1 Department of Neurology, The Affiliated Hospital of Hangzhou Normal University, 310015 Hangzhou, Zhejiang, China
2 School of Clinical Medicine, Hangzhou Normal University, 311121 Hangzhou, Zhejiang, China
3 Department of Obstetrics, The Affiliated Hospital of Hangzhou Normal University, 310015 Hangzhou, Zhejiang, China
4 Department of General Practice, The Affiliated Hospital of Hangzhou Normal University, 310015 Hangzhou, Zhejiang, China
5 Centre for Cognition and Brain Disorders, The Affiliated Hospital of Hangzhou Normal University, 310015 Hangzhou, Zhejiang, China
6 TMS Centre, Deqing Hospital of Hangzhou Normal University, 310018 Hangzhou, Zhejiang, China
Abstract
Background: Somatosensory deficits are common symptoms post stroke. Repetitive transcranial magnetic stimulation (rTMS) over the motor cortex is able to promote motor rehabilitation, whereby its impact on somatosensory functioning remains unknown. This study was designed to evaluate the association between somatosensory deficits and corticospinal excitability following stroke, with the purpose to provide insights on rTMS interventions for the management of somatosensory deficits. Methods: Somatosensory functioning and corticospinal excitability (motor-evoked potential, MEP; cortical silence period, CSP) were evaluated from a group of sixteen patients with unilateral ischemic stroke in the acute or subacute phase. Results: Results indicated that the uncommon presentation of larger MEPs in ipsilesional vs. contralesional motor cortex was associated with worse somatosensory function compared to those with a smaller MEP in ipsilesional motor cortex. Moreover, increased MEP ratio (ipsilesional vs. contralesional motor cortex) was associated with better somatosensory function in patients with well-preserved somatosensory function. Conclusions: In well-recovered patients, an increased MEP ratio between the ipsilesional and contralesional motor cortex could be an indicator of improved somatosensory functioning following stroke.
Keywords
- stroke
- somatosensory deficits
- TMS
- MEP
- CSP
Somatosensory deficits are common post-stroke symptoms characterised by sensory loses, numbness. It is estimated that about 50–80% of post-stroke survivors demonstrate somatosensory deficits, which have clear adverse influence on motor functioning and overall recovery from stroke [1, 2]. Repetitive transcranial magnetic stimulation (rTMS) is a safe and non-invasive form of brain stimulation which is able to induce neuroplastic changes [3, 4]. It has been used in the management of depression [5, 6], chronic pain [7, 8], and post-stroke motor rehabilitation [9, 10]. Studies have been focussed on motor rehabilitation with motor cortex rTMS (see reviews in [11, 12]), however, the potential benefits of rTMS on somatosensory deficits remain unclear. Overall, there is a lack of effective treatment for somatosensory deficits in clinical settings [13, 14, 15].
Although most rTMS studies aimed at improving somatosensory function following stroke have focused on targeting the primary somatorsensory cortex (S1) [16, 17, 18], the primary motor cortex (M1) may be an alternative target. Although the motor cortex is predominantly involved in motor control, it is also responsible for somatosensation via its anatomical and functional connections with somatosensory cortices and the thalamus [19]. In fact, motor cortex rTMS has a clear impact on the transmission of sensory information from the body parts [20, 21, 22]. This argument is also consistent with findings that motor recovery following stroke is positively associated with sensory functioning [2]. Moreover, motor cortex rTMS has demonstrated potential benefits on motor rehabilitation following stroke [12], although recent studies have called this argument into question [23, 24].
In addition to the site of stimulation, it is important to determine the
relationship between somatosensory deficits and corticospinal excitability, in
order to facilitate the design of stimulation protocols for somatosensory
improvement. Somatosensory deficits following stroke are associated with the
impairment of somatosensory pathways (e.g., the medial lemniscal pathway for
discriminative touch and proprioception, the spinothalamic pathway for pain and
temperature) [25, 26, 27], which transmits sensory signals from body parts. Our group
has previously demonstrated that bottom-up sensory transmission is able to
inhibit corticospinal excitability measured by motor-evoked potential (MEP) and
cortical silent period (CSP) [21]. MEP amplitude provides a simple and direct
measurement of the excitation of corticospinal pathways. Meanwhile, CSP is able
to indicate intracortical inhibition supported by gamma-aminobutyric acid
(GABA
This study was designed to evaluate the association between somatosensory deficits and corticospinal excitability following stroke. Somatosensory functioning and corticospinal excitability were evaluated from patients with unilateral ischemic stroke. We have specified this study to acute and subacute phases of stroke to reduce heterogeneity. It is hypothesised that decreased corticospinal excitability in the ipsilesional (vs. contralesional) motor cortex would be associated with poorer somatosensory functioning.
A total of thirty-nine patients were screened, among which sixteen participated
in this study. All patients had unilateral ischemic stroke observed on a
diffusion-weighted magnetic resonance imaging (MRI) scan. The inclusion criteria
were: (1) unilateral ischemic stroke in the acute and subacute phase of stroke
(
This was an observational study (Fig. 1). All the patients visited our research centre once. Somatosensory function was evaluated during the testing, and corticospinal excitability was recorded with single-pulse TMS-evoked MEPs.
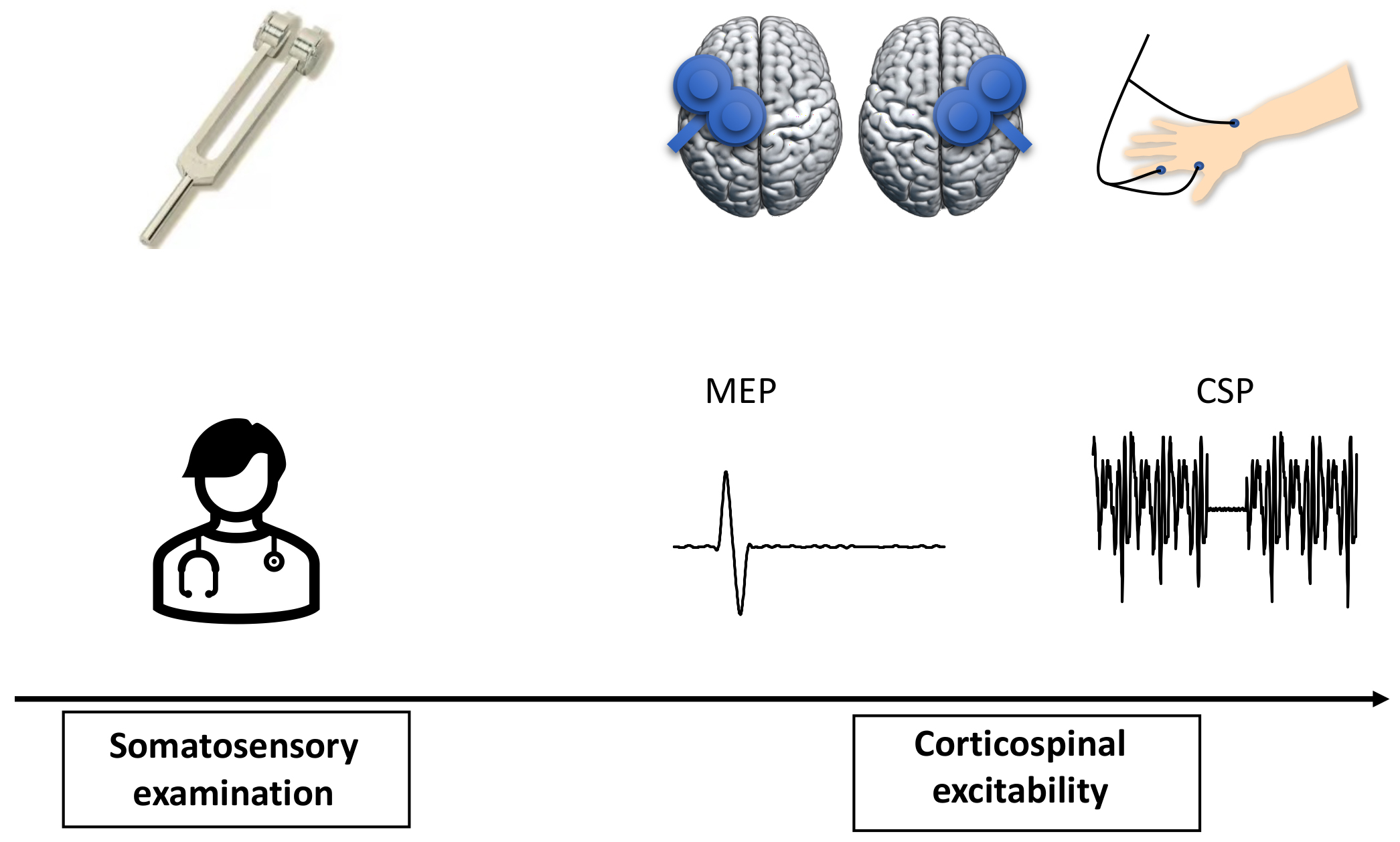 Fig. 1.
Fig. 1.Study design. Somatosensory function was initially evaluated on both sides of body. Corticospinal excitability was then recorded from both hemispheres with single-pulse TMS-evoked MEPs.
Somatosensory functioning was evaluated using the modified Fugl-Meyer and Lindmark Assessment [33, 34], which included superficial sensation (pain, temperature, and touch), deep sensation (proprioception, motion perception, and vibration), cortical sensation (two-point discrimination, stereognosis), and subjective sensation. Subjective sensation was added due to the fact that some patients (3/16 in this sample) tended to report somatosensory deficits that could not be identified by any of the three sensory dimensions (superficial, deep, and cortical sensation). The assessment of somatosensory function systematically covered trunk, limbs, and head with 29 items (superficial = 10; deep = 13, cortical = 3, and subjective = 3), and had a total score of 58 (three levels: 0, 1, 2). The score of somatosensory functioning assessed both the ipsilesional and contralesional sides, whereby deficit scores (determined by deficit item and level) were deducted from the total score of 58 [27]. It is noted that motor functioning was not systematically evaluated with Fugl-Meyer Assessment (FMA) as this type of patients generally had no complains on motor functioning compared to somatosensory deficits during the visit to our hospital.
Each session started with the assessment of resting motor threshold (RMT). RMT
was defined as the minimum intensity to induce motor-evoked potentials (MEPs)
Corticospinal excitability was measured with MEP amplitude and CSP at rest and during a sustained voluntary FDI muscle contraction respectively [21, 35]. A total of 40 single pulses (20 of each) were consecutively delivered to the hand region of the motor cortex at 110% RMT (45° to the midline, handle pointing backward). It is worth noting that CSP was evaluated following MEP as the muscle contraction during CSP may have an impact on MEP amplitude [36]. Corticospinal excitability was evaluated from both sides of the brain respectively and the sequence was counterbalanced across hemispheres.
MEP amplitude was calculated by peak-to-peak amplitude. The calculation of CSP
duration was based on the Mean Consecutive Difference (MCD) [37], which was
highly recommended by a recent expert review [35]. The MCD methodology is briefly
described here: (1) All silent period trials were rectified using the absolute
value and then were averaged; (2) The MCD of 100 ms EMG data before a TMS pulse
was calculated, in which the MCD is the mean successive difference between
individual data points; (3) Thresholds were set at:
Paired T-tests were performed to compare the ipsilesional and
contralesional motor cortex in terms of RMT, MEP amplitude, and CSP. We have also
calculated the ratio between the ipsilesional and contralesional motor cortex in
terms of corticospinal excitability and associated them with somatosensory
function using bivariate correlations and partial correlations controlling for
covariates. Independent T-tests were performed where possible when
patients were categorised based on results from corticospinal excitability (i.e.,
MEP ratio, see Results section). All the significant statistics were reported at
p
In addition to MEP amplitude and CSP, we have also evaluated MEP latency and its association with somatosensory functioning. MEP latency was defined as the time point where rectified EMG signals exceeded two standard deviations of the mean background EMG of 100 ms before the stimulus artifact [38, 39]. A paired T-test was performed to compare MEP latency from the ipsilesional and contralesional motor cortex.
The demographics and clinical information of patients were presented in Table 1. Patients had a mean age of 63.13 (male vs. female, 9:7). Lesions of the brain were mainly distributed in the thalamus, followed by damages to the brainstem and cortical regions such as the temporal cortex. Somatosensory functioning was overall preserved in our patients with a mean score of 51.88 out of a total score of 58. Patients had normal (5, n = 9/16) or slightly impaired (4, n = 7/16) muscle strength as indexed by the muscle strength grading scale (0–5).
| Gender | Age | Lesions | Duration (month) | Somatosensory function |
|---|---|---|---|---|
| F | 69 | Left thalamus | 2 | 57 |
| M | 70 | Right thalamus | 1 | 51 |
| M | 68 | Right temporal cortex | 1 | 50 |
| M | 58 | Left thalamus | 0.1 | 49 |
| F | 68 | Right pons | 0.5 | 49 |
| F | 73 | Left thalamus | 1 | 48 |
| F | 52 | Right pons | 1 | 51 |
| M | 66 | Right ventricle | 5 | 55 |
| M | 67 | Right basal ganglia | 1 | 53 |
| F | 57 | Left temporal cortex | 0.1 | 50 |
| M | 56 | Left pons | 0.5 | 56 |
| M | 57 | Left midbrain | 1 | 54 |
| F | 59 | Left ventricle | 0.1 | 49 |
| M | 56 | Left centrum semiovale | 0.3 | 51 |
| F | 67 | Right thalamus | 0.1 | 56 |
| M | 67 | Right thalamus | 0.1 | 51 |
A paired T-test indicated comparable MEP amplitude (p
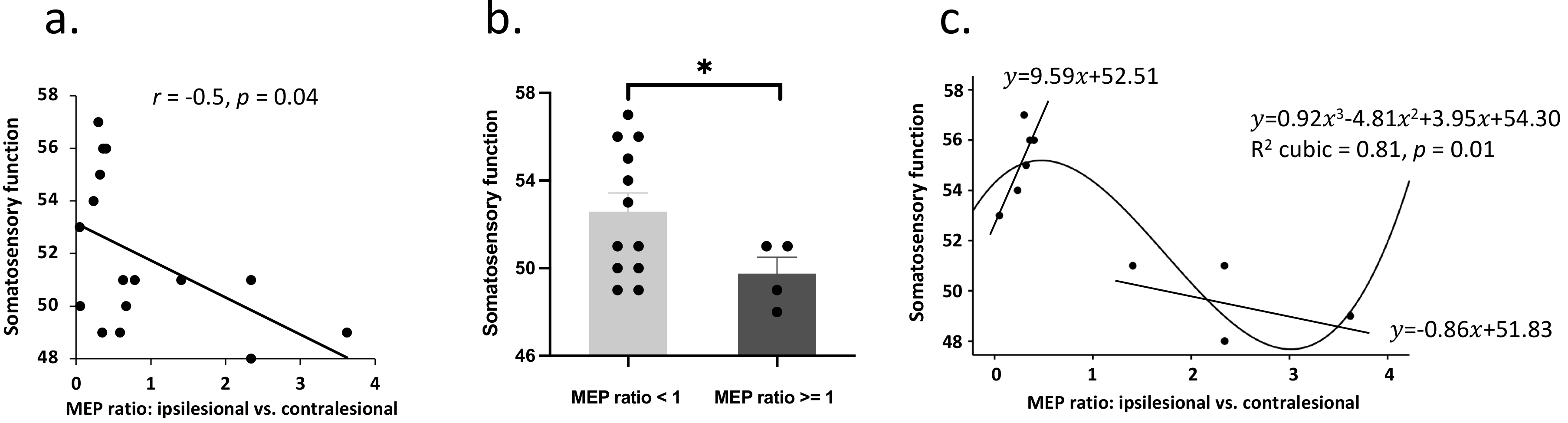
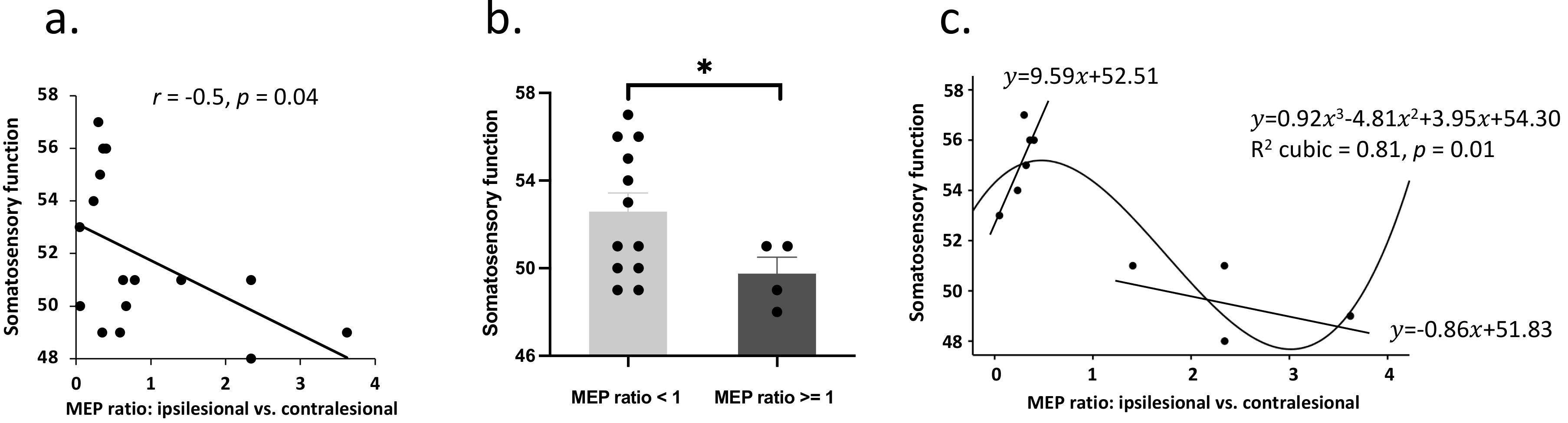 Fig. 2.
Fig. 2.Corticospinal excitability and the association with
somatosensory function. (a) group comparison indicated decreased somatosensory
functioning in patients with higher MEP amplitude on the ipsilesional
(vs. contralesional) motor cortex. (b) A linear model was fitted between
MEP ratio and somatosensory function in patients with a larger MEP amplitude in
the ipsilesional compared to the contralesional motor cortex. This model was not
significant potentially due to a small sample size. (c) In patients with well
preserved somatosensory function, increased MEP ratio was associated with better
somatosensory function. * denotes p
It is clear that this negative relationship between MEP ratio and somatosensory
function was driven by the uncommon presentation of patients with even larger
MEPs in ipsilesional vs. contralesional motor cortex. An independent
T-test (decided by equal MEP amplitude) was further performed on
somatosensory function, which revealed worse somatosensory function in patients
with a larger MEP amplitude in the ipsilesional compared to the contralesional
motor cortex (t
We further performed curve estimation between MEP ratio and somatosensory
function. A cubic model could best fit the relationship between MEP ratio and
somatosensory function (R
No significant relationship was found between CSP changes and somatosensory
functioning (p
A breakdown of somatosensory functioning indicated most damage to subjective sensations (percent change = 29.33%), followed by superficial sensations (16.65%), deep sensations (5.69%), and cortical sensations (1.67%).
Supplementary analyses indicated no significant difference in MEP latency
between the ipsilesional and contralesional motor cortex (t
This study was designed to evaluate the relationship between corticospinal excitability and somatosensory deficits following stroke. Results indicated that the uncommon presentation of larger MEPs in ipsilesional vs. contralesional motor cortex was associated with worse somatosensory function compared to those with a smaller MEP in ipsilesional motor cortex. Moreover, increased MEP ratio (ipsilesional vs. contralesional motor cortex) was associated with better somatosensory function in patients with well preserved somatosensory function. MEP and CSP changes in the ipsilesional (vs. contralesional) motor cortex were parallel following stroke.
Our data indicated a negative relationship between MEP ratio (ipsilesional vs. contralesional motor cortex) and somatosensory functioning. However, this finding does not necessarily have clinical implications for somatosensory improvement as it was driven by patients with larger MEPs in ipsilesional vs. contralesional motor cortex. Our data indicated individual differences in MEP ratio whereby some patients (4/16, 25%) demonstrated an even larger MEP amplitude in the ipsilesional vs. contralesional motor cortex (see Fig. 2). Further analysis demonstrated a significant lower somatosensory functioning in patients with a larger MEP amplitude in the ipsilesional motor cortex compared to those with a smaller one. This result highlights the clinical implications for MEP ratio in which a larger MEP amplitude in the ipsilesional veusus contralesional motor cortex indicates more prominent somatosensory deficits following stroke. The ipsilesional motor pathway is believed to generate a smaller MEP compared to the contralesional hemisphere [40]. However, it remains to be determined why a certain proportion of patients respond with a reverse pattern of MEP amplitude between the two hemispheres [41]. We also tried to model the relationship and present a potential negative relationship between MEP ratio and somatosensory functioning in patients with a larger MEP amplitude in the ipsilesional motor cortex. If this model reaches statistical significance in future studies with a larger sample, our preliminary finding could indicate the significance for somatosensory improvement by rebalancing corticospinal excitability between two hemispheres.
In addition, an increased MEP ratio (ipsilesional vs. contralesional motor cortex) was associated with better somatosensory function in patients with well-preserved somatosensory function as determined by the median of the overall sample. As discussed earlier, the effects of motor cortex rTMS have not been evaluated on somatosensory deficits in spite of a relatively large body of evidence on motor rehabilitation [11, 12]. Our data therefore provide novel findings that increasing MEP ratio (ipsilesional vs. contralesional motor cortex) may be able to indicate somatosensory improvement following stroke. In line with the motor recovery with rTMS, this could be achieved by excitating the ipsilesional motor cortex and/or inhibiting the contralesional motor cortex [9, 10]. Overall, our preliminary findings demonstrate the potential of corticospinal excitability in guiding rTMS treatments for somatosensory deficits following stroke.
It is interesting to find somatosensory functioning to be associated with MEP ratio but not with CSP ratio. It is consistent with our previous study in which sensory transmission was able to inhibit corticospinal excitation (i.e., MEP) rather than inhibition (i.e., CSP) [21]. Although we observed a significant covariance between changes in MEP ratio and CSP ratio, CSP measurements were not able to predict changes in somatosensory functioning whatsoever. These results indicate that MEP is more directly associated with changes in somatosensory functioning following stroke which could serve to direct TMS interventions for somatosensory improvement.
It would be more straightforward to consider targeting the primary sensory cortex (S1) for the improvement of somatosensory function following stroke. There is extensive evidence indicating TMS to be able to modulate S1 response in healthy subjects (for a review see [16]). For instance, a line of evidence indicates that stimulation of the sensory cortex is able to drive somatosensory cortex excitability and tactile sensations [42, 43, 44]. In stroke patients, one study further demonstrated daily S1-TMS for five days to facilitate motor learning [17], and one recent study combined S1-TMS with sensory stimulation (including sensory training, mirror therapy, and transcutaneous electrical nerve stimulation) to improve somatosensory function [18]. Overall, the efficacy of S1 stimulation is well supported for the improvement of somatosensory function following stroke. Moreover, stimulation of the dorsolateral prefrontal cortex is also able to modulate sensations like pain experience [45, 46, 47]. These findings are not mutually exclusive with our data. Future studies may wish to evaluate rTMS efficacy in post-stroke somatosensory functioning by targeting different brain regions.
This study was limited as it only evaluated MEP and CSP of the corticospinal
pathway. Other protocols like short interval intracortical inhibition (SICI) and
intracortical facilitation (ICF) are further able to evaluate GABA
Our findings provide insights for future studies. Our findings indicate the need for future investigations with a larger sample and more diversity in the phase of stroke. Building on these findings, future TMS protocols could be designed to improve somatosensory deficits by targeting corticospinal excitability following stroke.
To conclude, MEP ratio between the ipsilesional and contralesional motor cortex could indicate the improvement for somatosensory functioning following stroke. These findings indicate the importance to increase MEP ratio in patients with a lower ipsilesional MEP amplitude, as well as to rebalance corticospinal excitability in patients with an excessive ipsilesional MEP amplitude.
The datasets used and/or analyzed during the current study are available from the corresponding author (XC) on reasonable request.
ZG, JW, and XC contributed to study design, data collection, data analysis, and writing-up. QC, HF, JH, ZH, YJ, BT, YW, and YC contributed to data collection and manuscript drafting. All authors listed here have contributed significantly to this study and have agreed for this study to be published. All authors read and approved the final manuscript.
This study was approved by the Ethics Committee in the Affiliated Hospital of Hangzhou Normal University (2021-E2-HS-029) and was conducted in accordance with the Declaration of Helsinki. All participants provided a written informed consent before study commencement.
Not applicable.
ZG was supported by the Hangzhou Municipal Health Commission (2022WJCY011). XC was supported by the National Natural Science Foundation of China (4045F41120040), the Key Research and Development Program of Zhejiang Province (2022C03038), and Hangzhou Municipal Health Commission (2021WJCY130). JH was supported by the Hangzhou Municipal Health Commission (2022WJCY012), and Huzhou Science and Technology Bureau (2021GY63). The authors declare no competing financial interests.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.