1 The Second School of Clinical Medical of Binzhou Medical University, 264003 Yantai, Shandong, China
2 Department of Anesthesiology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, 264000 Yantai, Shandong, China
3 Department of Psychiatry, Zhenjiang Mental Health Center, The Fifth People’s Hospital of Zhenjiang City, 212000 Zhenjiang, Jiangsu, China
†These authors contributed equally.
Abstract
Perioperative neurocognitive disorders (PND) are a cognitive impairment that occurs after anesthesia, especially in elderly patients and significantly affects their quality of life. The hippocampus, as a critical region for cognitive function and an important location in PND research, has recently attracted increasing attention. However, in the hippocampus the impact of anesthesia and its underlying mechanisms remain unclear. This review focuses on investigation of the effects of anesthesia on the hippocampal dopamine (DA) system and explores its potential association with PND. Through comprehensive review of existing studies, it was found that anesthesia affects the hippocampus through various pathways involved in metabolism, synaptic plasticity and oxygenation. Anesthesia may also influence the DA neurotransmitter system in the brain which plays a role in emotions, rewards, learning and memory functions. Specifically, anesthesia may participate in the pathogenesis of PND by affecting the DA system within the hippocampus. Future studies should explore the molecular mechanisms of these effects through techniques such as neuroimaging to study real-time effects to improve animal models to better simulate clinical observations. For clinical application, it is recommended that physicians exercise caution when selecting and managing anesthetic drugs by adopting comprehensive cognitive assessment methods to reduce post-anesthesia cognitive risk. Overall, this review provides a better understanding of the relationship between the hippocampal DA system and perioperative neurocognitive function and provides valuable guidance for prevention and treatment strategies for PND.
Keywords
- perioperative neurocognitive disorders
- hippocampus
- dopamine
- anesthesia
- sevoflurane
- isoflurane
Perioperative neurocognitive disorders (PND) refer to a new
cognitive disorder after anesthesia, involving one or more cognitive domains such
as orientation, memory, calculation, attention, language, executive function,
reasoning and visuospatial function [1]. The incidence of PND in elderly patients
aged
The hippocampus has been widely recognized as the main brain area in the
formation of cognitive function and it has become a focus of study in
cognition-related impairment. Currently, increasing attention is being directed
towards PND, with a growing research focus on the hippocampus in related
research. As a common medical method, anesthesia is widely used in surgery and
other medical procedures. Studies have found that the structure of the
hippocampus is damaged after anesthesia, which leads to cognitive
dysfunction [4]. Currently, it is believed that the main cause of PND is
neuroinflammatory reaction. In related experiments, the authors of this review
have found that the use of anesthetic drugs leads to the activation of microglia
and astrocytes in brain tissue, thus producing related inflammatory factors and
causing the occurrence of PND [5]. In further studies, they have found that the
anesthetic process mainly leads to the release of Interleukin-1
Although the research on the relationship between PND and neuroinflammation is comprehensive, the authors have also found a close correlation between PND and neuronal inhibition. Isaeva et al. [8] have found that the use of inhaled anesthetics such as isoflurane inhibits neuronal activity in the CA3 region of the hippocampus. There is also a large body of research that has found that anesthesia not only affects the tissue structure of the hippocampus, but also its neurotransmitter release and reuptake [9, 10, 11]. Among recent studies, it has been observed that the neurotransmitter dopamine (DA), which is the primary focus here, plays a crucial role [12]. The release of DA is closely linked to the reward mechanism, regulating positive reinforcement of behavior and motivation formation [13]. It plays a key role in motor control, sending synaptic signals to the caudate and putamen of the dorsal striatum via the substantia nigra pathway, helping to maintain coordinated and fluid movements [14]. Additionally, DA is also involved in the process of learning and memory. Therefore, the DA nervous system provides a key pathway for regulating mood, reward and motivation and may also have an impact on cognitive function during anesthesia. Consequently, this review concentrates on a pivotal and persuasive theme: the correlation between an impaired hippocampal DA system and perioperative neurocognitive impairment. Existing research and literature on the impact of anesthesia on the hippocampal DA system, is aimed at investigating its potential contribution to PND. This exploration underscores the significance of the hippocampus and DA in the realm of anesthesia and their role in cognitive dysfunction, thus aiming to advance understanding of the intricate relationship between anesthesia technology and brain function.
Currently, there are many types of anesthetic drugs used clinically, among which
the inhalation anesthetic drugs, propofol and opioids are the main ones. For
example, sevoflurane affects Ca
As an intervention, anesthesia not only triggers an inflammatory response and
the release of inflammatory factors affecting hippocampal function but also
induces neuronal apoptosis and alters the hippocampal volume. Primarily,
anesthesia may influence the metabolic activity of hippocampal tissue cells,
causing shifts in energy metabolism. For instance, isoflurane, a commonly used
inhaled anesthetic in clinical practice, has been demonstrated to impact the
metabolomics of the hippocampus by elevating glutamate and lactate levels in this
region [19]. Beyond direct metabolic impact, anesthesia also exerts an indirect
influence on hippocampal metabolic processes through modulation of cellular gene
expression, consequently influencing hippocampal function [20, 21]. Experiments
have shown that inhalation of sevoflurane causes oxidative stress, increases the
apoptosis of hippocampal neurons and significantly reduces the expression of
BDNF, resulting in learning and memory disorders [22]. Peng et al. [23]
discovered in mouse hippocampal neurons that sevoflurane alters mRNA expression
levels by inhibiting the acetylcholine receptor (mAChR
| Influencing factors | Subject | Anesthetic drugs | Medication dosage | Experimental methods | Conclusions | Data sources |
| Anesthesia temperature | Newborn mice | Propofol | 25 mg/kg | Water maze test and Western blot | Hypothermia during anesthesia increases apoptosis rate of hippocampal neurons in neonatal rats | Liu, Wenbo et al. [37] |
| Adult rat | Isoflurane | 1.50% | The Y-maze test and Western blot experiment | Hypothermia during anesthesia induction leads to cognitive dysfunction in rats | Tan, Wenfei et al. [36] | |
| Types of narcotic drugs | Adult rat | Isoflurane | 1.20% | Barnes maze and fear conditioning test, Western blot | Isoflurane increases expression of inflammatory cytokines, which leads to cell damage in the hippocampus and cognitive dysfunction in rats | Lin, Daowei et al. [38] |
| Adult rats | Sevoflurane | 3% | Reverse Transcription-Polymerase Chain Reaction (RT-PCR) | Sevoflurane can affect the cognitive function of rats by inhibiting the expression of M1 muscarinic acetylcholine receptor (mAChR·M1) mRNA in the hippocampus | Peng, Sheng et al. [23] | |
| Young rats | Midazolam | 10 mg/kg | Immunohistochemical method | Midazolam affects the expression of hippocampal neural stem cells, results in cognitive impairment in mice that can last into adulthood | Doi, Hiroyoshi et al. [39] | |
| Prolonged continuous anesthesia | Adult rats | Propofol | 90 mg/kg | Immunofluorescence staining | Prolonged use of propofol produced rapid but transient cognitive impairment | Kim, Jenny L et al. [4] |
| Adult rats | Midazolam | 8.0 mg/kg | Immunofluorescence staining | Prolonged use of midazolam produced cognitive changes that were delayed by several weeks | Kim, Jenny L et al. [4] | |
| Adult rats | Dexmedetomidine | 0.1 mg/kg | Immunofluorescence staining | Prolonged administration of dexmedetomidine produced cognitive changes that were delayed for several weeks | Kim, Jenny L et al. [4] |
Anesthesia impairs concentration and executive ability. It manifests, for example, as decreased ability to assign and perform tasks, poor concentration and prolonged reaction time. Cognitive dysfunction caused by anesthesia can be divided into long and short-term cognitive impairment according to the duration of the effect. Karaman et al. [40] performed water maze experiments on rats anesthetized by sevoflurane and found it to cause obvious defects in short-term spatial learning ability and short-term memory. Additionally, a prospective study of 46 patients undergoing general anesthesia in clinical studies showed that repeated exposure to general anesthesia can lead to short-term postoperative cognitive impairment [41]. In clinical trials, Linassi et al. [42] conducted Montreal Cognitive Assessment (MoCA) and Trail Making Test B (TMT-B) tests on 151 adult patients undergoing elective cardiac surgery; results showed that isoflurane caused long-term cognitive dysfunction. As a critical neurotransmitter in the central nervous system, DA plays a pivotal role in several key functions, including emotion, reward, motor control, learning and memory. The release of DA is closely linked to the reward mechanism and regulates the positive reinforcement and motivation formation of behaviors [13]. From Donald Hebb’s first proposal of homo-synaptic plasticity, to Kandel and Tauc’s proposal of the hetero-synaptic rule, to a large number of experiments demonstrating that plasticity is a homeostatic synaptic scaling, increasing evidence suggests that neural plasticity plays a crucial role in brain learning and memory abilities [14, 43, 44, 45, 46]. DA, as an important neurotransmitter, participates in motivation and stimulus-reward learning processes while also possessing the ability to regulate synaptic plasticity [47]. It also affects the synaptic efficacy of neuronal circuits, enabling them to produce LTP and Long Term Depression (LTD) [48]. Therefore, DA is an indispensable part of learning and memory processes.
Narcotic drugs affect the DA nervous system through a variety of pathways. First, some narcotic drugs may reduce the number of DA neurons. By comparing experimental data, Zhou et al. [49] confirmed that due to the neurotoxicity of anesthetic drugs, DA neurons undergo apoptosis during anesthesia, so the function of DA is affected. Second, anesthetics may also disrupt the release of neuronal electrical signals in the DA nervous system [49]. For example, cocaine inhibits the function of neurons by reducing the firing frequency and the number of bursts of neurons, while Bao et al. [50] found through clinical studies that sevoflurane reduces the electrical signal release of neurons and is also one of the reasons for inhibiting neurons [51]. He et al. [52] also confirmed that the use of anesthetics such as isoflurane to inhibit the discharge of D2-SPNs (spin-projecting neurons expressing d2 type DA receptors) and the broadband oscillation rhythm of the local field potential resulted in the inhibition of DA release. Additionally, anesthetics may also have an effect on DA transporters. Dopamine Transporter (DAT), which removes DA from the synaptic cleft, ensures proper DA function and coordinates spatial and temporal modulation of DA neurotransmission [53]. In related experiments, the use of anesthetics resulted in abnormal DAT function. As a result, the transmission and regulation of DA signaling are affected [53, 54]. Taken together, these effects may have multiple impacts on cognitive function, including emotion, learning, memory and movement. Additionally, the effects of anesthesia and DA are also summarized in this review as well as the effects of anesthesia and DA. We provide a summary of recent correlation studies exploring the relationship between anesthesia and DA (Table 2) [51, 55, 56, 57].
| Pathways of influence | Subject | Anesthetic drugs | Medication dosage | Experimental Methods | Conclusions | Data sources |
| Neuronal firing | Adult rats | Cocaine | 10 mg/kg | Multielectrode array with telemetry recording system | Cocaine increases the firing and burst rates of most DA neurons | Koulchitsky et al. [51] |
| Dopamine uptake and release | Adult rats | Isoflurane | 1.50% | Quick scan cyclic voltammetry | Prolonged use of isoflurane alters DA release and uptake | Brodnik et al. [55] |
| Dopamine concentration | Middle-aged rats | Sevoflurane | 3% | High Performance Liquid Chromatography (HPLC) with electrochemical detector (ECD-300, EICOM) | Isoflurane anesthesia significantly increases extracellular DA concentrations in middle-aged rats | Kimura-Kuroiwa et al. [56] |
| Young rats | Sevoflurane | 3% | HPLC with electrochemical detector (ECD-300, EICOM) | In young rats, isoflurane significantly enhances methamphetamine (MAPT)-induced DA | Kimura-Kuroiwa et al. [56] | |
| Dopamine receptors | Adult rats | Morphine | 3 mg/kg | In vivo electrophysiological recording techniques | Systemic injection of morphine can induce DA D1 receptor-mediated synaptic potentiation in hippocampal neurons | Hu et al. [57] |
First, the hippocampus receives dopaminergic innervation and DA plays a crucial role in hippocampal-dependent plasticity and related learning and memory processes [12]. In the mammalian central nervous system, the dopaminergic pathway between the ventral tegmental area of the midbrain and the hippocampus in mice is involved in the formation of cognition, memory and learning, reward and other functions (Fig. 1). Second, a large number of experimental data confirm the existence of DA receptor subtypes in the hippocampus. In the dorsal hippocampus, D1 receptors were significantly expressed in the granule cells of the dentate gyrus. D2 receptors were mainly found in hilar mossy cells [58]. D1/D2 heterodimers were mainly distributed in the dorsal hippocampal hilar region. The ventral hippocampus plays a key role in addiction and other DA-dependent psychiatric disorders [59, 60]. DA is a key regulator of memory function in the hippocampus and plays diverse roles in various aspects of memory and cognition [55, 56, 57, 58, 59, 60]. The hippocampus is involved in the formation of spatial memory and innovative learning based on synaptic plasticity. In response to novel information and motivational events (rewards), signals at hippocampal CA1 synapses are mediated by DA [61]. Additionally, DA induces the protein synthesis required for late LTP within hippocampal neuron dendrites. In conclusion, the hippocampal DA system as a whole is mainly involved in the formation of cognitive function.
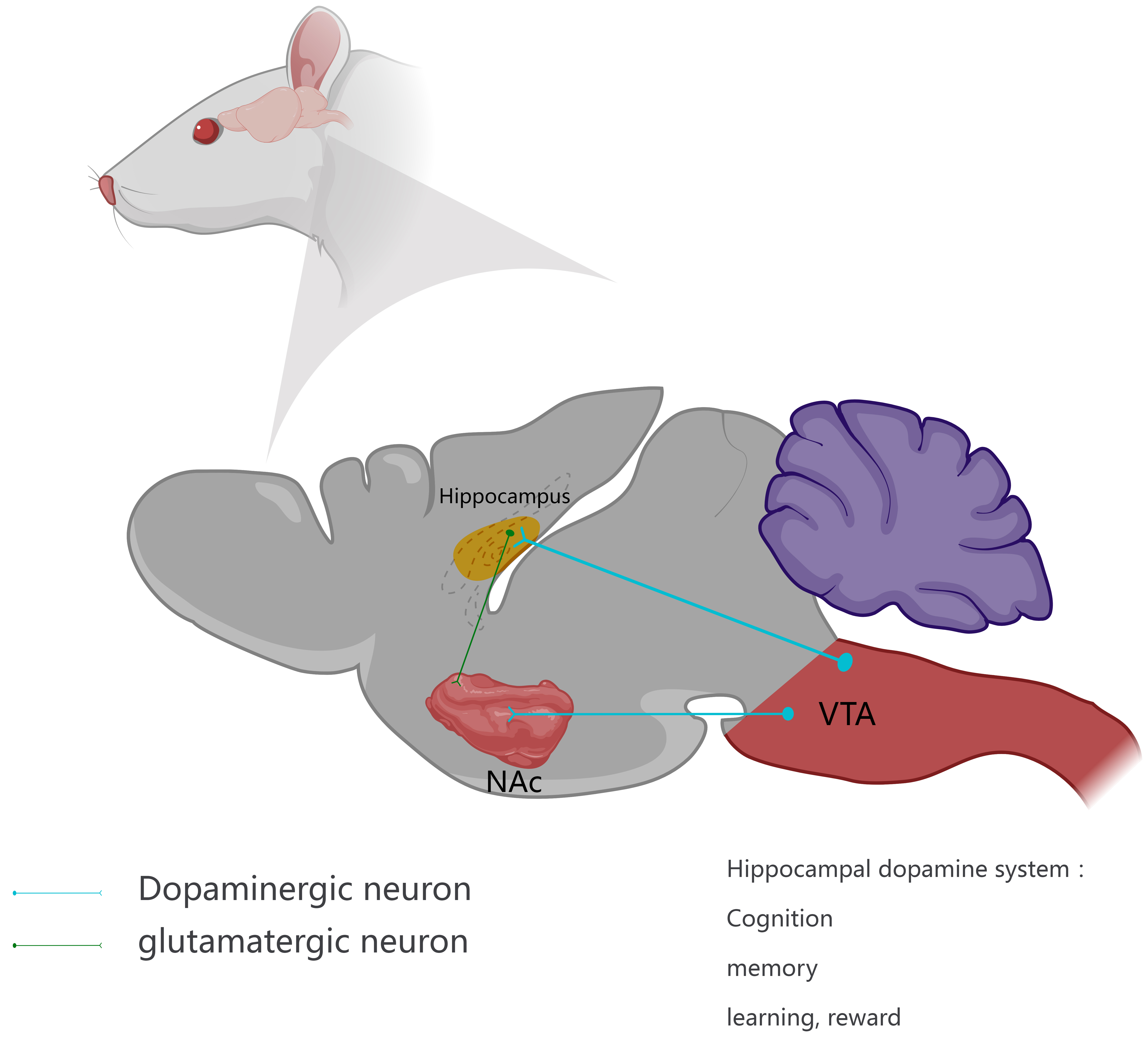 Fig. 1.
Fig. 1.The hippocampal dopamine system. The dopamine nervous system in the hippocampus mainly comes from the ventral tegmental area (VTA), and there are abundant nerves between the two. At the same time, DA-binding DA receptors (DAR) are also present in the nucleus accumbens (NAc) (ventral striatum), receiving innervation from DAergic pathways in the VTA and dense innervation from glutamatergic neurons in the hippocampus to release dopamine (DA) in response to reward-related stimuli. Thus, DAergic pathways control cognition, memory and learning, and reward. The diagram was meticulously crafted and designed in-house, using Medpeer.cn.
Anesthesia, as an intervention, may cause PND in patients by affecting the function of hippocampal DA system. As shown in Fig. 2, firstly, the neurons in the ventral CA1 of the hippocampus and the medial prefrontal cortex are regulated by the DA system. DA activates synaptic plasticity in hippocampal neurons after acting on D1-like receptors [62]. During anesthesia, anesthetic drugs may impede the function of DAT and obstruct its transport function, resulting in diminished recovery of DA neurotransmitters. Consequently, this reduction in DA neurotransmitter release may impair synaptic plasticity of ventral CA1 and medial prefrontal cortex neurons [43]. Therefore, the reason for PND due to anesthesia may be the inhibition of synaptic plasticity in the hippocampus by affecting the hippocampal DA system. Secondly, Hu et al. [57] found that the use of morphine and other narcotic drugs leads to the abnormal enhancement of NMDA receptor-dominated neurons in the hippocampus and the hippocampal DA D1 receptor plays an important role in the synaptic potentiation induced by morphine. Experiments have also found that DA binds to DA D1-like receptors after morphine treatment, which enhances NMDA current and stimulates cAMP-dependent signaling [57]. Therefore, it is hypothesized here that morphine and other anesthetics promote the release of electrical signals from DA neurons by acting on dopamine D1 receptors, thereby affecting cognitive functions such as emotion, reward, learning and memory. However, this effect may also be achieved through a variety of other mechanisms, including the regulation of neurotransmitters and changes in synaptic plasticity.
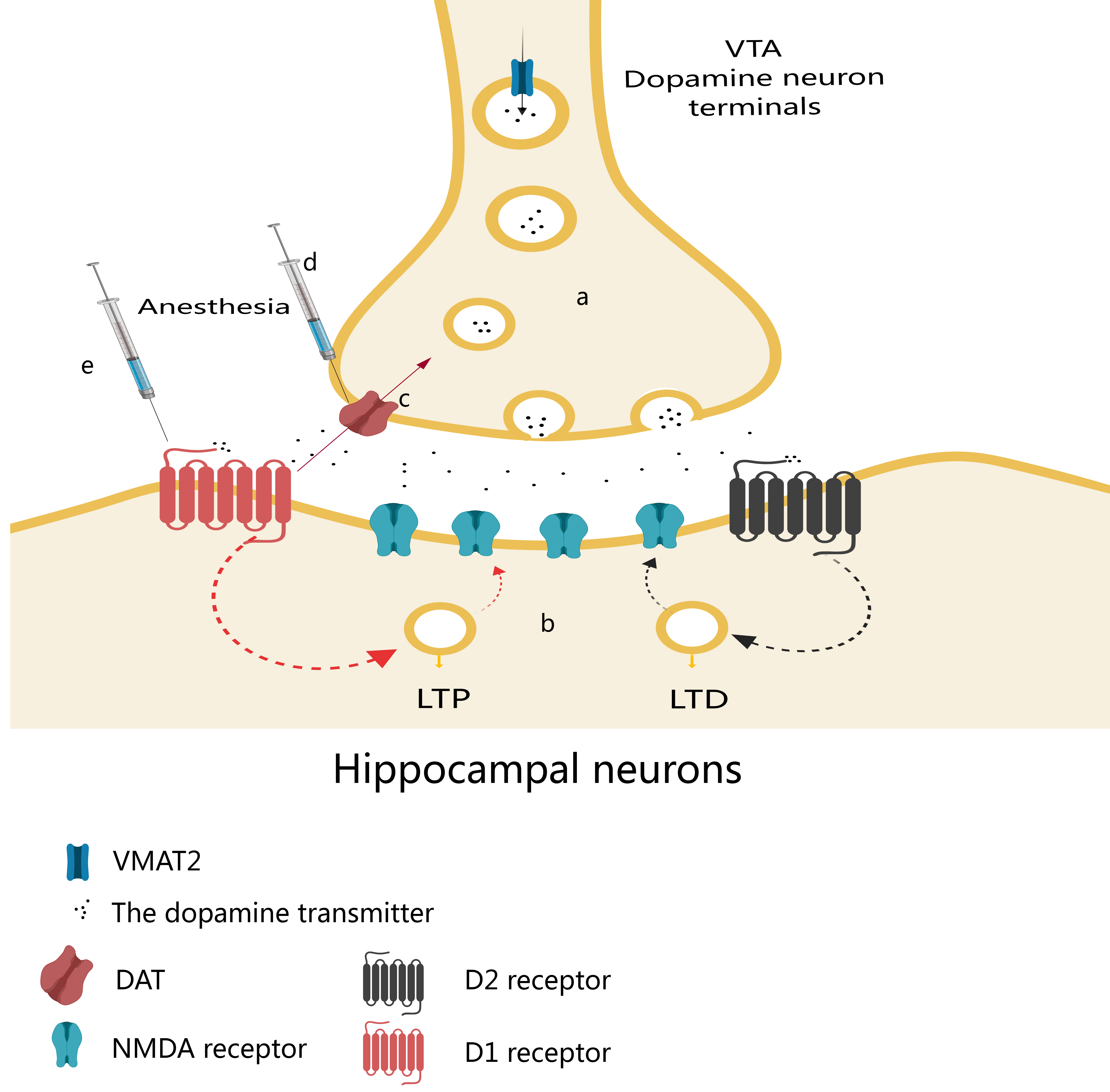 Fig. 2.
Fig. 2.Effects of narcotic drugs on the hippocampal dopamine system. (a) After dopamine (DA) synthesis, vesicular monoamine transporter 2 (VMAT2) transports DA from the cytoplasm to synaptic vesicles at the synaptic terminal, where it is finally released. (b) Neurons in the ventral hippocampal CA1 (vCA1) and medial prefrontal cortex (mPFC) are regulated by the DA system, which acts on D1-like and D2-like receptors to activate synaptic plasticity—long-term potentiation (LTP) and long-term depression (LTD). (c) DA is transported back to the presynaptic terminals of DA neurons by DA transporter (DAT). (d) During anesthesia, anesthetics may inhibit the function of DAT, block its transport function and reduce the recovery of DA neurotransmitters, thereby reducing their release, resulting in the impairment of synaptic plasticity in vCA1 and mPFC neurons. (e) Anesthetics can lead to abnormal synaptic potentiation of NMDA receptor-dominated neurons in the hippocampus and hippocampal DA D1 receptor (D1R) plays an important role in the synaptic potentiation. DA can bind to hippocampal D1R receptors, enhance NMDA currents, and stimulate cAMP-dependent signaling. VTA, ventral tegmental area; NMDA, N-methyl-D-aspartate; cAMP, cyclic adenosine monophosphate.
In conclusion, by investigating the relationship between the hippocampus, DA and perioperative neurocognitive dysfunction, as well as potential mechanisms, a more comprehensive understanding is gained of the potential impact of anesthesia on cognitive function. This, in turn, offers valuable insights for clinical practice and treatment. Future research should focus on elucidating the mechanisms of action of the effects of anesthesia on the hippocampus and DA system, thereby enhancing the understanding of the relationship between hippocampus, DA, and cognitive function.
First, the mechanism of anesthesia on the hippocampus and the DA system should be further studied along with the specific signaling pathways, subpathways and related cellular and molecular changes. Second, with the help of advanced neuroimaging techniques, such as positron emission tomography, magnetic resonance imaging and electroencephalography, the real-time effects of anesthesia on the activity of hippocampal DA system are studied, so as to better understand its role in cognitive function [63, 64]. Thirdly, better animal models should be designed to better simulate the anesthetic process in clinical practice, so as to study the relationship between hippocampus, DA and cognitive function in experiments.
On the clinical side, the use of DA drugs can reduce PND and patients with
dopaminergic system disorders, such as Parkinson’s disease, have also received a
lot of attention. Currently, the number of middle-aged and elderly patients is
gradually increasing, as is the proportion of elderly patients with Parkinson’s
disease. Tinkhauser et al. [65] have found that during surgery, DA drugs
can inhibit the excessive activity of basal ganglia
This review examines the relationship between the hippocampal DA system and perioperative neurocognitive function, with the aim of understanding how the two interact and elucidating possible mechanisms that contribute to PND. Here, it is believed that the hippocampus plays an important role in cognitive functions such as learning, memory and spatial navigation and many types of neurotransmitters in the hippocampus are associated with PND. By summarizing the previous literature, it is concluded that DA neurotransmitters are closely related to emotion, reward and learning. Therefore, special attention should be paid to the hippocampal DA system. Physiological and metabolic effects of anesthesia on the hippocampus and DA system are discussed, along with their potential impact on synaptic plasticity and neurotransmitter regulation. The mechanisms through which anesthesia induces an inflammatory response are summarized. The review demonstrates how anesthesia can influence the hippocampus, highlighting the potential effects of anesthesia drugs on cognitive function by affecting the hippocampus and DA system. Finally, a direction for future research is highlighted, including an in-depth study of molecular mechanisms, application of neuroimaging techniques and improvement of animal models; it is suggested that the selection and administration of anesthetic drugs should be optimized, cognitive function assessed and interventions developed to reduce the cognitive risk of patients after anesthesia in clinical practice.
In summary, this review provides a more comprehensive understanding of the complex relationship between the hippocampal DA system and perioperative neurocognitive function by exploring the foregoing aspects in depth. This not only has important implications for improving clinical practice and surgical management, but also provides useful guidance for future research on drugs to treat PND or anesthesia methods to assist prevention of PND. It is hoped that further research reveals more detail of the link between the multi-systems and provides a more complete strategy for the prevention and treatment of PND, ultimately improving the quality of life of patients.
FNJ and ARC found references and drafted the manuscript. FNJ drew figures. CCY and HHL designed literature retrieval strategy, reviewed the manuscript and obtained fundings. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We would like to express my gratitude to all those who helped me during the writing of this manuscript.
This work was supported by the Yantai city science and technology innovation development plan (Grant no. 2023YTYD06000871), Zhenjiang Social Development Project (Grant no. SH2023083).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


