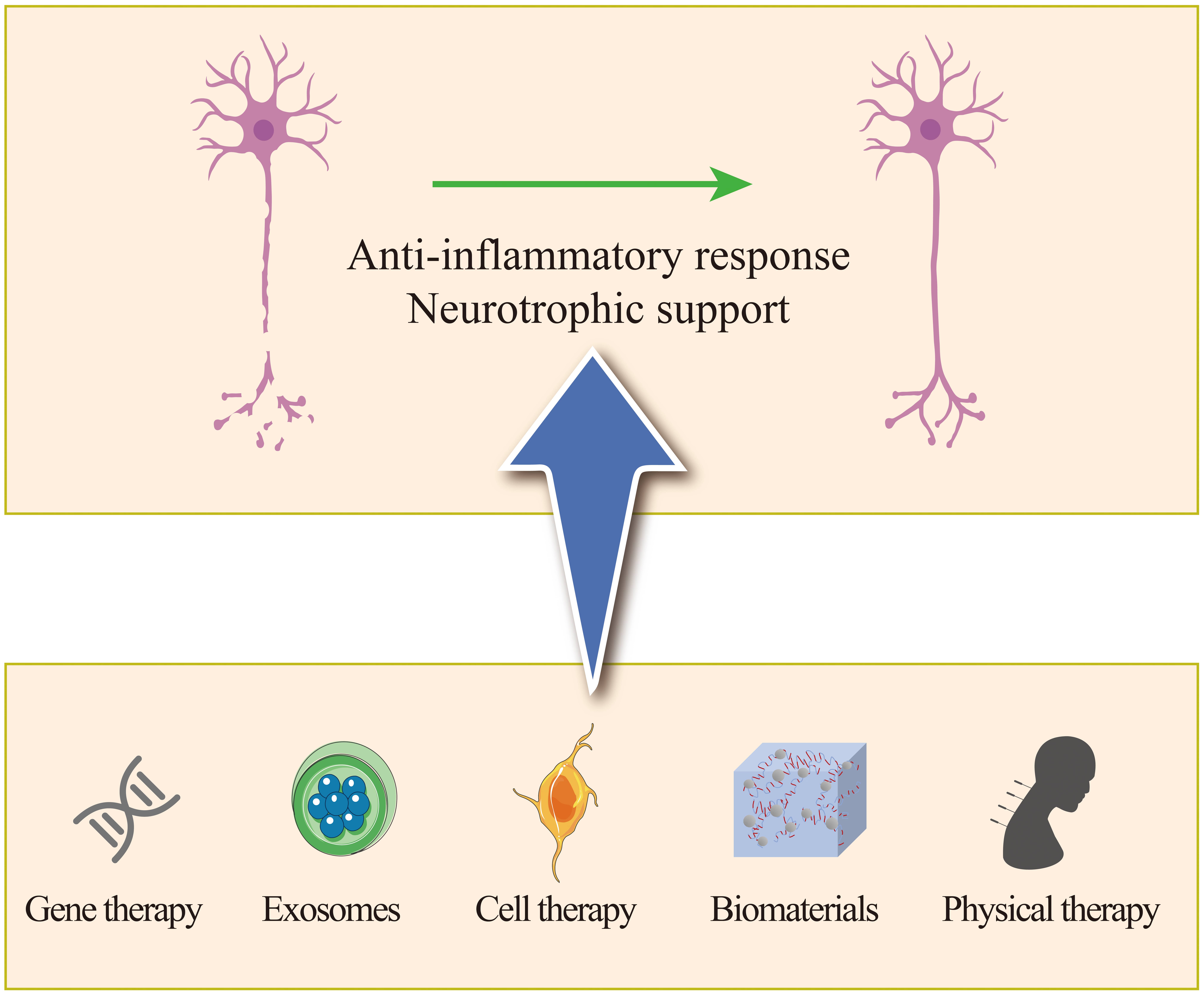
In clinical practice, physical therapy is often applied to SCI patients once their condition has stabilised with the main aim of promoting the recovery of motor function and preventing complications. Physical therapy represents the most common therapeutic strategy for spinal cord injuries, due to its non-invasive nature and feasibility [1]. The repair of SCI is a complex pathophysiological process. Over the past few decades, numerous research efforts have been conducted, achieving certain success through strategies such as gene therapy, biomaterials, and exosomes to modulate the phenotype of microglia and promote SCI repair (Fig. 3). However, due to the complexity of SCI repair, no ideal repair strategy has yet been found to fully repair and regenerate SCI. Hence, the combination and synergy of physiotherapy with other therapies may represent a promising avenue for future research in SCI therapy [108, 109].
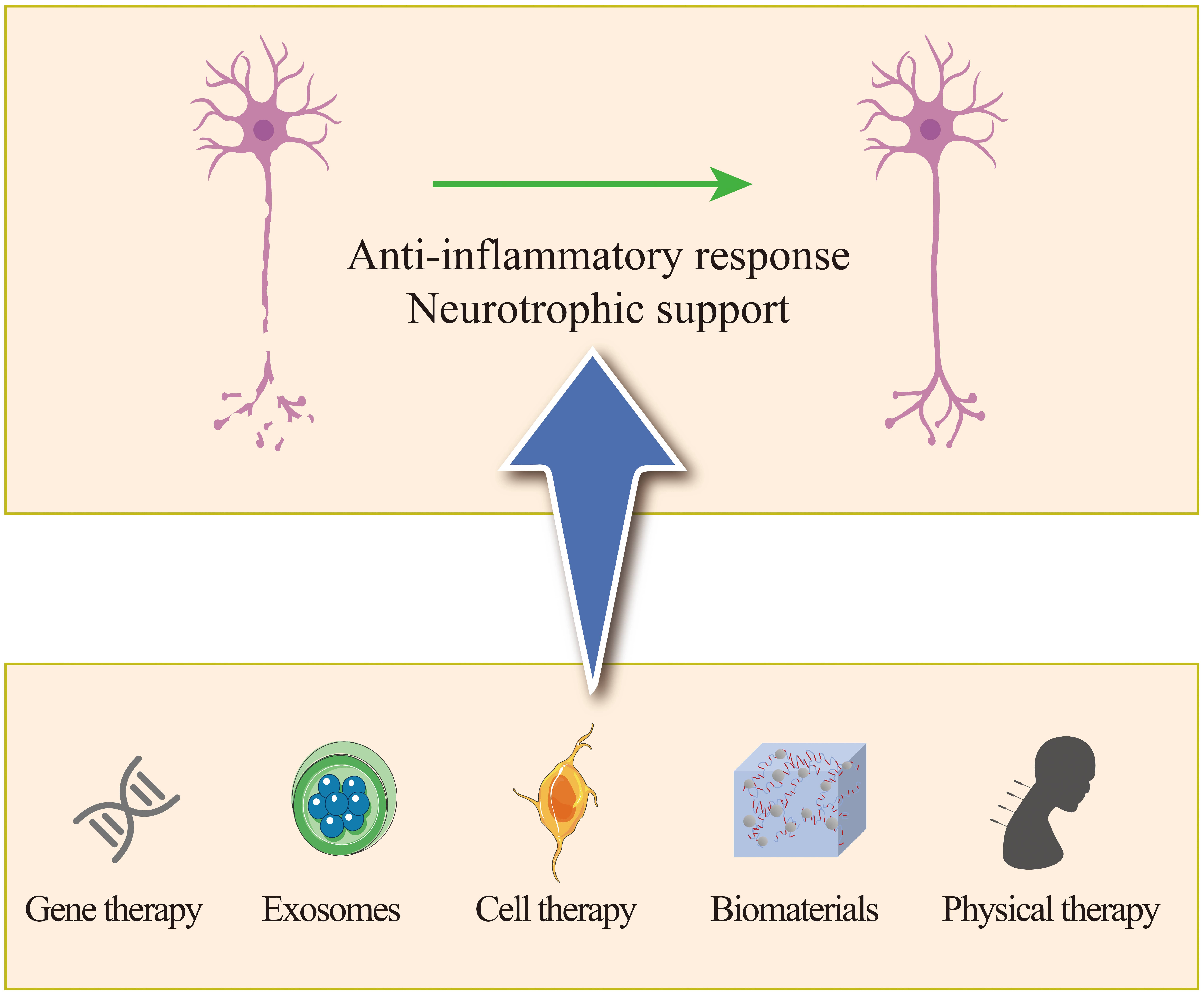 Fig. 3.
Fig. 3.
Introduce the current methods for treating spinal cord injury. The methods for treating spinal cord injury are mainly divided into 5 categories, including gene therapy, exosomes, cell therapy, biomaterials, and physical therapy, all of which can inhibit inflammatory reactions and provide neuroprotective effects.
SCI is a severe condition that can lead to paralysis of the limbs, respiratory system impairment, and restricted mobility, imposing significant physical, psychological, and economic burdens on patients. SCI current clinical treatments, including surgical decompression, drug therapy, and physical rehabilitation, have limited effectiveness. SCI-associated inflammation can exacerbate tissue damage and functional loss, leading to secondary injury. Studies indicate that reducing inflammation and immune cell infiltration in the damaged CNS may improve neuronal regeneration. M1 microglia secrete inflammatory factors at the injury site, exacerbating spinal cord damage. In contrast, M2 macrophages secrete IL-4, IL-10, and neurotrophic factors to suppress inflammation and neuronal apoptosis. Increasing evidence suggests that inhibiting microglia M1 polarization to suppress the release of pro-inflammatory mediators may have neuroprotective effects. Meanwhile, M2 microglia can produce anti-inflammatory cytokines and promote angiogenesis, facilitating neural recovery in injured spinal cords and inhibiting neuronal death. We have summarized recent research on regulating microglia polarization for SCI treatment (Table 1, Ref. [13, 29, 31, 33, 37, 38, 45, 47, 51, 55, 58, 62, 64, 66, 67, 69, 70, 73, 74, 75, 80, 81, 82, 83, 88, 89, 90, 97, 98, 100, 101, 106, 107]). Despite the several studies aimed at elucidating the regulatory mechanisms of microglia, there is currently no clinically effective method to promote microglia transition into a neuroprotective phenotype to stimulate neural regeneration. Clinical trials have been carried out to test advanced treatments such as biomaterial, cell therapy, and physical therapy. However, it is still challenging to remodel neurological function due to the complex pathological process of SCI. It is difficult to translate animal studies to clinical practice owing to species differences. In the future, more research is needed to further analyze the pathological mechanisms of SCI to explore new therapeutic targets and approaches. Modulation of microglial function is an emerging and promising treatment strategy for SCI recovery. In conclusion, targeted modulation of microglial polarization holds promise as a future therapeutic approach for SCI aimed at promoting neural regeneration and improving functional recovery.
| Category | Type of research | Model | Type of injury | Treatment | Effect | Reference |
| Molecules | Preclinical study | Spinal cord injury in mice | Contusion | Administration of extracellular vesicles, released from MSCs treated with TGF- |
Increased the transition of reactive microglia from M1 polarization to M2 polarization, alleviated neuroinflammation, and enhanced the neuroprotective effect of residual cells in the acute phase. | [13] |
| Preclinical study | Spinal cord injury in rats | Contusion | Local injection of recombinant human BMP7 | Suppressed the viability of microglia cells and increased the proportion with the M2 phenotype, thus reducd neuron loss in the injured spinal cord and promoted functional recovery after SCI. | [29] | |
| Preclinical study | Spinal cord injury in mice | Complete transection | Injection of IL-10-releasing hydrogel | Promoted the M2 macrophage/microglia phenotype, and led to neural regeneration and axon growth. | [31] | |
| Preclinical study | Spinal cord injury in mice/Experiments on microglia In vitro | Contusion/Treatment of microglia using IL-4 In vitro | Injection of PARP14 shRNA-carrying lentivirus to silence PARP14 expression/Transfected with an adenovirus PARP14 overexpression vector | PARP14 knockdown activated microglia in the spinal cord and promoted a shift from M2-polarized to M1-polarized/IL-4 treatment promoted M2 polarisation in microglia. In addition, PARP14 overexpression made microglia more prone to M2 polarization. | [33] | |
| Preclinical study | Spinal cord injury in mice | Hemisection | Transplantion of GDNF-loaded nanoparticles | GDNF-loaded nanoparticles promoted microglia M2 polarization, thereby inhibited inflammatory response at the injury site. | [37] | |
| Preclinical study | Spinal cord injury in rats | Contusion | Epidural injection of nanomedicines loaded with NGF | A strong anti-inflammatory effect was observed in the short term with a reduction of type M1 microglia. | [38] | |
| Preclinical study | Spinal cord injury in mice | Contusion | Intraperitoneal injection of melatonin | Melatonin activated the Nrf2/Keap1 signaling pathway and promoted microglia polarization towards the M2 phenotyp. | [45] | |
| Preclinical study | Spinal cord injury in rats | Complete transection | Transplantion of induced pluripotent stem cells (iPSCs) infected with lentivirus bearing an apelin expression vector | Transplantation of transfected iPSCs in situ immediately after SCI reduced polarization of M1 microglia, facilitated recovery of motor function. | [47] | |
| Preclinical study | Spinal cord injury in mice | Contusion | Intraperitoneal injection of lupenone | Lupenone enhanced the conversion of proinflammatory M1 microglial cells into anti-inflammatory M2 microglial cells, and protect against spinal cord injury by inhibiting inflammasomes. | [51] | |
| Preclinical study | Spinal cord injury in rats | Ischemia-reperfusion injury | Intraperitoneal injection of curcumin | Curcumin restrained microglia M1 activation and neuroinflammation in spinal cord tissues. | [55] | |
| Molecules | Preclinical study | Spinal cord injury in rats | Contusion | Intraperitoneal injection of GA | GA promoted recovery in SCI rats by promoting microglia M2 polarisation and inhibiting M1 polarisation. | [58] |
| Preclinical study | Spinal cord injury in rats | Contusion | Gavage administration of naringin | Naringin effectively inhibited microglial activation and expression of M1 markers in spinal cord tissues. It also elevated M2 polarization-related gene expression and significantly lowered the levels of inflammatory factors. | [62] | |
| Preclinical study | Spinal cord injury in mice | lateral compression | Injection of butylphthalide through the caudal vein | Treatment with butylphthalide could reduce pro-inflammatory cytokine release after SCI and could facilitate macrophage/microglia M2 polarization and inhibit M1 polarization after SCI. | [64] | |
| Preclinical study | Spinal cord injury in rats | Contusion | Oral treatment with glycyrrhizin | Oral treatment with glycyrrhizin promoted microglial M2 polarization and improved functional recovery after traumatic SCI. | [66] | |
| Preclinical study | Spinal cord injury in mice | Contusion | Intraperitoneal injection of parthenolide | Parthenolide promoted axonal regeneration, increased myelin reconstitution, and facilitated shift from M1 to M2 polarization of microglia. | [67] | |
| Preclinical study | Spinal cord injury in rats | Contusion | Intraperitoneal injection of riluzole | Riluzole applied in a single dose immediately post-SCI reduced the destruction of neurons, and reduced the activation of microglia M1 expression at day 1 post-SCI. | [69] | |
| Preclinical study | Spinal cord injury in rats | Contusion | Intraperitoneal injection of riluzole | Riluzole upregulated the mRNA levels of M2 markers, but downregulated that of M1 markers. | [70] | |
| Gene therapy | Preclinical study | Spinal cord injury in rats/Experiments on microglia In vitro | Contusion/Lipopolysaccharide treatment | Detection of SP1 expression in injured spinal cord/Silencing SP1 in microglia using siRNA-Sp1 | SP1 was highly expressed in SCI rats. Sp1 knockdown restrained M1 polarization of microglia and its associated inflammation. | [73] |
| Preclinical study | Spinal cord injury in rats/Experiments on microglia In vitro | Contusion/Lipopolysaccharide treatment | Detection of Htr2b expression in injured spinal cord/Inhibition of Htr2b by shRNA-Htr2b | Htr2b was highly expressed in SCI rats. In addition, inhibition of Htr2b reduced M1 polarization of microglia. | [74] | |
| Gene therapy | Preclinical study | Spinal cord injury in rats/Experiments on microglia In vitro | Contusion/Lipopolysaccharide treatment | Detection of TNIP2 expression in injured spinal cord/Transfection of microglia with TNIP2-overexpressing lentiviral | TNIP2 expression was increased during SCI in rats and that overexpression of TNIP2 inhibited M1 polarization and pro-inflammatory cytokine production in microglia. | [75] |
| Preclinical study | Spinal cord injury in mice | Contusion | Injection of PARP14 shRNA-carrying lentivirus | PARP14 knockdown promoted microglia M1 polarization. | [33] | |
| Exosomes | Preclinical study | Spinal cord injury in rats | Contusion | Injection of adipose-derived MSCs exosomes via tail veins | Adipose-derived MSCs exosomes inhibited the expression of both inflammatory factors in the spinal cord tissues and M1 microglia, promoted the expression of M2 microglia. | [80] |
| Preclinical study | Spinal cord injury in rats | Contusion | Injection of miR-216a-5p-overexpressed BMSCs-exosomes via tail veins | The injection of miR-216a-5p-overexpressed BMSCs-exosomes improved locomotor performance, while inhibiting neuronal apoptosis and microglia M1 polarization. | [81] | |
| Preclinical study | Spinal cord injury in rats | Contusion | Injection of Schwann cell-derived exosomes via tail veins | Schwann cell-derived exosomes could attenuate the inflammation in SCI rats by suppressing M1 polarization and stimulating M2 polarization. | [82] | |
| Preclinical study | Spinal cord injury in mice | Hemisection | Transplantion of hydrogels loaded with BMSC-exosomes | Exosomes-loaded hydrogels modulate microglial M2 polarization. | [83] | |
| Cell therapy | Preclinical study | Spinal cord injury in rats | Complete transection | Transplantion of NSCs | NSCs reduced the number of infiltrated immune cells, biased microglia towards a regenerative M2 phenotype. | [88] |
| Preclinical study | Spinal cord injury in rats | Contusion | Transplantion of OECs | OECs modulated microglial polarization from the M1 to M2 phenotype. | [89] | |
| Preclinical study | Spinal cord injury in rats | Contusion | Transplantion of MSCs | MSCs increased numbers of M2 microglia and decreased numbers of M1 microglia. | [90] | |
| Biomaterials | Preclinical study | Experiments on microglia In vitro | None | Treatment of microglia with nanoparticles incorporating carbohydrate antigens | Resting microglia exposed to nanoparticles can be activated and polarized toward M2 state. | [97] |
| Biomaterials | Preclinical study | Spinal cord injury in rats | Contusion | Injection of gold nanoclusters loaded with berberine via tail veins | Nanoclusters reduced M1 protein marker CD86, increased M2 protein marker CD206, reduced inflammation and apoptotic cytokines. | [98] |
| Preclinical study | Spinal cord injury in mice | Contusion | Treatment with a biocompatible hydrogel loaded with fat extract | The composite promoted the polarization of microglia from an inflammatory M1 phenotype to an anti-inflammatory M2 phenotype. | [100] | |
| Preclinical study | Spinal cord injury in rats | Complete transection | Treatment with fibronectin hydrogel containing lycium barbarum oligosaccharide and nasal mucosa-derived MSCs | The hydrogel possesses a synergistic effect on M2 polarization of microglia. | [101] | |
| Physical therapy | Preclinical study | Spinal cord injury in rats | Clip-compression | Transcranial direct current stimulation | Transcranial direct current stimulation reduced the proportion of the M1 phenotype of microglia and increase the proportion of the M2 phenotype. | [106] |
| Preclinical study | Spinal cord injury in rats | Complete transection | Electroacupuncture | Electroacupuncture improved BBB scores, inhibited the proportion of M1 microglia. | [107] |
TGF-
QY and ZC conducted a literature review, wrote the manuscript and designed the figures and tables. XL, SL, PL, YR and JL conducted literature searches and assisted in writing. XH and WL was responsible for the conceptualization, supervision and reviewing the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research was funded by National Natural Science Foundation of China (No. 81771323), Natural Science Foundation of Guangdong Province of China (No. 2021A1515010722), and Natural Science Foundation of Shenzhen Municipality (No. JCYJ20190813112401660).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.