1 The Second Clinical Medical College, Zhejiang Chinese Medicine University, 310053 Hangzhou, Zhejiang, China
2 Department of Neurology, Zhejiang Hospital, 310025 Hangzhou, Zhejiang, China
3 Department of Neurology, Zhejiang Chinese Medicine and Western Medicine Integrated Hospital, 310020 Hangzhou, Zhejiang, China
4 Department of Neurology, Second Affiliated Hospital, School of Medicine, Zhejiang University, 310027 Hangzhou, Zhejiang, China
5 Department of Neurology, First Affiliated Hospital, School of Medicine, Zhejiang University, 310027 Hangzhou, Zhejiang, China
6 Hospital of the Chinese People's Liberation Army Unit 71217, 265200 Laiyang, Shandong, China
†These authors contributed equally.
Abstract
Alzheimer’s disease (AD) is recognized as the leading cause of dementia, imposing a significant economic toll on society. Despite the emergence of novel therapeutic approaches for AD, their efficacy and safety mandates further validation through rigorous clinical trials. In this context, hypertension (HTN) has garnered considerable attention as an amendable risk factor for AD. Research indicates that hypertension during midlife is associated with an elevated risk of AD in later years, influencing both the onset and progression of the disease. Nevertheless, the relationship between AD and hypertension in the later stages of life remains a subject of debate. Moreover, the consequences of blood pressure reduction on cognitive function, along with the optimal pharmacological interventions and therapeutic thresholds for hypertension, have emerged as pivotal areas of inquiry. This review synthesizes findings on epidemiology, neuroimaging, and biomarkers, and the effects of antihypertensive medications to elucidate the link between hypertension and cognitive performance. We particularly investigate how hypertension and AD are related by plasma sulfide dysregulation, offering possible indicators for future diagnosis and therapy.
Keywords
- Alzheimer's disease
- hypertension
- biomarkers
- neuroimaging
- pathophysiology
Alzheimer’s disease (AD), recognized as the primary cause of dementia, imparts a
substantial economic strain on society [1, 2]. Extensive research has highlighted
several interconnected neurodegenerative processes, leading to the identification
of common pathological features in AD, notably beta-amyloid plaques and
neurofibrillary tangles (NFT) within nerve cells [3, 4]. The accumulation of
abnormal forms of amyloid beta (A
Hypertension (HTN) is one of the most prevalent chronic diseases, affecting as many as one billion adults. Currently, around 75% to 85% of people over the age of 65 years have hypertension [11, 12]. HTN is also the most preventable and modifiable risk factor for cognitive impairment stemming from neurodegenerative diseases, including AD, stroke, Parkinson’s disease (PD), and small cerebral vessel disease (SCVD) [2].
Interestingly, AD and HTN are frequent comorbidities among the elderly [11, 13]. Current evidence indicates that HTN not only affects neurobiological substrates but also plays a role in the clinical progression of cognitive impairment and dementia. Further investigation into the mechanisms underlying this impact could provide valuable insights for developing more effective strategies to prevent and diagnose AD.
Neuropsychological testing is a potent tool for measuring cognitive functioning and can capture even the most subtle cognitive changes to uncover evidence of cognitive dysfunction [14]. Cognitive decline (cognitive functions such as memory, thinking, and reasoning may decline over years to decades to a greater extent than would be expected due to age alone) is assessed by longitudinally comparing the degree of cognitive decline in a patient. It does not require the patient to meet definitive criteria for dementia or mild cognitive impairment (MCI). Studies on cognitive decline have helped to identify the danger of preclinical cognitive impairment in advance, providing a simpler technique of testing cognitive function and allowing for a more accurate assessment of the relationship between hypertension and cognitive performance [14, 15]. However, clinical diagnosis of MCI or dementia is equally crucial, as both diagnoses have far-reaching public health consequences [16]. In the case of hypertension, current evidence from epidemiologic studies has found that hypertension is strongly associated with cognitive decline [14], MCI [17], and dementia [2, 18, 19].
Prospective cohort studies may be preferable to cross-sectional studies when examining the causal relationship between hypertension and AD, so we focused primarily on evidence from longitudinal cohort studies. While the correlation between hypertension and cognitive impairment was first established in patients with stroke and Vascular Cognitive Impairment (VCI), subsequent investigations have determined that cognitive decline attributed to hypertension could also occur in the absence of stroke [20]. Multiple studies have found that there may be an age-dependent relationship between hypertension and the risk of AD, and that the association between hypertension and AD at different ages of onset is complex and variable [21, 22].
Several studies have arrived at more consistent conclusions about the
relationship between midlife hypertension and the risk of AD. In a meta-analysis
of prospective cohort studies, middle-aged hypertension was found to be more
strongly associated with AD [19]. In the Honolulu-Asia Aging Study, untreated
hypertension levels in midlife were found to be associated with an increased risk
of AD 25 years later, and it was suggested that hypertension may be an important
mediator or pathologic contributor to AD [23]. Another study with 21 years of
follow-up found that elevated systolic blood pressure (SBP) in midlife was an
independent risk factor for AD in later life [24]. Moreover, the Atherosclerosis
Risk in Communities (ARIC) study revealed that hypertension and prehypertension
among middle-aged individuals confer a comparable risk of dementia, with both
groups displaying a higher risk relative to normotensive individuals [14]. A
national cohort study in Korea also found an increased risk of AD with SBP
As for the effect of late-life hypertension on AD there is currently
inconsistency. A few studies have found late-life hypertension to be associated
with AD. In the Kungsholmen Project, a community-based cohort of 75-year-olds
followed for 6 years found that subjects with an SBP
Collectively, the above results highlight the potential detrimental impacts of hypertension acquired during midlife. Nevertheless, due to the absence of a gold standard for stratifying dementia subtypes, the subset of studies investigating the correlation between hypertension and specific dementia subtypes might inadvertently complicate the assessment of hypertension’s role in other forms of dementia and cases with mixed underlying causes. Therefore, more rigorous criteria are needed to aid in the diagnosis of dementia subtypes. Furthermore, age-stratified and long-term follow-up studies focused on hypertension could serve as valuable tools for enhancing our comprehension of the intricate connection between hypertension and AD.
Research suggests that HTN may have a global impact on cognitive performance and in global cognitive assessments, such as the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA), people with vascular risk including hypertension score lower. Individuals with more factors also score lower, which is largely influenced by difficulties related to attention (in the MMSE assessment) and visuospatial executive functioning (in those with lower MoCA scores) [31]. Broader neuropsychological assessments suggest that hypertension most significantly affects executive function [16], motor speed, and attention [32, 33]. These cognitive domains are often associated with subcortical disorders such as common vascular diseases or pure vascular dementia (VaD) [34].
The pattern of cognitive impairment in hypertension-associated dementia is
complicated by potential overlapping etiologies and the high prevalence of mixed
dementia (MD). Although memory impairment is considered a typical feature of AD
compared with VaD [35, 36], it has been found that situational memory is
significantly affected in cognitively impaired hypertensive participants and is
associated with elevated A
Studies focusing only on specific cognitive domains are therefore prone to errors in findings due to misdiagnosis or the effect of difficulties in determining the exact cause throughout an individual’s lifetime. Further incorporation of biomarkers (including serology, cerebrospinal fluid, and imaging) can help to better characterize the disease [38].
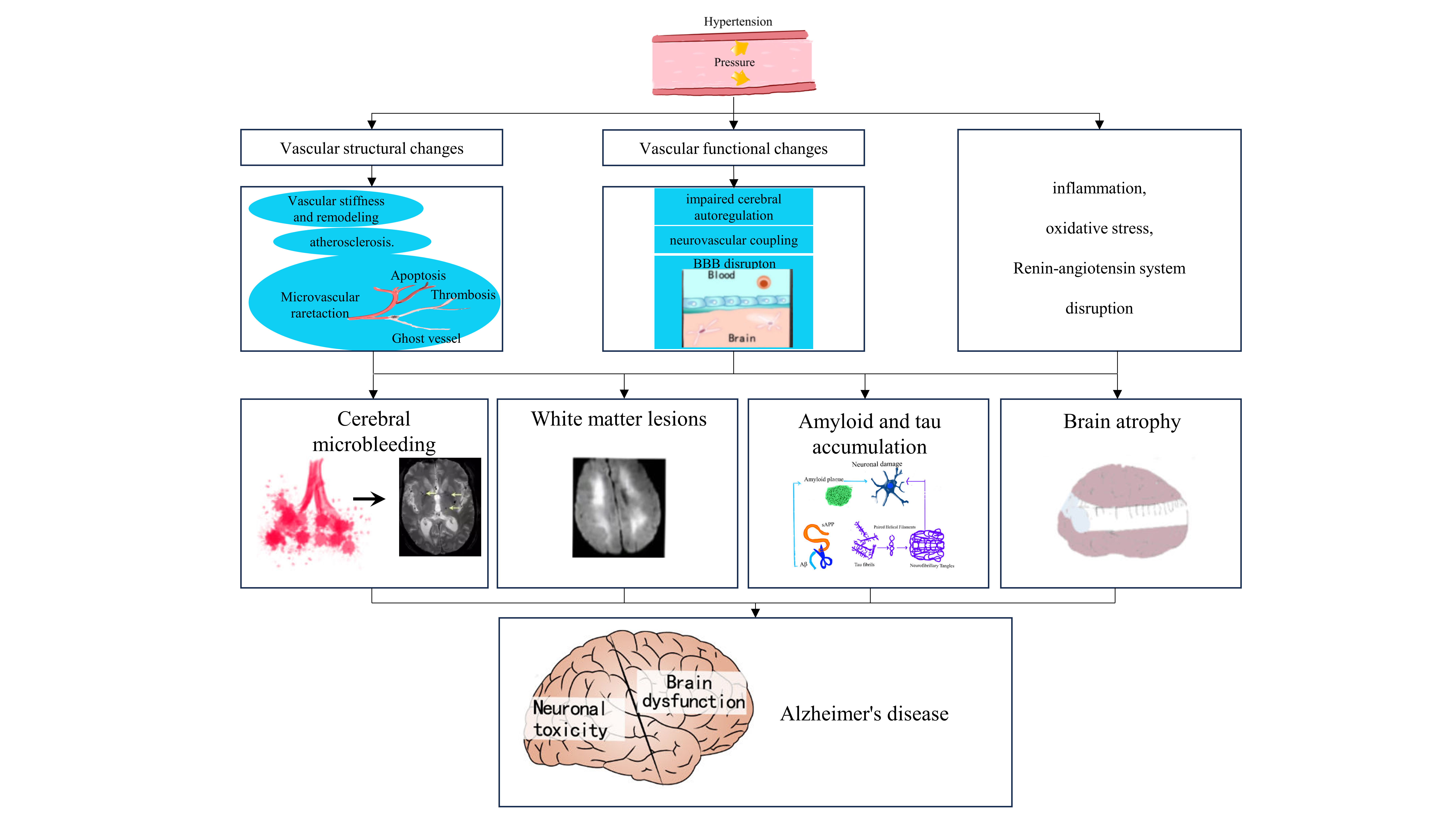
The potential mechanisms linking hypertension and AD are complex and multifaceted. Current evidence suggests that a synergistic interplay among various pathological factors may underlie the cognitive impairments associated with hypertension and there are extensive detailed reviews on this topic [39]. Below, we provide a summary of the key points and an overview of the current evidence using neuroimaging markers to elucidate the connection between hypertension and AD (Fig. 1) [40].
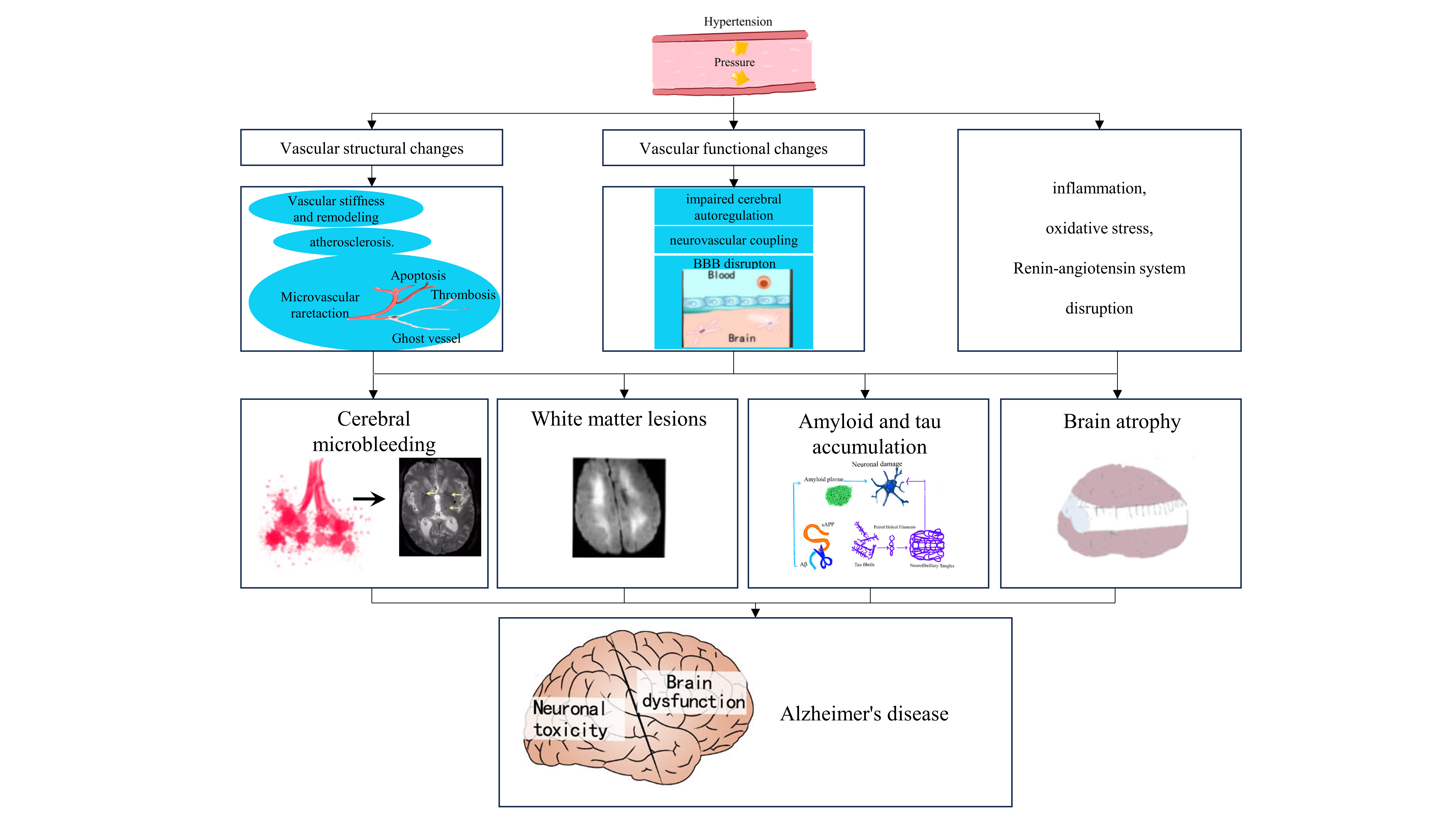 Fig. 1.
Fig. 1.
Common imaging features and potential mechanistic links between
hypertension and Alzheimer’s disease (AD). Hypertension exerts a multifaceted
influence on the brain, manifesting through alterations in the structure and
function of the cerebrovasculature, perturbations in the renin-angiotensin
system, the emergence of inflammation, and the induction of oxidative stress.
Such pathological processes can lead to the development of cerebral white matter
lesions, brain atrophy, microhemorrhages, and the accumulation of pathologic
proteins associated with AD. These conditions are discernible through
neuroimaging technologies and are recognized for their potential to affect the
initiation or advancement of AD. BBB, blood-brain barrier; A
The relationship between hypertension and brain structure and functions is not completely understood and current evidence suggests that several pathological factors can interact [41]. Hypertension can produce vascular and functional changes in large and small cerebral vessels in the brain, and small cerebral vessels and arterioles are more vulnerable to the mechanical stress associated with hypertension [42]. It is relevant to highlight the strong relationship between arterial hypertension and cerebral small vessel disease (cSVD); the characteristic findings of cSVD in magnetic resonance imaging (MRI) include lacunar infarcts, white matter hyperintensities (WMH), cerebral microbleeds (CMB), enlarged perivascular spaces, and brain atrophy [42]. Conversely, chronic hypertension induces a rightward shift in the autoregulatory curve and, consequently, an increment of vulnerability to sudden changes in blood pressure, resulting in ischemia or increased risk of brain hemorrhage.
The modified structure of the perivascular space, often observed in conditions
such as hypertension, heightens the possibility that the perivascular and
paravascular clearance systems, which are vital for the removal of waste products
from the brain, may be disrupted, potentially contributing to white matter injury
[43, 44]. Specifically, in hypertension, the perivascular spaces become enlarged
and distorted [43, 45], a change that could hinder the effective elimination of
neurotoxic metabolic byproducts. This is significant as hypertension is also
linked to the accumulation of A
In conclusion, vascular damage caused by hypertension results in brain
dysfunction due to hypoxia-ischemia and increased production of A
Hypertension impairs the structural and functional integrity of the cerebral microcirculation, promoting microvascular thinning, cerebral microvascular endothelial dysfunction, and neurovascular uncoupling, as well as impairing cerebral blood supply [39]. This insufficient blood supply, in turn, triggers metabolic irregularities within the brain parenchyma. Consequently, there is an increased activity of beta-secretase 1, an enzyme responsible for cleaving APP into beta-amyloid, thus promoting the formation of amyloid plaques [46]. HTN also increases the pressure in the lumen of cerebral vessels, leading to increased formation of reactive oxygen species and weakening of the vessel wall, resulting in disruption of the blood-brain barrier (BBB). This allows the contents of the plasma to enter the central nervous system (CNS) thereby triggering neuroinflammation. Mouse model studies have effectively demonstrated that neuroinflammation is a pivotal link connecting hypertension and AD. Additionally, BBB disruption impedes the efficient clearance of beta-amyloid, which further promotes its deposition. Taken together, these series of events significantly contribute to both the onset and progression of AD [20].
While the heightened risk of AD associated with hypertensive patients is evident and the impact of hypertension on cognition is relatively well-established, a degree of uncertainty remains regarding whether HTN directly influences AD neuropathology or simply serves as a concurrent factor contributing to cognitive impairment and dementia [47].
An autopsy study reported that midlife hypertension was associated with more
senile plaques and NFT pathology at death, showing that the apolipoprotein E
(APOE)
Several epidemiologic and clinicopathologic studies have reported an association between hypertension and AD. However, a challenge to the interpretation of the relationship between hypertension and AD is the substantial lag in time from the start of hypertension studies to the pathologic diagnosis of AD. The increasing popularity of amyloid PET imaging and structural MRI provides an opportunity to better understand the role of hypertension and brain pathology in vivo. In population-based or special (enriched) cohort studies, large sample sizes are needed to distinguish the separate roles of SBP, DBP, pulse pressure, mean arterial pressure, and carotid-femoral pulse wave velocity (PWV) for each imaging feature. Longitudinal studies that prioritize the assessment of perfusion deficit, white matter changes, cortical volume changes, microbleeds, and amyloid accumulation are needed to understand their interrelationships. Future imaging techniques that can detect the involvement of specific targets will be needed to understand which cells or cellular components are the sites of initial pathogenic injury in hypertension. The cell types and endpoints most suitable for treatment remain undetermined.
Although most of the current research has concluded that a direct association
between hypertension and tau is lacking [60], a recent study from China has
introduced a novel perspective. The study reported a noteworthy link between a
history of hypertension and elevated systolic blood pressure with tau levels in
the CSF, specifically in individuals under the age of 65 years [53]. Furthermore,
other studies have suggested that hypertension may influence tau levels under
certain conditions. For instance, individuals in the hypertension group who are
carriers of the APOE
Hydrogen sulfide (H
The recent availability of imaging and biochemical biomarkers of AD has provided the opportunity to investigate the relationship between AD pathology and hypertension [77, 78, 79, 80]. From the beginning of the 20th century to the present, functional MRI (fMRI) has increased in popularity [81], as it has been shown that brain functional connectivity has been altered in hypertensive patients who show no signs of macroscopic damage on structural MRI [82, 83]. At the same time, fMRI signals are generated by neurovascular coupling, making it sensitive to the effects of hypertension and more favorable for observing the effects of hypertension on brain function.
Several studies have highlighted the structural brain alterations that are characteristic of AD, which include diminished hippocampal volume, reduced cortical thickness, and disruptions in the microstructure of white matter fiber tracts [2, 82]. These suggest the presence of alterations in functional brain activity as well as in brain network connectivity, particularly within the default network (DMN) [84, 85]. Shah et al. [86] previously observed distinctive cerebral white matter lesions in hypertensive patients. They further uncovered a significant correlation between cerebral white matter load and DMN regions by studying the relationship between cerebral white matter load and functional cerebral networks in hypertensive patients, revealing that greater white matter lesion volumes correlated with lower functional connectivity (reflects the functional relationship between brain regions, FC) in the DMN [87]. Moreover, this was accompanied by poorer performance on cognitive tests, suggesting that WMH could be a potential mechanism through which hypertension impacts the progression of cognitive decline in AD.
Another study delved into the interaction between hypertension and individuals
carrying the APOE
Collectively, these studies underscore the structural and functional transformations that hypertension induces in the brain, although there is currently a lack of convincing large cohort prospective studies exploring the causal relationship between hypertension and cognitive function.
Analyses of structural brain studies conducted on individuals with both AD and hypertension have highlighted distinct patterns of atrophy when compared with AD patients without hypertension. Notably, in the hypertensive group, significant atrophy was observed primarily in the temporal lobe, frontal lobe, and cingulate gyrus [88, 89, 90]. These findings imply that hypertension might play a role in the initiation and progression of AD by influencing critical brain regions. While research exploring brain function in individuals with combined AD and hypertension is more limited and currently exhibits some variation due to diverse analytical approaches, it remains crucial to acknowledge the impact of hypertension on resting-state brain function in AD patients, especially the disruption of the DMN region [82, 84]. However, some literature has reported enhanced the posterior cingulate cortex (PCC) functional connectivity in AD/MCI patients, with areas such as the ventral medial prefrontal, bilateral dorsolateral frontal, and left middle cingulate gyrus. Moreover, heightened connectivity has been noted between the left inferior parietal lobule and the PCC in AD patients with hypertension [91, 92]. These findings are indicative of a potential compensatory mechanism, suggesting that these regions might enhance their function in response to early-stage AD/MCI-associated damage to other brain areas. However, other studies did not identify this compensatory phenomenon. Variations in sample sizes and analytical methodologies are factors that might contribute to these discrepancies. This area of research currently features substantial gaps, and further studies are essential to provide deeper insights and more conclusive findings.
The introduction of multimodal imaging techniques has helped us to better understand the pathogenesis shared in the imaging features of hypertension and AD. Notably, these shared features include WMH, cerebral microhemorrhages, and atrophy. Meanwhile, diffusion tensor imaging is another useful imaging technique developed for assessing the anatomic integrity of white matter [93]. Some parameters of the microstructural abnormalities derived from diffusion tensor imaging (DTI) have been reported to help differentiate between dementias. Some studies have found that compared with conventional MRI, DTI may provide a more objective method for the differential diagnosis of VaD and AD disease patients who have only mild white matter alterations on T2-weighted imaging. Compared with VaD patients, AD patients had lower fractional anisotropy (FA) values in the anterior frontal lobe, temporal lobe, hippocampus, inferior-fronto-occipital fascicles (IFOF), genu of the corpus callosum (GCC), and the cingulate fasciculus (CF); and higher apparent diffusion coefficient (ADC) values in the temporal lobe and hippocampus [94]. Parahippocampal tracts were found to be mainly affected in AD, while VaD showed more spread white matter damages associated with thalamic radiation involvement. The genu of the corpus callosum was predominantly affected in VaD, while the splenium was predominantly affected in AD, revealing the existence of specific patterns of alteration useful in distinguishing between VaD and AD [95]. Based on the above evidence, cerebrovascular dysfunction, including HTN, is associated with the accumulation of AD pathology. The “vascular dysregulation hypothesis” proposes that an imbalance between blood flow-based substrate supply and brain energy demand caused by vascular risk factors such as hypertension exacerbates AD and ADRD [96, 97]. The current view is that cerebrovascular dysfunction occurs early in ADRD and therefore may be a potential diagnostic marker and target for intervention. Cerebrovascular dysfunction induces pathophysiology that may contribute to the common symptoms of AD, including cerebral atrophy, cortical thinning, and white matter deterioration. These can be observed by multimodal magnetic resonance, facilitating exploration of the potential link between hypertension and AD [76].
DTI, fMRI, and PET are three imaging techniques widely used in neuroscientific research and clinical diagnostics, and despite their unique advantages, they have some limitations. DTI is primarily used to depict white matter fiber bundles in the brain, but its ability to image grey matter and subcortical structures is limited and it is not sensitive enough for certain types of white matter lesions. fMRI can non-invasively measure brain activity, but its signal can be affected by a variety of physiological and operational factors, such as vascular saturation, the complexity of the task design, and the level of participant cooperation, which can lead to challenges in data interpretation. PET assesses metabolic and neurochemical activity in the brain using radiotracers, and although it provides detailed information about brain function, it carries radiation risks, is costly, and often requires special radiopharmaceuticals, which limits its use in some situations. The introduction of multimodal imaging techniques has helped us to better understand the pathogenesis shared in the imaging features of hypertension and AD.
The pathology of AD is indeed intricate, encompassing not only the accumulation
of A
NFL, a biomarker associated with significant axonal injury, has gained attention
due to its increased levels in the CSF of individuals with AD [91]. Recent
studies have facilitated the analysis of NFL not only in the CSF but also in the
blood (serum and plasma), offering a more accessible means of monitoring [104].
An intriguing link has been suggested between NFL levels and white matter
alterations in AD [87, 105, 106]. A recent study by Walsh et al. [99]
found a significant positive correlation between NFL and WMH volume in a
population with different cognitive impairments and a large proportion of
age-related, independent vascular risk factors (including hypertension). However,
further analysis by the same research group found that the combination of
hypertension and the APOE
Cerebral microhemorrhages have traditionally been attributed to two main causes:
cerebral amyloid vascular degeneration and hypertension [98, 108].
Hypertension-related microhemorrhages are typically found deep within the brain
parenchyma, often localized in regions such as the internal capsule. Notably,
microhemorrhages in cortical sites are more often associated with the presence of
the APOE
The report found that patients with vascular or MD presented with a more severe load of white (periventricular and deep) and grey matter lesions compared with AD. The high number of juxtacortical microhemorrhages observed in the group with MD suggests that cerebral amyloid angiopathy (CAA) may be a relevant pathological finding within this group [111]. Microhemorrhages may cause cognitive impairment by affecting executive function, information processing, and memory [112]. However, the mechanisms by which microhemorrhages alter cognitive function remain to be determined, and it is currently believed that the direct cognitive effects of microhemorrhages are related to localized damage or dysfunction of adjacent brain tissue. Alternatively, microhemorrhages may be a more general marker of the severity of small-vessel pathology associated with CAA, modulating altered vascular supply and reduced cortical function [113].
Indeed, neuropathological studies have recognized CAA as a major cause of MD in
combination with Alzheimer’s or Lewy body pathology [114, 115]. Furthermore, CAA
has been significantly associated with subcortical white matter damage,
particularly in APOE
Hypertension is currently thought to cause brain atrophy [117, 118], but the exact mechanism remains elusive [118]. Part of the reason is that hypertension-mediated WMH leads to a reduction in brain volume due to white matter thinning. Other potential factors include reduced brain volume due to brain tissue destruction caused by cerebral microinfarcts, cerebral atherosclerosis, and impaired clearance of abnormal proteins in AD patients [119].
A greater number of periventricular lesions, deep white matter lesions, deep grey matter lesions, and enlarged perivascular spaces was observed in vascular dementia compared with AD, while MD showed a significantly greater number of periventricular lesions, deep white matter lesions, deep gray matter lesions, and deep and juxtacortical microhemorrhages. Comparing VaD and MD, VD showed a higher number of perivascular spaces in the basal ganglia and centrum semiovale, while MD showed more deep and juxtacortical microhemorrhages. Gray and white matter lesions predominate in vascular and MD, while deep and juxtacortical microhemorrhages predominate in MD, suggesting that cerebral amyloid angiopathy may be the main underlying pathology in cases of mixed-based cognitive decline.
In several observational studies, the use of antihypertensive medication has
been correlated with a reduced decline in cognitive abilities. In the
ARIC study, participants who were on
antihypertensive medications experienced a 20-year cognitive decline equivalent
to that of the prehypertensive group (higher than those with normal blood
pressure but lower than those with untreated hypertension) [14]. The Northern
Manhattan Study found that SBP was negatively associated with cognitive function
in the elderly, both cross-sectionally and longitudinally. Antihypertensive
treatment eliminated the longitudinal negative correlation between SBP and the
functions of processing speed and visual motor integration [120]. In the
Epidemiology of Vascular Aging (EVA) study group, treated hypertension was
associated with less cognitive decline over a four-year period compared with
untreated hypertension [121]. Another study indicated that in patients with
various forms of dementia, including AD, who received permanent correction of
hypertension, the cognitive decline could be alleviated [122]. The results of the
Systolic Blood Pressure Intervention Trial to Reduce Memory and Cognition in
Hypertension (SPRINT MIND) study support intensive SBP reduction (goal:
Lowering blood pressure may lead to a lower risk of dementia or cognitive impairment, as suggested by several recent meta-analyses, but there is no evidence that a specific class of antihypertensive drugs is more effective than any other in reducing dementia risk. These findings challenge the hypothesis that renin-angiotensin system blockade may be more likely to preserve cognition [126, 129, 130, 131]. The limitations of some of the methods described above are worth noting. Subgroup and meta-regression analyses from systematic evaluations may be prone to ecological bias, and the use of randomized controlled trial (RCT) data rather than observational data may be more appropriate for addressing some challenging and unresolved questions of interest.
The above studies consider the role of antihypertensive drug therapy, although all of them are susceptible to some indication bias: individuals prescribed and taking antihypertensive drugs are different from those not taking antihypertensive drugs; beyond that, many aspects are open to statistical adjustment. Clinical trials would therefore be an ideal forum to answer this question, but given that the evidence reviewed above suggests that consideration of the relationship between hypertension and dementia is strongest in middle age, decades before the development of dementia, and that clinical trials cannot randomize and follow participants for that long, some type of long-term observational study design is needed to take into account these lifespan considerations. These studies would also allow for consideration of age at treatment or duration of treatment, which may be beyond what clinical trials currently allow.
Hypertension and AD are two major health concerns within the global aging population, showing high rates of co-morbidity. Hypertension has been shown to increase the risk of AD by two-fold. Moreover, when hypertension and AD co-occur, their combined impact appears to magnify. Structural and functional changes induced by hypertension play a substantial role in elucidating the pathophysiological connection between these two diseases. The integration of multiple biomarkers in scientific research has provided valuable insights into the synergistic effects arising from the interplay of hypertension and AD. While studies investigating brain function within the context of AD and hypertension co-morbidity are relatively scarce, ongoing and future research will likely aim to unravel this relationship in greater detail. In the absence of definitive therapeutic approaches for AD, evidence strongly indicates that achieving treatment targets for hypertension holds significant importance since this not only aids in reducing the incidence of cardiovascular disease but also plays a crucial role in curbing the occurrence of AD.
Although the body of knowledge on hypertension and cognitive outcomes, dementia, and AD has expanded considerably in recent years, several key questions remain. The answers to some of the questions listed below are critical to better understand how hypertension affects cognitive function and to best recommend preventive and management strategies, suggesting some possible paths for future research. (1) Is hypertension-induced neurovascular dysfunction sufficient to cause cognitive impairment? Which are the best surrogate endpoints or biomarkers for measuring brain or cognitive benefit in hypertension treatment? (2) Is lowering blood pressure alone sufficient to maintain cognitive performance? Should other dementia risk factors be considered? When is it necessary to start antihypertensive therapy to reduce the risk of cognitive impairment? Which class of antihypertensive medications is most effective in preventing cognitive impairment?
SCY, LL and GLF equally contributed to the primary study design of this review. SCY, LYZ, and SQ equally performed the literature search and wrote the manuscript. STW, JJW, QLL, LC, YJM, TYZ, YXZ and PPG were involved in conceptualizing and designing the manuscript, data collection, or data analysis and interpretation, and added important intellectual content to the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This work was supported by the Zhejiang Science and Technology Project [2023RC120] and Zhejiang Provincial Natural Science Foundation [LQ19H090006] and Zhejiang Provincial Health Commission [NO. 2023KY006].
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.