1 Department of Neurosurgery, Paracelsus Medical University, 90471 Nuremberg, Germany
2 Department of Neurology, Paracelsus Medical University, 90471 Nuremberg, Germany
Abstract
Background: Non-invasive brain mapping using navigated transcranial
magnetic stimulation (nTMS) is a valuable tool prior to resection of malignant
brain tumors. With nTMS motor mapping, it is additionally possible to analyze the
function of the motor system and to evaluate tumor-induced neuroplasticity.
Distinct changes in motor cortex excitability induced by certain malignant brain
tumors are a focal point of research. Methods: A retrospective
single-center study was conducted involving patients with malignant brain tumors.
Clinical data, resting motor threshold (rMT), and nTMS-based tractography were
evaluated. The interhemispheric rMT-ratio (rMT
Keywords
- navigated transcranial magnetic stimulation
- nTMS motor mapping
- neuronavigation
- neurooncology
- functional imaging
- glioma
In the preoperative diagnostic workflow of brain tumor patients, navigated transcranial magnetic stimulation (nTMS) has emerged to a valuable tool for non-invasive mapping of motor and language function [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18].
Using nTMS motor mapping data, the clinical course of patients with motor-eloquent malignant brain tumors can be improved significantly. Preoperative visualization of motor eloquent cortex results in low rates of permanent surgery-related motor deficits while achieving a high extent of resection [17, 19, 20, 21, 22, 23, 24, 25, 26].
In the routine clinical workflow, the patient-individual motor cortex is mapped using nTMS and the cortical muscle representations are used as a seeding region for nTMS-based diffusion tensor imaging (DTI)-fibertracking (nTMS-based-DTI-FT) of the corticospinal tract (CST) [14, 21, 27, 28, 29, 30, 31].
Analysis of both cortical and subcortical motor areas is correlated with postoperative outcome after resection of the lesion. This makes nTMS-based DTI-FT a diagnostic and prognostic tool in neurosurgery [8, 9, 21, 22, 26, 32, 33]. Key parameters of nTMS-based risk assessment are the resting motor threshold (rMT) and the interhemispheric rMT-ratio of the tumorous and the non-affected hemisphere [8, 9, 32]. This ratio is considered pathological if it exceeds 110% or falls below 90%. A pathological interhemispheric rMT-ratio is associated with an increased risk of permanent surgery-related motor deficits [8]. Furthermore, the distance between the lesion and the CST (lesion-to-tract-distance – LTD) correlates inversely with the risk for surgery-related paresis [8, 9, 21, 32, 34].
Apart from risk stratification and preoperative visualization of motor cortex and motor pathways, nTMS mapping serves as a tool to evaluate motor function and motor cortex reorganization in brain tumor patients [6, 35, 36, 37, 38, 39, 40, 41, 42, 43]. nTMS data correspond with tumor grading and motor status: high grade gliomas are associated with higher rates of pathological rMT-ratios of upper and lower limb muscles compared with lower grade gliomas, indicating a measurable distraction of the motor system induced by more aggressive tumors [37, 38, 39]. Additionally, edema, intake of anticonvulsive medication, and tumor location significantly influence the rMT of the tumorous hemisphere [36, 43]. To the best of our knowledge, there are no data regarding the influence of different tumor entities, such as low grade gliomas, high grade gliomas and cerebral metastases, on cortical excitability and interhemispheric motor threshold ratio using nTMS. Consequently, we conducted this study to add further information to this phenomenon of alterations in cortical excitability and nTMS-based-DTI-fibertracking in patients with brain tumors.
The study design was approved by the institutional review board of Paracelsus Medical University Nuremberg (IRB-2024-10). The study was conducted in accordance with the Declaration of Helsinki. Informed consent was waived due to the retrospective study design.
Patients that were treated surgically between July 2021 and March 2024 for histologically confirmed glioma or metastases and received navigated transcranial magnetic stimulation for mapping the motor cortex were included into this retrospective study. Inclusion criteria were age over 18 years and presence of one single supratentorial lesion. Patients with multiple lesions or infratentorial lesions were excluded from the analysis. Patients with motor deficits that were related to a previous surgery were excluded.
Apart from demographic data, intake of anticonvulsive medication, tumor histology, and the presence of motor deficits were registered. All motor deficits were considered to be induced by the tumor.
Bilateral nTMS motor mapping using the Nexstim NBS 5 system (Nexstim Oy, Helsinki, Finland) was performed in each patient as part of the preoperative diagnostic workflow. All examinations were conducted by one investigator (T.E.). The workflow was in accordance with published consensus guidelines [44]; however, some modifications were made to the mapping procedure. These are outlined in the following.
The patient’s head was co-registered with the magnetic resonance (MR) reference exam. Pre-glued surface electrodes (Ambu Neuroline 700, Ambu, Ballerup, Denmark) were attached to the target muscles (abductor pollicis brevis (APB), abductor digiti minimi (ADM), one forearm muscle, and the tibialis anterior (TA) and gastrocnemius muscle (GC)) in a belly-tendon-fashion. The ground electrode was placed at the patient’s elbow. In all examinations, the non-affected hemisphere was mapped first.
The first step of the mapping procedure was to determine the hand motor hotspot
within the primary motor cortex. The spot which elicited the greatest motor
evoked potential (MEP) amplitudes during the first round of mapping was
considered as the motor hotspot. Stimulations were repeated at that spot to
confirm the location of the hotspot. The next step was the determination of the
rMT using the NBS 5’s inbuilt motor threshold hunting algorithm. The stimulation
intensity which elicited MEPs with amplitudes
The contralateral hemisphere was mapped analogously.
The interhemispheric rMT-ratio was calculated for each extremity as
(rMT
All examinations were evaluated post-hoc and motor positive spots (MEPs with a
peak-to-peak amplitude
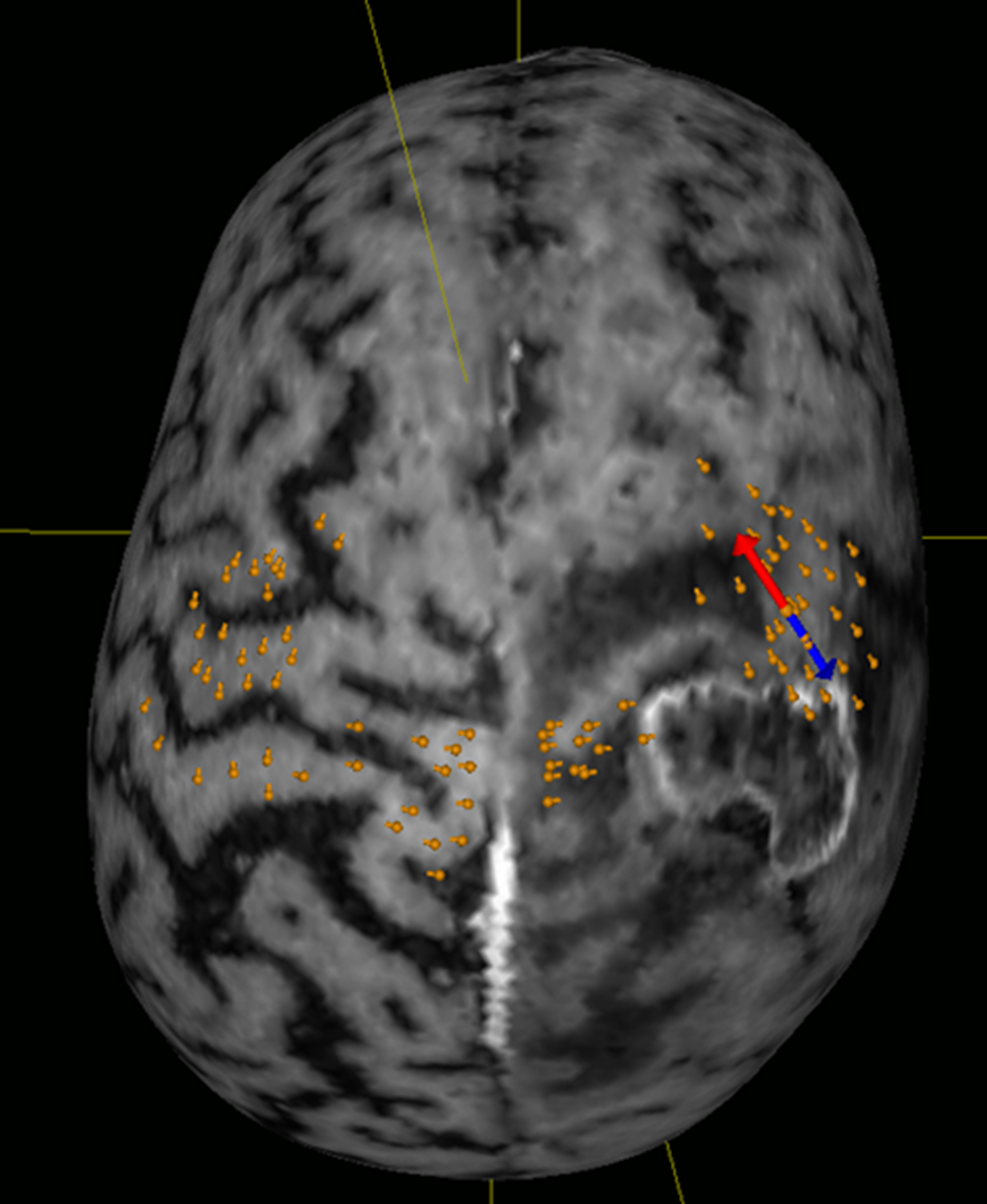
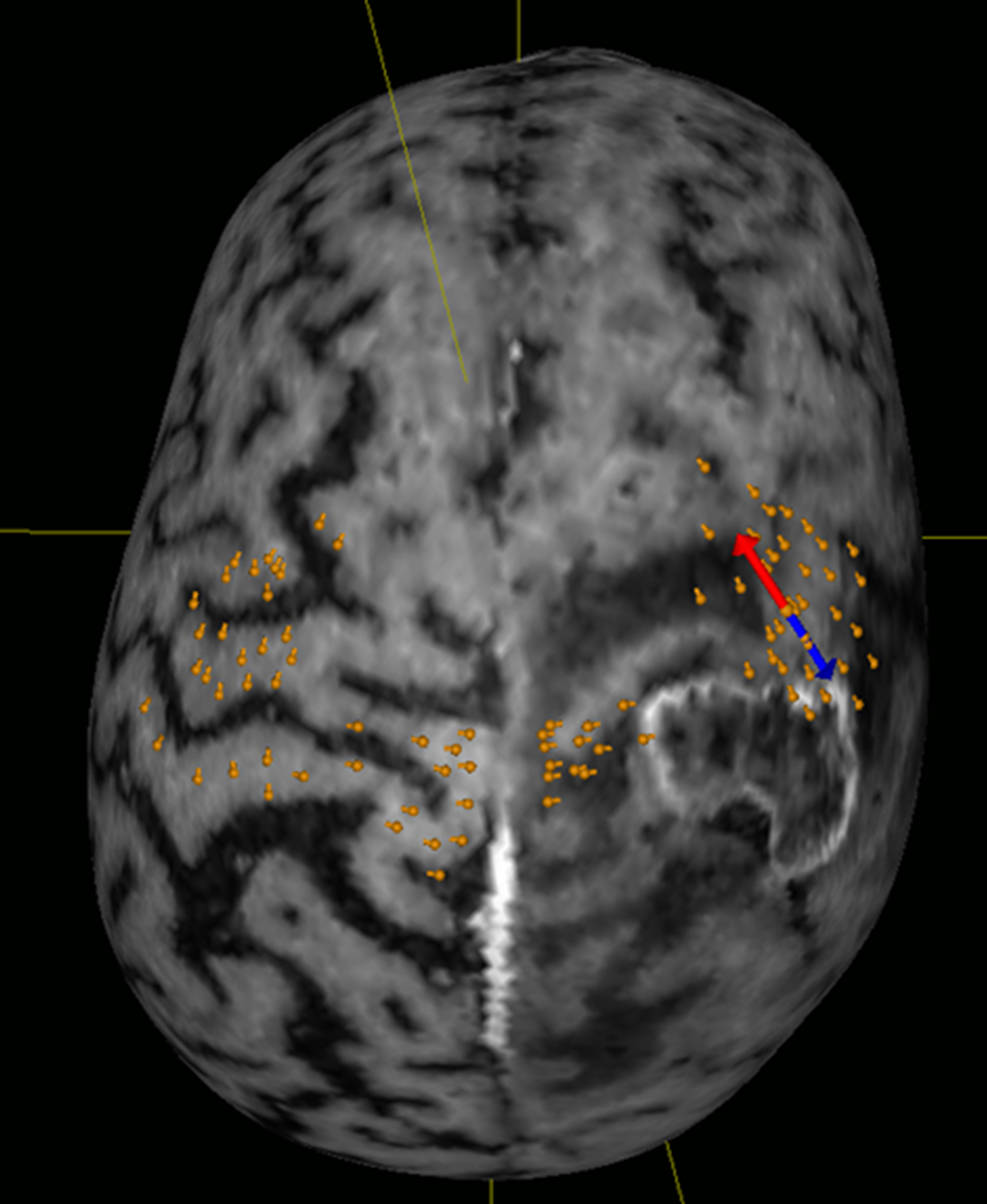 Fig. 1.
Fig. 1.Visualization of the motor cortex. Orange spots indicate motor-positive spots. The red-blue double arrow indicates the position and orientation of the stimulation coil.
All patients received contrast enhanced MR-imaging, with a voxel size of 1
All nTMS motor positive spots were imported into the Medtronic® Stealthviz™ software (Medtronic Inc., Louisville, CO, USA), enlarged to a diameter of 6 mm per spot and defined as a cortical region of interest. A second region of interest was placed into the caudal pons. CSTs of both hemispheres were visualized at 75%, 50% and 25% of the patient’s individual fractional anisotropy threshold (FAT, i.e., the highest fractional anisotropy to visualize fibers belonging to the corticospinal tract; 75%FAT, 50%FAT, 25%FAT) at a directional angle of 60° (upper extremity) or 45° (lower extremity) and a minimum fiber length of 110 mm. The minimum distance between the CST and the tumor (LTD) was measured for each fractional anisotropy (FA)-value. For distinct analyses, the LTDs were measured for upper and lower limb CST-fibers separately.
The data analysis was subdivided into two parts.
(1) Influence of motor status and tumor subtype on cortical excitability measured with rMT and interhemispheric rMT-ratio.
(2) Influence of motor status and tumor subtype on CST reconstructions measured in FAT and LTD.
IBM SPSS Version 29.0 (IBM Corp., Chicago, IL, USA) for Microsoft Windows was
used for statistical analysis. Metric variables are shown as means
A p-value
49 patients were included. 29 (59.2%) were female and the mean age was 60.3
| Mean (SD) | N (%) | ||
| Demographic data | |||
| Age (years) | 60.3 (17.0) | ||
| Female | 29 (59.2) | ||
| Intake of antiepileptic drugs | 15 (30.6) | ||
| Motor deficit | 16 (32.7) | ||
| Tumor characteristics | |||
| Histology | |||
| Cerebral Metastasis | 16 (32.7) | ||
| Glioma CNS WHO grade 2 | 6 (12.2) | ||
| Glioma CNS WHO grade 3 | 5 (10.2) | ||
| Glioma CNS WHO grade 4 | 22 (44.9) | ||
| Recurrence | 5 (10.2) | ||
| Left-hemispheric | 27 (55.1) | ||
| Tumor location | |||
| Frontal outside precentral gyrus | 20 (40.8) | ||
| Precentral gyrus | 7 (14.3) | ||
| Postcentral gyrus | 4 (8.2) | ||
| Parietal outside postcentral gyrus | 8 (16.3) | ||
| Temporal | 10 (20.4) | ||
| Tumor volume (cm |
30.5 (29.2) | ||
| Edema volume (cm |
58.3 (53.1) | ||
| nTMS parameter | |||
| rMT in % upper extremity tumor hemisphere | 33.2 (7.3) | ||
| rMT in V/m (20 mm coil to cortex distance) upper extremity tumor hemisphere | 72.9 (19.4) | ||
| rMT in % upper extremity unaffected hemisphere | 34.6 (11.0) | ||
| rMT in V/m (20 mm coil to cortex distance) upper extremity unaffected hemisphere | 75.2 (23.1) | ||
| rMT in % lower extremity tumor hemisphere | 53.9 (13.4) | ||
| rMT in V/m (20 mm coil to cortex distance) lower extremity tumor hemisphere | 107.7 (28.8) | ||
| rMT in % lower extremity unaffected hemisphere | 61.4 (13.2) | ||
| rMT in V/m (20 mm coil to cortex distance) lower extremity unaffected hemisphere | 123.7 (30.2) | ||
SD, standard deviation; N, number; CNS, central nervous system; nTMS, navigated transcranial magnetic stimulation; rMT, resting motor threshold.
nTMS was successfully conducted in all patients of the cohort. Motor evoked
potentials could be elicited from each monitored muscle. Mean rMT
Patients with motor deficits did not exhibit altered rMT-values and interhemispheric rMT-ratios only tended towards significance in these patients. No patient with a tumor-induced motor deficit had normal interhemispheric rMT-ratios for both the upper and the lower extremities (Table 2).
| Motor deficit (N = 16) | No motor deficit (N = 33) | p-value | |||
| Mean (SD) | N (%) | Mean (SD) | N (%) | ||
| Patient characteristics | |||||
| Age (years) | 65.6 (10.1) | 57.7 (19.0) | 0.32 | ||
| Female | 8 (50) | 21 (63.6) | 0.54 | ||
| Cerebral metastasis | 7 (43.8) | 9 (27.3) | 0.26 | ||
| Glioma CNS WHO grade 2 | 0 (0) | 6 (18.2) | |||
| Glioma CNS WHO grade 3 | 1 (6.3) | 4 (12.1) | |||
| Glioma CNS WHO grade 4 | 8 (50) | 14 (42.4) | |||
| Tumor volume (cm |
27.9 (25.3) | 31.7 (31.2) | 0.81 | ||
| Edema volume (cm |
74.8 (52.0) | 50.3 (52.6) | 0.06 | ||
| nTMS data | |||||
| rMT in % upper extremity tumor hemisphere | 32.4 (6.3) | 33.6 (7.8) | 0.7 | ||
| rMT in % upper extremity unaffected hemisphere | 34.7 (9.9) | 34.5 (11.7) | 0.77 | ||
| rMT in % lower extremity tumor hemisphere | 53.8 (14.4) | 53.9 (13.1) | 0.98 | ||
| rMT in % lower extremity unaffected hemisphere | 63.4 (14.8) | 60.5 (12.5) | 0.35 | ||
| rMT-ratio upper extremity (%) | 97.8 (21.9) | 100.2 (16.1) | 0.63 | ||
| rMT-ratio 90–110% | 6 (37.5) | 17 (51.5) | 0.38 | ||
| rMT-ratio lower extremity (%) | 86.6 (19.9) | 30.4 (19.0) | 0.46 | ||
| rMT-ratio 90–110% | 2 (12.5) | 12 (36.4) | 0.1 | ||
| LTD measurements | |||||
| LTD 75%FAT upper extremity (mm) | 10.3 (6.8) | 14.8 (9.2) | 0.09 | ||
| LTD 75%FAT lower extremity (mm) | 7.9 (7.7) | 14.7 (7.9) | 0.005 | ||
| LTD 50%FAT upper extremity (mm) | 8.2 (6.5) | 12.9 (8.9) | 0.07 | ||
| LTD 50%FAT lower extremity (mm) | 6.5 (7.1) | 13.6 (7.7) | 0.002 | ||
| LTD 25%FAT upper extremity (mm) | 6.0 (5.5) | 11.3 (8.6) | 0.044 | ||
| LTD 25%FAT lower extremity (mm) | 5.7 (6.8) | 13.0 (7.1) | |||
Categorial variables were tested with the Fisher Exact Test. Continuous variables were tested for significance using the Mann-Whitney-U-Test. LTD, lesion to tract distance; FAT, fractional anisotropy threshold.
We did not discover differences in rMT-values for any extremities in patients
with gliomas or metastases (Fig. 2, Table 3). In contrast, patients with cerebral
metastases were found to have higher rates of pathological rMT-ratios for the
upper extremity muscles compared with the other tumor entities of the patient
sample (p = 0.002). In the binary logistic regression model, which
comprised age, sex, edema volume, LTD, and histological diagnosis, cerebral
metastasis remained the only significant variable (OR = 21.67, 95% CI =
1.8–260.57, p = 0.015, Nagelkerke’s R
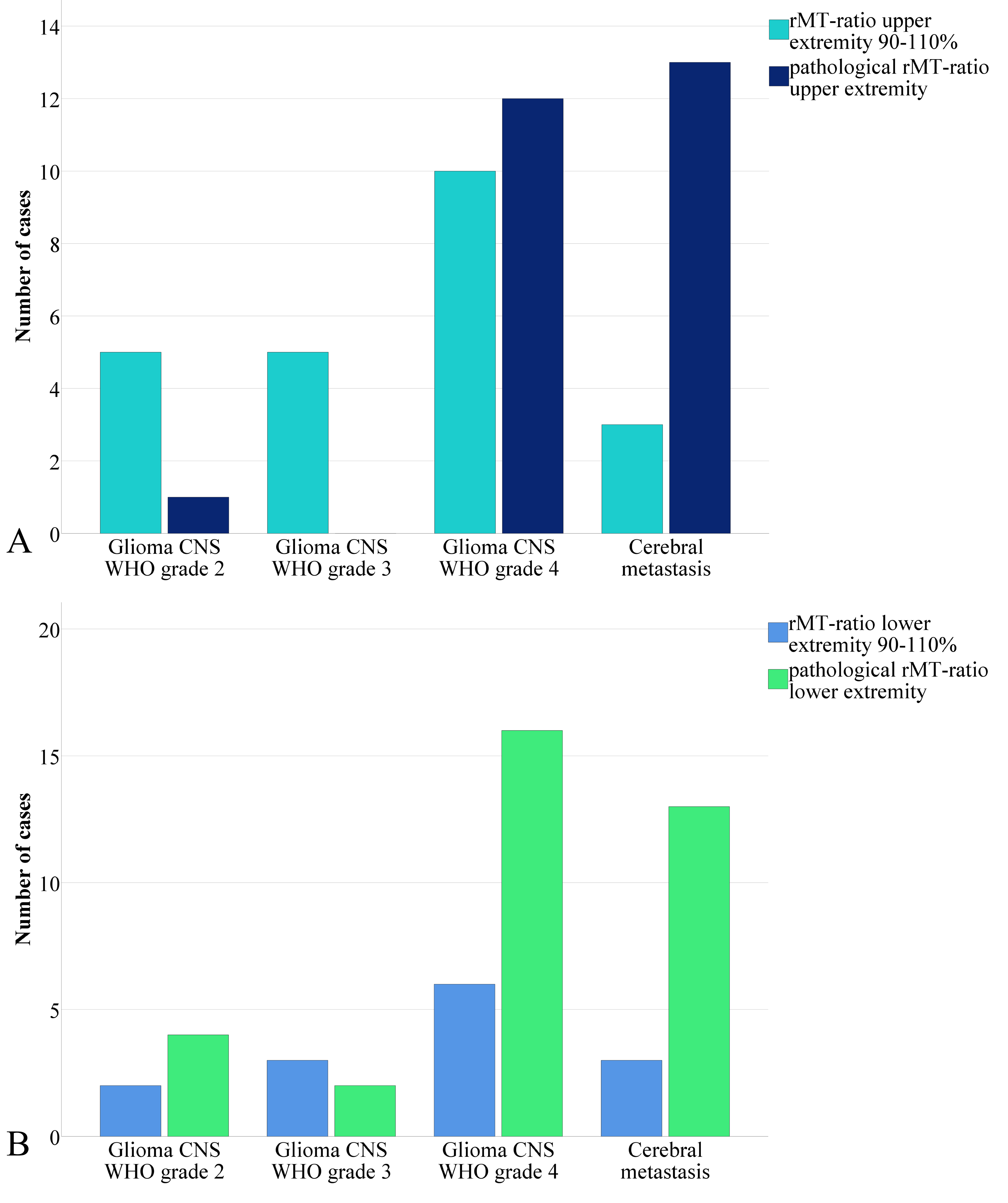
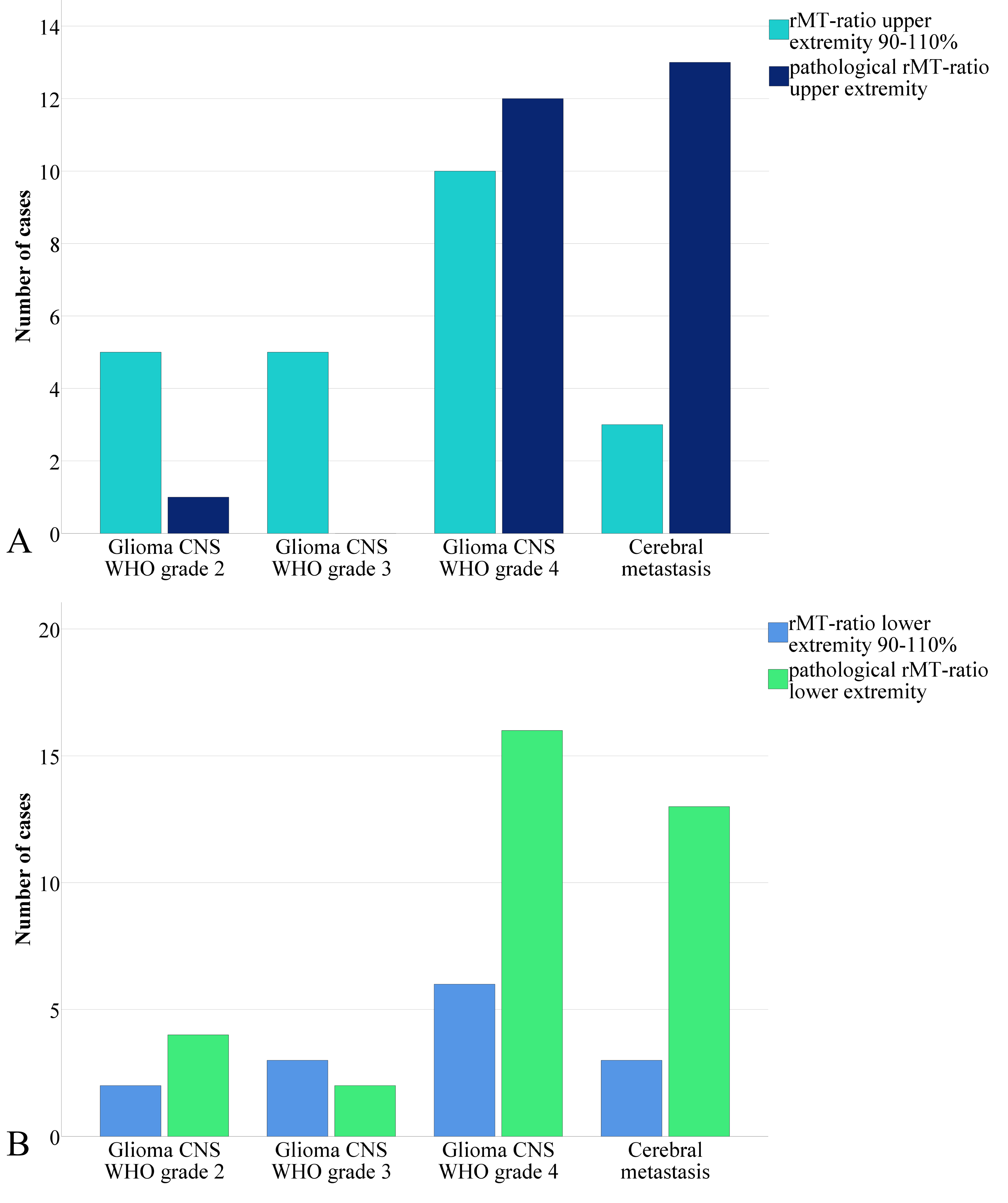 Fig. 2.
Fig. 2.Pathological interhemispheric rMT-ratios depending on tumor histology. (A) Patients with cerebral metastases had higher rates of pathological interhemispheric rMT-ratios of the upper extremity compared with glioma patients (p = 0.002). (B) The distribution of pathological interhemispheric rMT-ratios for the lower extremity muscles was not significantly different among different tumor entities (p = 0.36). Fisher-Freeman-Halton-Test. rMT, resting motor threshold.
| Metastasis (N = 16) | Glioma CNS WHO Grade 4 (N = 22) | Glioma CNS WHO Grade 3 (N = 5) | Glioma CNS WHO Grade 2 (N = 6) | p-value | |||||
| Mean (SD) | N (%) | Mean (SD) | N (%) | Mean (SD) | N (%) | Mean (SD) | N (%) | ||
| Patient characteristics | |||||||||
| Age (years) | 65.8 (10.4) | 67.4 (14.3) | 39.6 (10.5) | 37.0 (10.0) | |||||
| Female | 11 (68.8) | 14 (63.6) | 2 (40) | 2 (33.3) | 0.38 | ||||
| Motor deficit | 7 (43.8) | 8 (36.4) | 1 (20) | 0 (0) | 0.26 | ||||
| Tumor volume (cm |
17.2 (14.3) | 34.8 (32.0) | 53.7 (45.5) | 30.7 (20.0) | 0.16 | ||||
| Edema volume (cm |
70.7 (55.5) | 68.2 (53.6) | 39.4 (34.0) | 4.8 (11.7) | 0.004 | ||||
| nTMS data | |||||||||
| rMT in % upper extremity tumor hemisphere | 32.9 (8.8) | 34.1 (6.4) | 29.8 (7.6) | 33.5 (6.7) | 0.47 | ||||
| rMT in % upper extremity unaffected hemisphere | 35.3 (16.3) | 35.0 (7.3) | 30.8 (7.6) | 34.2 (9.1) | 0.45 | ||||
| rMT in % lower extremity tumor hemisphere | 54.6 (15.6) | 54.2 (10.2) | 49.2 (20.2) | 55.0 (14.6) | 0.88 | ||||
| rMT in % lower extremity unaffected hemisphere | 59.4 (13.6) | 64.1 (11.8) | 56.0 (13.5) | 61.7 (17.8) | 0.49 | ||||
| rMT-ratio upper extremity (%) | 100.1 (23.6) | 99.5 (18.2) | 96.7 (2.7) | 99.3 (6.4) | 0.94 | ||||
| rMT-ratio 90–110% | 3 (18.8) | 10 (45.5) | 5 (100) | 5 (83.3) | 0.002 | ||||
| rMT-ratio lower extremity (%) | 93.2 (22.0) | 86.6 (18.4) | 85.2 (15.4) | 91.0 (19.0) | 0.81 | ||||
| rMT-ratio 90–110% | 3 (18.8) | 6 (27.3) | 3 (60) | 2 (33.3) | 0.36 | ||||
| LTD measurements | |||||||||
| LTD 75%FAT upper extremity (mm) | 13.7 (7.8) | 14.5 (7.8) | 9.7 (7.0) | 12.0 (15.0) | 0.34 | ||||
| LTD 75%FAT lower extremity (mm) | 11.3 (7.7) | 13.5 (8.1) | 10.8 (6.6) | 14.6 (12.8) | 0.87 | ||||
| LTD 50%FAT upper extremity (mm) | 11.6 (7.2) | 12.7 (7.9) | 6.5 (7.2) | 10.7 (14.0) | 0.31 | ||||
| LTD 50%FAT lower extremity (mm) | 9.5 (7.7) | 12.3 (7.8) | 13.5 (8.1) | 13.8 (12.2) | 0.83 | ||||
| LTD 25%FAT upper extremity (mm) | 9.1 (7.6) | 10.7 (7.1) | 6.1 (7.4) | 10.2 (13.4) | 0.57 | ||||
| LTD 25%FAT lower extremity (mm) | 8.9 (7.3) | 11.8 (7.8) | 10.2 (6.3) | 12.7 (10.1) | 0.83 | ||||
Categorial variables were tested with the Fisher-Freeman-Halton-Test. Continuous variables were tested for significance using the Kruskal-Wallis-Test.
| Logistic regression backward elimination model (final model, step 5: Nagelkerke R | |||||
| Regression coefficient B | p | OR | 95% CI | ||
| Step 1 | Age | 0.01 | 0.86 | 1.0 | 0.95–1.07 |
| Female | 1.46 | 0.07 | 4.32 | 0.87–21.44 | |
| Edema volume | 0.01 | 0.15 | 1.01 | 1.0–1.03 | |
| Glioma CNS WHO grade 2 | 0.38 | ||||
| Glioma CNS WHO grade 3 | –20.1 | 1.0 | 0.0 | 0.0 | |
| Glioma CNS WHO grade 4 | 0.71 | 0.66 | 2.03 | 0.09–47.2 | |
| Metastasis | 1.991 | 0.23 | 7.32 | 0.28–194.48 | |
| LTD 75%FAT | –0.04 | 0.51 | 0.97 | 0.87–1.07 | |
| Intercept | –2.26 | 0.16 | 0.1 | ||
| Step 2 | Gender female | 1.46 | 0.07 | 4.32 | 0.87–21.38 |
| Edema volume | 0.01 | 0.15 | 1.01 | 1.0–1.03 | |
| Glioma CNS WHO grade 2 | 0.32 | ||||
| Glioma CNS WHO grade 3 | –20.08 | 1.0 | 0.0 | 0.0 | |
| Glioma CNS WHO grade 4 | 0.86 | 0.52 | 2.37 | 0.17–33.47 | |
| Metastasis | 2.15 | 0.13 | 8.58 | 0.52–140.66 | |
| LTD 75%FAT | –0.03 | 0.52 | 0.97 | 0.88–1.07 | |
| Intercept | –2.08 | 0.10 | 0.13 | ||
| Step 3 | Gender male | 1.35 | 0.09 | 3.86 | 0.83–18.0 |
| Edema volume | 0.01 | 0.19 | 1.01 | 1.0–1.03 | |
| Glioma CNS WHO grade 2 | 0.31 | ||||
| Glioma CNS WHO grade 3 | –20.15 | 1.0 | 0.0 | 0.0 | |
| Glioma CNS WHO grade 4 | 0.82 | 0.54 | 2.27 | 0.17–30.83 | |
| Metastasis | 2.12 | 0.13 | 8.29 | 0.53–128.96 | |
| Intercept | –2.24 | 0.06 | 0.11 | ||
| Step 4 | Gender female | 1.04 | 0.14 | 2.84 | 0.7–11.46 |
| Glioma CNS WHO grade 2 | 0.14 | ||||
| Glioma CNS WHO grade 3 | –19.67 | 1.0 | 0.0 | 0.0 | |
| Glioma CNS WHO grade 4 | 1.56 | 0.2 | 4.78 | 0.45–50.69 | |
| Metastasis | 2.86 | 0.03 | 17.5 | 1.38–221.47 | |
| Intercept | –2.04 | 0.08 | 0.13 | ||
| Step 5 | Glioma CNS WHO grade 2 | 0.09 | |||
| Glioma CNS WHO grade 3 | –19.59 | 1.0 | 0.0 | 0.0 | |
| Glioma CNS WHO grade 4 | 1.79 | 0.13 | 6.0 | 0.6–60.16 | |
| Metastasis | 3.08 | 0.015 | 21.67 | 1.8–260.57 | |
| Intercept | –1.6 | 0.14 | 0.2 | ||
Model fit and significant predictors did not change for 75%FAT, 50%FAT or 25%FAT (data not shown). OR, odds ratio; CI, confidence intervals.
In the subgroup of glioma patients, pathological rMT-ratios for the upper extremity were more frequent in glioblastomas compared with grade 2 and 3 tumors (p = 0.04).
The results of the nTMS examinations are shown in Tables 2,3,4.
In the tractography analysis, we observed lower LTDs if patients had motoric deficits, whereas FATs did not differ (Fig. 3). Regarding the different histological tumor types, there were no alterations in FATs between gliomas or metastases and no differences in involvement of the corticospinal tract (Fig. 3B, Table 3).
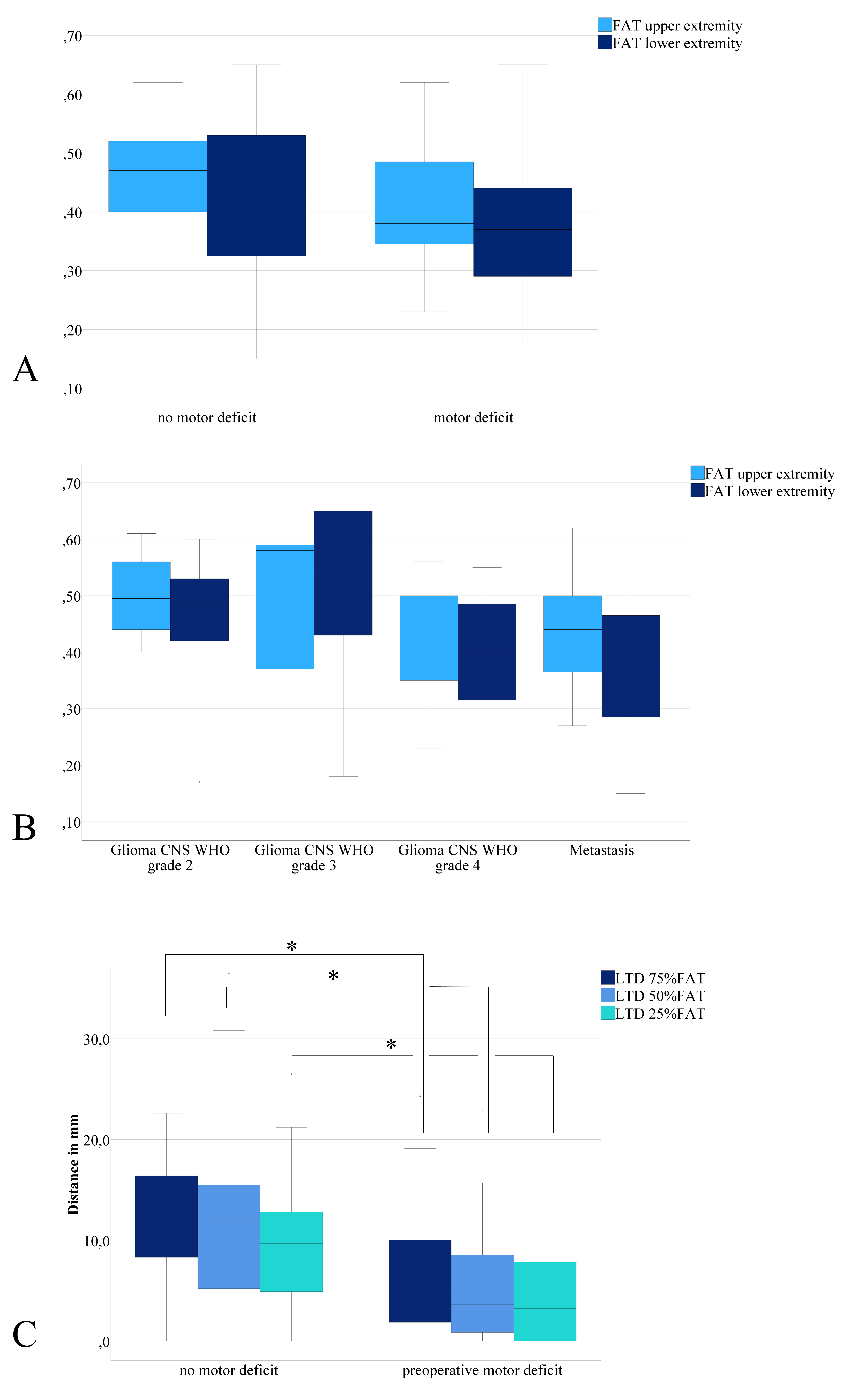
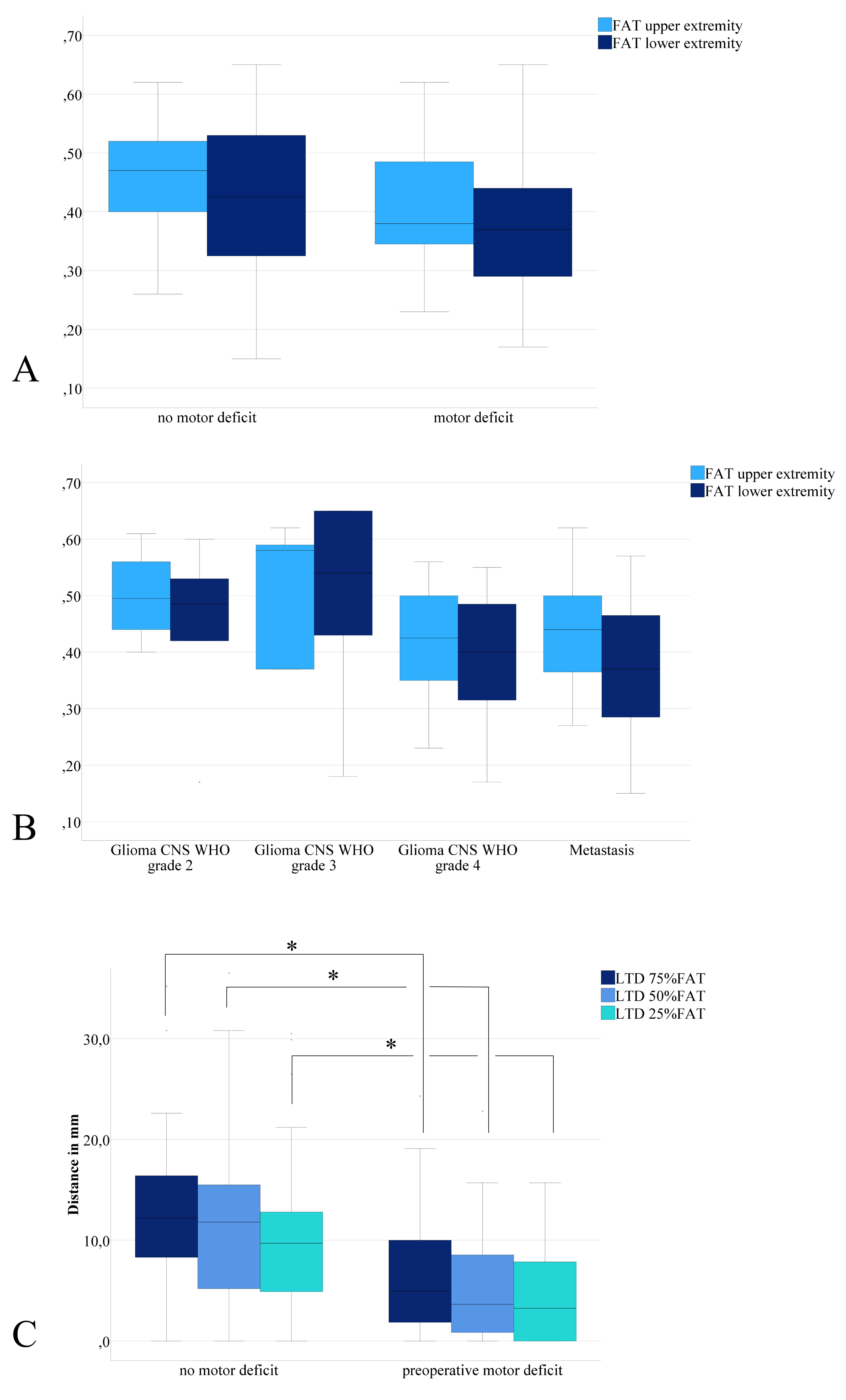 Fig. 3.
Fig. 3.Comparison of fibertracking results according to motor status
and tumor entity. (A) FATs showed no difference in patients with tumor-induced
motor deficits (all p
Taking the results of the nTMS examinations into account, there were no
differences in upper-extremity LTDs, if the interhemispheric rMT-ratio for the
upper extremity was pathological (Fig. 4A), whereas LTDs were significantly
shorter for the lower limb CST if the lower extremity had a pathological
interhemispheric rMT-ratio (Fig. 4B). LTD measurements only tended towards
shorter distances if patients developed new motoric deficits postoperatively
(p = 0.16 for 75%FAT, p = 0.096 for 50%FAT, and p = 0.11 for 25%FAT for worsening in motor function at discharge; and all p
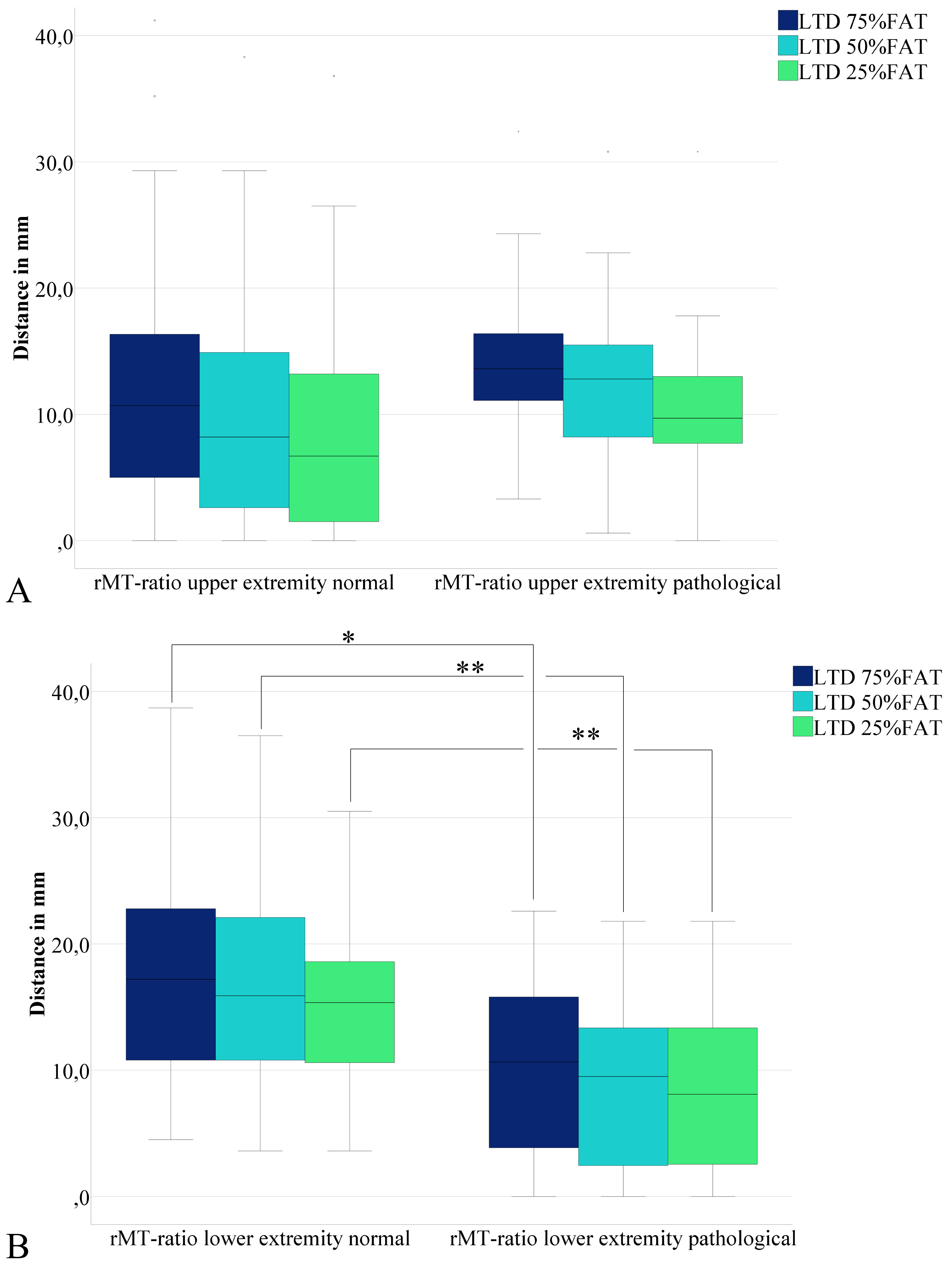
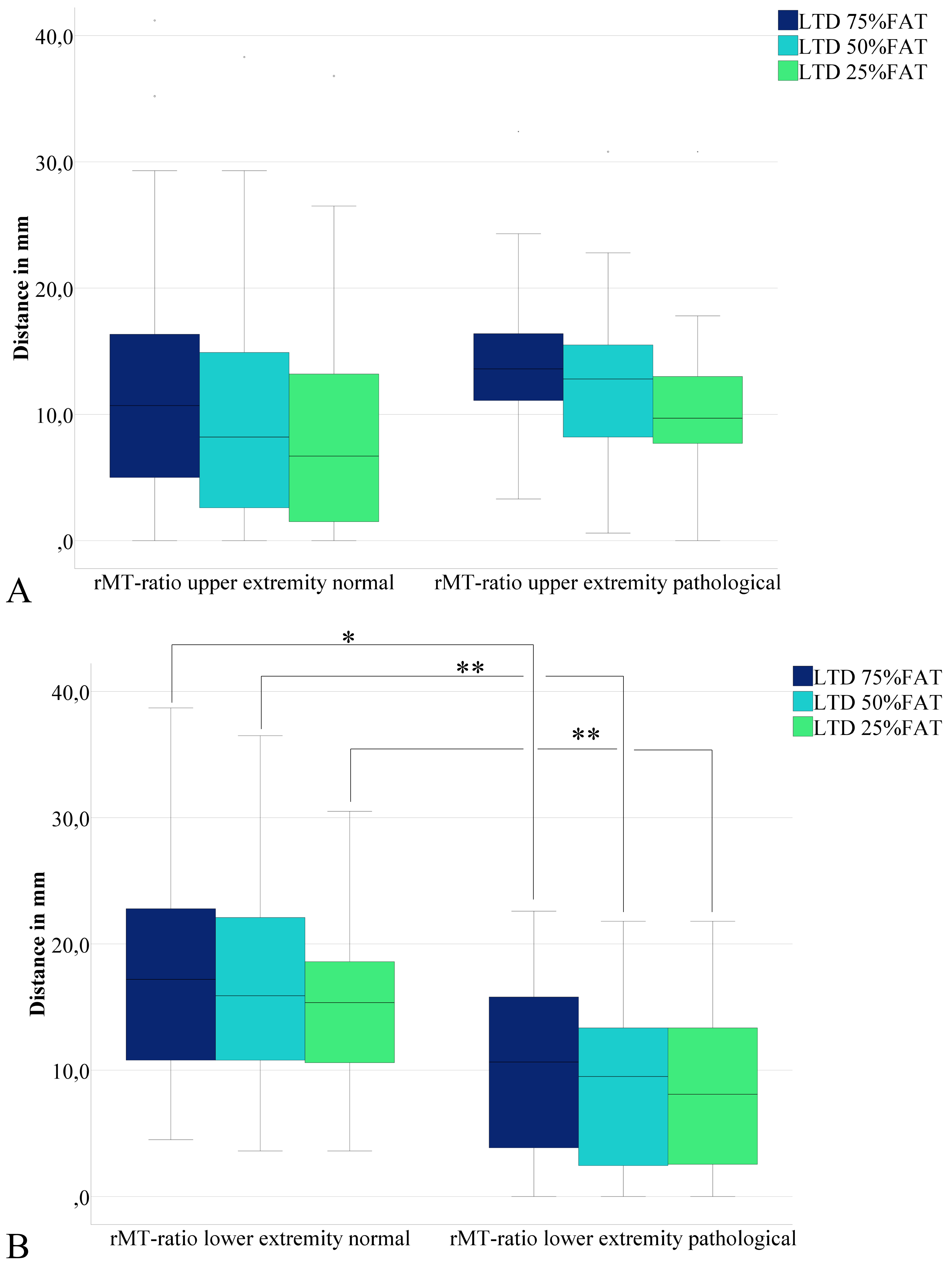 Fig. 4.
Fig. 4.Comparison of LTD measurements in patients with and without a
pathological interhemispheric rMT-ratio. (A) LTDs for the upper limb showed no
difference if rMT-ratios were pathological for the upper extremity. (all
p
The aim of this study was to analyze the cortical excitability among patients with and without tumor-induced motoric deficits and to further differentiate the nTMS- and fibertracking-derived characteristics of different malignant brain tumors.
In our cohort, all patients with tumor-induced motor deficits had abnormal interhemispheric rMT-ratios of the upper or lower limb. Additionally, infiltration of the nTMS-positive cortex was associated with tumor-induced motor symptoms as a proof of concept of nTMS. Regarding tumor histology, patients with cerebral metastases were more likely to have a pathological rMT-ratio of the upper extremity compared with glioma patients. The results of the tractography analyses confirmed the closer spatial relation between motor eloquent brain and motor symptoms, and LTD were shorter in patients with pathological cortical excitability.
The results of our study partly support the results published by Lavrador and Mirchandani et al. [37, 38, 39]. In their study of intrinsic brain tumors, higher tumor grading is associated with pathological cortical excitability, especially for the lower limb muscles, whereas we found this result for the upper but not for the lower extremity. Additionally, we found alterations in metastases compared with gliomas, which has not been shown before. Furthermore, tractography reveals changes in CST diffusion parameters in glioma patients compared with healthy participants [39]. The pathological changes of the CST correlated well with the nTMS-parameters in their study [39]. Using nTMS and nTMS-based tractography, it is possible to analyze and measure tumor-induced alterations in the motor system.
Glioma patients had altered excitability compared with patients with cerebral metastases indicating different impact on the motor system by different tumors, whereas the spatial distance between the lesion and the motor pathways was not different among the tumor entities in our cohort. We hypothesize that there are different neuroplastic compensation mechanisms in gliomas compared with cerebral metastases in order to counterbalance the disruption of the motor system by an intrinsic brain tumor. Neuroplasticity is well-described by nTMS in glioma patients [40, 41, 42, 45].
Previously published studies found associations of motor status, tumor
histology, and altered cortical excitability [6, 36, 43]. Higher motor thresholds
are associated with motoric deficits and an interhemispheric rMT-ratio of
The results of our study are limited by the retrospective study design and the small sample size. It was not possible to further differentiate between the intensity of motor symptoms. In contrast, the nTMS workflow and nTMS-based fibertracking followed a strict and standardized protocol and all examinations were conducted by the same examiner, ensuring the highest possible standards and comparability. Additional limitations arise from the fact that DTI tractography is based upon software algorithms with known limitations in voxels of crossing fibers and areas of extensive edema [47, 48]. Taking the limitations of DTI tractography into account, we might have underestimated the impact of altered nTMS-parameters and pathological rMT-ratios on the results of tractography.
The results of our study indicate altered cortical excitability in patients with gliomas and metastases. Cerebral metastases were associated with abnormal rMT-ratio of the upper extremity, whereas the cortical excitability was only partly associated with the tractography analysis. Tractography analyses further correlated well with the motor status of the patients.
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
All authors contributed to the study conception and design. Research, data collection, and data analyses were performed by TE. nTMS examinations were conducted by TE. TE, AL, LR and K-MS prepared the original draft. Figures and Tables were prepared by TE. Supervision was performed by H-HS, RL, MS and K-MS. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study design was approved by the institutional review board of Paracelsus Medical University Nuremberg (IRB-2024-10). The study was conducted in accordance with the Declaration of Helsinki. Informed consent was waived due to the study design.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.