1 Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 430030 Wuhan, Hubei, China
Abstract
Objectives: Intracranial hemorrhage is the second most common stroke subtype following ischemic stroke and usually induces high mortality and disability. Here, we conducted a retrospective study to establish a nomogram clinical prediction model. Methods: First, the baseline data of patients who presented to our hospital in 2015–2021 were collected and compared (789 patients for the training cohort and 378 patients for the validation cohort). Second, univariate and binary logistic analyses were performed to screen out alternative indicators. Finally, a clinical prediction model by nomogram was established that included such indicators to estimate the prognosis of intracranial hemorrhage patients. Results: Univariate logistic analysis was used to screen several possible impact factors, including hypertension, hematoma volume, Glasgow Coma Scale (GCS) score, intracranial hemorrhage (ICH) score, irregular shape, uneven density, intraventricular hemorrhage (IVH) relation, fibrinogen, D-dimer, low density lipoprotein (LDL), high-density lipoprotein (HDL), creatinine, total protein, hemoglobin (HB), white blood cell (WBC), neutrophil blood cell (NBC), lymphocyte blood cell (LBC), the neutrophil lymphocyte ratio (NLR), surgery, deep venous thrombosis (DVT) or pulmonary embolism (PE) rate, hospital day, and hypertension control. Further binary logistic analysis revealed that ICH score (p = 0.036), GCS score (p = 0.000), irregular shape (p = 0.000), uneven density (p = 0.002), IVH relation (p = 0.014), surgery (p = 0.000) were independent indicators to construct a nomogram clinical prediction model. The C statistic was 0.840. Conclusions: ICH score, GCS score, irregular shape, uneven density, IVH relation, surgery are easily available indicators to assist neurologists in formulating the most appropriate therapy for every intracranial hemorrhage patient. Further large prospective clinical trials are needed to obtain more integrated and reliable conclusions.
Keywords
- intracerebral hemorrhage
- nomogram
- prognosis
- clinical study
- retrospective
Intracranial hemorrhage is the second most common type of stroke after ischemic stroke. It is estimated that intracranial hemorrhage occurs in approximately 10–30 per 100,000 people each year, with a mortality rate of up to 50% within one month of intracranial hemorrhage, and functional independence is achieved in less than 40% of patients [1, 2]. The incidence rate is increasing, especially in developing countries [3]. A total of 60–70% of cases of intracranial hemorrhage are attributed to hypertension, usually located in the caudate-putamen (basal nuclei), thalamus, cerebellum and pons [4, 5, 6], which presents more serious performance issues and poor prognosis. However, there is still no appropriate treatment to improve the prognosis of patients with intracerebral hemorrhage [2, 7]. Due to the sudden onset of cerebral hemorrhage and poor prognosis, it is very important for the neurologist and the patient’s family to accurately evaluate the prognosis of the patient, especially their ability to live independently. There are several clinical prediction models [8], and the most commonly used model is Hemphill’s ICH (intracranial hemorrhage) score [9]. However, these scores are not very conveniently or widely used. Nomograms are visual displays of regression equations, which has gained widespread attention in recent years [10, 11]. Here, we used several common indicators to create a nomogram clinical prediction model to assist clinicians in assessing the prognosis of intracranial hemorrhage.
This retrospective study was approved by the Ethics Committee of Tongji Hospital of the Huazhong University of Science and Technology (TJ-IRB20220118). Informed consents were obtained from patients.
We continuously enrolled all spontaneous intracranial hemorrhage patients (confirmed by non-contrast brain computerized tomography (CT), including supratentorial and infratentorial hemorrhage) who presented to our hospital in the period from 2015–2021. A total of 789 patients who came from the Tongji Hospital affiliated to Tongji Medical College of Huazhong University of Science & Technology were included in the training cohort, and 378 patients who came from Optics Valley Hospital of HUST Tongji Hospital and Sino-French New City Campus (two branch of Tongji Hospital) were included in the validation cohort.
The inclusion criteria were as follows: (1) patients aged
The exclusion criteria were as follows: (1) patients with primary IVH (intraventricular hemorrhage) or subarachnoid hemorrhage (SAH); (2) patients with secondary intracranial hemorrhage (arteriovenous malformation (AVM), moyamoya disease, aneurysm, coagulopathy, brain tumor and amyloid angiopathy); (3) patients with incomplete medical records.; and (4) patients with a lack of follow-up data.
Baseline data was collected for the following: sex, age, medical history (hypertension, diabetes, ischemic stroke, intracranial hemorrhage), personal history (smoking, drinking), drug use history (antihypertension drugs, hypoglycemic agents, antiplatelet drugs), systolic blood pressure, diastolic blood pressure, hematoma volume, Glasgow Coma Scale (GCS) score, hemorrhage location, irregular shape [12], uneven density [13], IVH relation, blood test indicators (activated partial thromboplastin time (APTT), prothrombin time (PT), the international normalized ratio (INR), fibrinogen, D-dimer, low density lipoprotein (LDL), triglyceride, high-density lipoprotein (HDL), total cholesterol, creatinine, aspartate aminotransferase (AST), total protein, calcium, hemoglobin (HB), white blood cell (WBC), neutrophil blood cell (NBC), lymphocyte blood cell (LBC), the neutrophil lymphocyte ratio (NLR), red blood cell distribution width (RDW), platelet distribution width (PDW), platelet (PLT)), surgery, deep venous thrombosis (DVT) or pulmonary embolism (PE) rate, hospital day, hypertension control, and follow-up data (modified Rankin Scale (mRS) score at six months follow-up).
The categorical data is expressed as percentages. If the continuous data
satisfied the normal distribution and equal variances, the mean
In the period from 2015–2021, 2114 ICH patients presented to our hospital. 947 patients were excluded as follows: 39 patients had primary IVH; 120 had primary SAH; 427 had incomplete records; 98 had secondary ICH; 12 had drug-induced ICH; 24 had amyloid angiopathy; 9 had intracranial venous sinus thrombosis; 196 had cerebral infarction hemorrhage transformation; and 22 had neoplastic bleeding. A total of 1167 patients were ultimately enrolled and divided into a training cohort and a validation cohort according to the visiting branch.
The baseline characteristics between the training cohort and validation cohort were compared (Table 1). The mean ages were 56.31 years old and 58.74 years old. The sex ratio was approximately 2:1. The alcohol, hematoma volume, ICH score, IVH relation, fibrinogen, HDL, creatinine, total protein, calcium, WBC, NBC, NLR, RDW, surgery, hospital day were higher in the training cohort than in the validation cohort (p = 0.01, p = 0.000, p = 0.000, p = 0.000, p = 0.000, p = 0.005, p = 0.028, p = 0.000, p = 0.000, p = 0.000, p = 0.000, p = 0.000, p = 0.000, p = 0.000, p = 0.019 respectively), and the GCS score, irregular shape, uneven density, D-dimer, AST, LBC was lower in the training cohort than in the validation cohort (p = 0.000, p = 0.000, p = 0.01, p = 0.015, p = 0.019, p = 0.000 respectively). The remaining indicators were not significantly different.
| Indicators | Training | Validation | Test value | p | |
|---|---|---|---|---|---|
| Age | 56.31 |
58.74 |
3.313 | 0.74 | |
| Sex | Male | 553 | 250 | 1.859 | 0.173 |
| Female | 236 | 128 | |||
| Hypertension | 563 | 258 | 1.179 | 0.278 | |
| Diabetes | 84 | 35 | 0.537 | 0.464 | |
| Ischemic | 74 | 24 | 3.05 | 0.081 | |
| Intracranial hemorrhage | 57 | 23 | 0.52 | 0.471 | |
| Smoke | 265 | 123 | 0.126 | 0.722 | |
| Alcohol | 250 | 92 | 6.659 | 0.01 | |
| Antihypertensive drugs | 310 | 146 | 0.048 | 0.827 | |
| Glucose-lowering drugs | 66 | 22 | 2.374 | 0.123 | |
| Antiplatelet agents | 57 | 21 | 1.141 | 0.285 | |
| SBP | 155.26 |
155.52 |
0.183 | 0.569 | |
| DBP | 91.52 |
93.08 |
1.735 | 0.309 | |
| Hematoma volume | 11.16 | 8.2 | –5.005 | 0.000 | |
| GCS score | 11.94 |
13.72 |
7.351 | 0.000 | |
| ICH score | 1 | 0 | –7.484 | 0.000 | |
| Hemorrhage | 1 | 644 | 325 | 3.443 | 0.064 |
| Location | 2 | 145 | 53 | ||
| Irregular shape | 216 | 173 | 38.896 | 0.000 | |
| Uneven density | 171 | 108 | 6.685 | 0.01 | |
| IVH | 258 | 82 | 14.995 | 0.000 | |
| APTT | 36.6 |
36.8 |
0.828 | 0.408 | |
| PT | 13.54 |
13.62 |
1.022 | 0.307 | |
| INR | 1.09 |
1.07 |
0.375 | 0.708 | |
| Fibrinogen | 3.8 |
3.34 |
6.292 | 0.000 | |
| D-dimer | 0.62 | 0.7 | 2.431 | 0.015 | |
| LDL | 2.8 |
2.76 |
0.783 | 0.434 | |
| Triglycerides | 1.5 |
1.44 |
0.887 | 0.375 | |
| HDL | 1.19 |
1.13 |
2.842 | 0.005 | |
| Total cholesterol | 4.38 |
4.32 |
1.059 | 0.29 | |
| Creatinine | 73 | 71.5 | 2.204 | 0.028 | |
| AST | 17 | 18 | 2.34 | 0.019 | |
| Total protein | 72.31 |
69.71 |
6.092 | 0.000 | |
| Calcium | 2.28 |
2.18 |
4.923 | 0.000 | |
| HB | 138.94 |
137.79 |
0.956 | 0.339 | |
| WBC | 9.80 |
8.42 |
5.183 | 0.000 | |
| NBC | 7.82 |
6.10 |
8.13 | 0.000 | |
| LBC | 1.24 |
1.49 |
4.648 | 0.000 | |
| NLR | 6.1 | 3.8 | 8.363 | 0.000 | |
| RDW | 43.22 |
41.71 |
6.135 | 0.000 | |
| PDW | 13.75 |
13.75 |
0.004 | 0.997 | |
| PLT | 216.24 |
210.71 |
1.245 | 0.213 | |
| Surgery | 148 | 23 | 16.791 | 0.000 | |
| DVT or PE | 85 | 31 | 1.889 | 0.169 | |
| Hospital day | 17.43 |
15.77 |
2.35 | 0.019 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; GCS, Glasgow Coma Scale; ICH, intracranial hemorrhage; IVH, intraventricular hemorrhage; APTT, activated partial thromboplastin time; PT, prothrombin time; INR, international normalized ratio; LDL, low density lipoprotein; HDL, high-density lipoprotein; AST, aspartate aminotransferase; HB, hemoglobin; WBC, white blood cell; NBC, neutrophil blood cell; LBC, lymphocyte blood cell; NLR, neutrophil lymphocyte ratio; RDW, red blood cell distribution width; PDW, platelet distribution width; PLT, platelet; DVT, deep venous thrombosis; PE, pulmonary embolism.
Then, we used univariate logistic analysis to identify possible indicators that
influenced the mRS score at six months follow-up. Hypertension (p =
0.005), hematoma volume (p = 0.000), GCS score (p = 0.000), ICH
score (p = 0.000), irregular shape (p = 0.000), uneven density
(p = 0.000), IVH relation (p = 0.000), fibrinogen (p =
0.003), D-dimer (p = 0.011), LDL (p = 0.039), HDL (p =
0.000), creatinine (p = 0.003), total protein (p = 0.014), HB
(p = 0.018), WBC (p = 0.000), NBC (p = 0.000), LBC (p = 0.000), NLR (p = 0.000), surgery (p
= 0.000), DVT or PE rate (p = 0.004), hospital day (p = 0.011),
and hypertension control (p = 0.000) were significantly different
between patients with a good prognosis (mRS score at six months follow-up
| Indicators | B | Error | Wald | p | OR | Lower | Upper |
|---|---|---|---|---|---|---|---|
| Hypertension | 0.452 | 0.161 | 7.924 | 0.005 | 1.572 | 1.147 | 2.154 |
| Hematoma volume | –0.069 | 0.007 | 91.881 | 0 | 0.933 | 0.92 | 0.946 |
| GCS score | 0.405 | 0.039 | 106.24 | 0 | 1.499 | 1.388 | 1.619 |
| ICH score | –0.888 | 0.087 | 104.731 | 0 | 0.411 | 0.347 | 0.488 |
| Irregular shape | 1.733 | 0.204 | 72.264 | 0 | 5.658 | 3.794 | 8.437 |
| Uneven density | 1.233 | 0.206 | 35.912 | 0 | 3.433 | 2.293 | 5.139 |
| IVH | 0.817 | 0.163 | 25.226 | 0 | 2.263 | 1.646 | 3.113 |
| Fibrinogen | –0.191 | 0.064 | 9.049 | 0.003 | 0.826 | 0.729 | 0.935 |
| D-dimer | –0.066 | 0.026 | 6.472 | 0.011 | 0.936 | 0.89 | 0.985 |
| LDL | 0.186 | 0.09 | 4.268 | 0.039 | 1.204 | 1.01 | 1.436 |
| HDL | –0.842 | 0.23 | 13.356 | 0 | 0.431 | 0.274 | 0.677 |
| Creatinine | –0.002 | 0.001 | 8.909 | 0.003 | 0.998 | 0.996 | 0.999 |
| Total protein | –0.028 | 0.011 | 6.086 | 0.014 | 0.973 | 0.951 | 0.994 |
| HB | 0.009 | 0.004 | 5.639 | 0.018 | 1.009 | 1.002 | 1.017 |
| WBC | –0.193 | 0.025 | 61.298 | 0 | 0.825 | 0.786 | 0.865 |
| NBC | –0.235 | 0.026 | 79.612 | 0 | 0.79 | 0.751 | 0.832 |
| LBC | 1.25 | 0.162 | 59.866 | 0 | 3.49 | 2.543 | 4.791 |
| NLR | –0.207 | 0.021 | 93.213 | 0 | 0.813 | 0.78 | 0.848 |
| Surgery | 2.358 | 0.292 | 65.182 | 0 | 0.095 | 0.053 | 0.168 |
| DVT or PE | 1.051 | 0.267 | 15.497 | 0 | 2.859 | 1.695 | 4.824 |
| Hospital day | –0.031 | 0.007 | 19.659 | 0 | 0.969 | 0.956 | 0.983 |
| Hypertension control | 1.312 | 0.192 | 46.709 | 0 | 0.269 | 0.185 | 0.392 |
OR, odds ratio; GCS, Glasgow Coma Scale; ICH, intracranial hemorrhage; IVH, intraventricular hemorrhage; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HB, hemoglobin; WBC, white blood cell; NBC, neutrophil blood cell; LBC, lymphocyte blood cell; NLR, neutrophil lymphocyte ratio; DVT, deep venous thrombosis; PE, pulmonary embolism.
ICH score (p = 0.036, Odds Ratio (OR) = 1.472, 95% confidence interval (CI) 1.026–2.11), GCS score
(p = 0.000, OR = 1.503, 95% CI 1.352–1.671), irregular shape
(p = 0.000, OR = 2.652, 95% CI 1.65–4.262), uneven density (p
= 0.002, OR = 2.171, 95% CI 1.326–3.554), IVH relation (p = 0.014, OR
= 1.93, 95% CI 1.142–3.262), surgery (p = 0.000, OR = 3.903, 95% CI
2.039–7.474) were independent risk factors for the mRS score (
| Indicators | B | Error | Wald | p | OR | Lower | Upper |
|---|---|---|---|---|---|---|---|
| ICH score | 0.386 | 0.184 | 4.416 | 0.036 | 1.472 | 1.026 | 2.11 |
| GCS score | 0.407 | 0.054 | 56.702 | 0.000 | 1.503 | 1.352 | 1.671 |
| Irregular shape | 0.975 | 0.242 | 16.227 | 0.000 | 2.652 | 1.65 | 4.262 |
| Uneven density | 0.775 | 0.252 | 9.497 | 0.002 | 2.171 | 1.326 | 3.554 |
| IVH | 0.658 | 0.268 | 6.039 | 0.014 | 1.93 | 1.142 | 3.262 |
| Surgery | 1.362 | 0.331 | 16.884 | 0.000 | 3.903 | 2.039 | 7.474 |
ICH, intracranial hemorrhage; GCS, Glasgow Coma Scale; IVH, intraventricular hemorrhage.
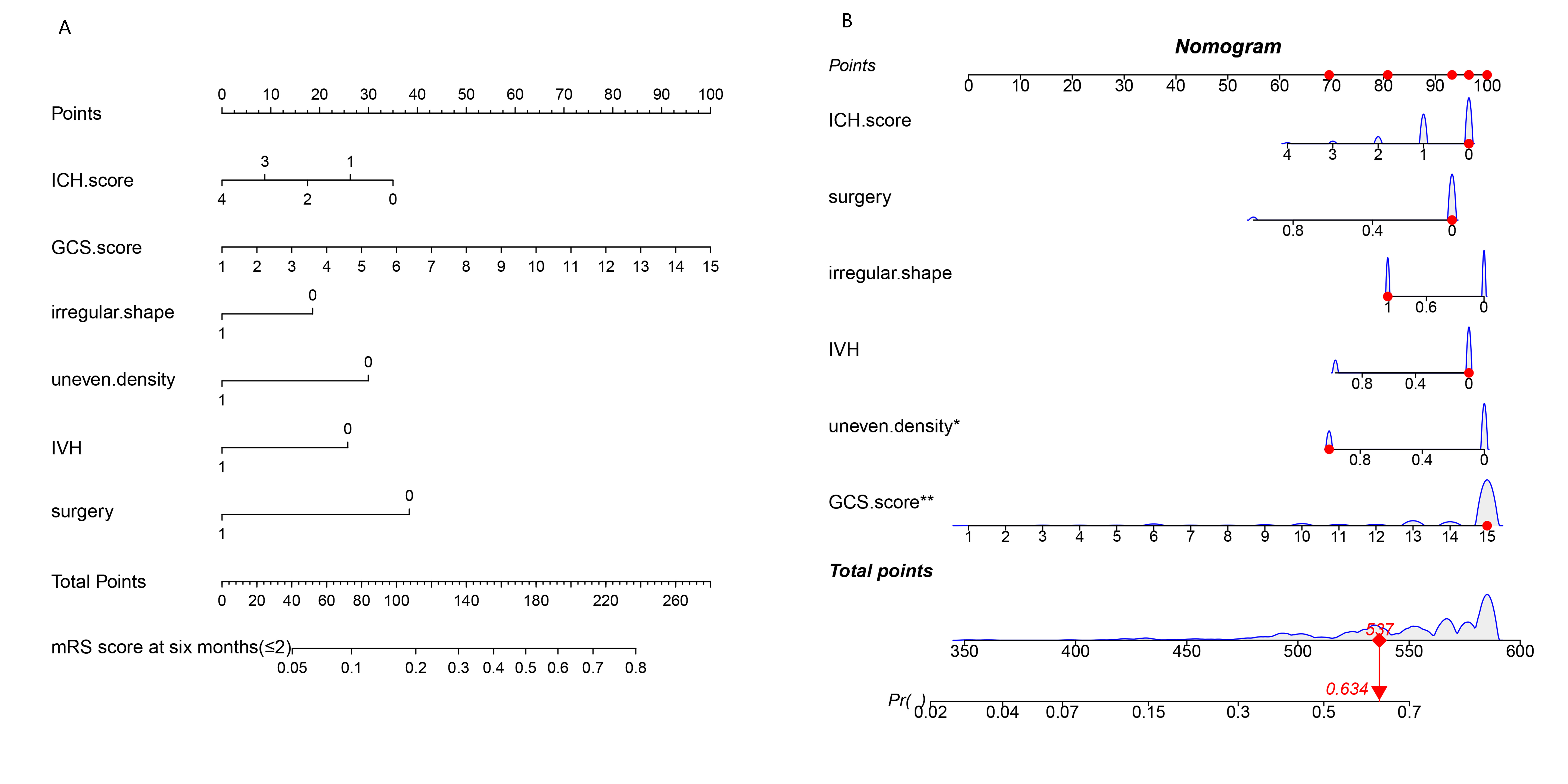
A nomogram to predict good prognosis (mRS score at six months follow-up
 Fig. 1.
Fig. 1.Nomogram plot. (A) Conventional nomogram plot. (B) Pattern nomogram plot. Total points indicate the total points and are the sum of the four indicators (hemorrhage location, IVH, D-dimer, hypertension control rate). Pr is the probability of obtaining a good prognosis from the total points.
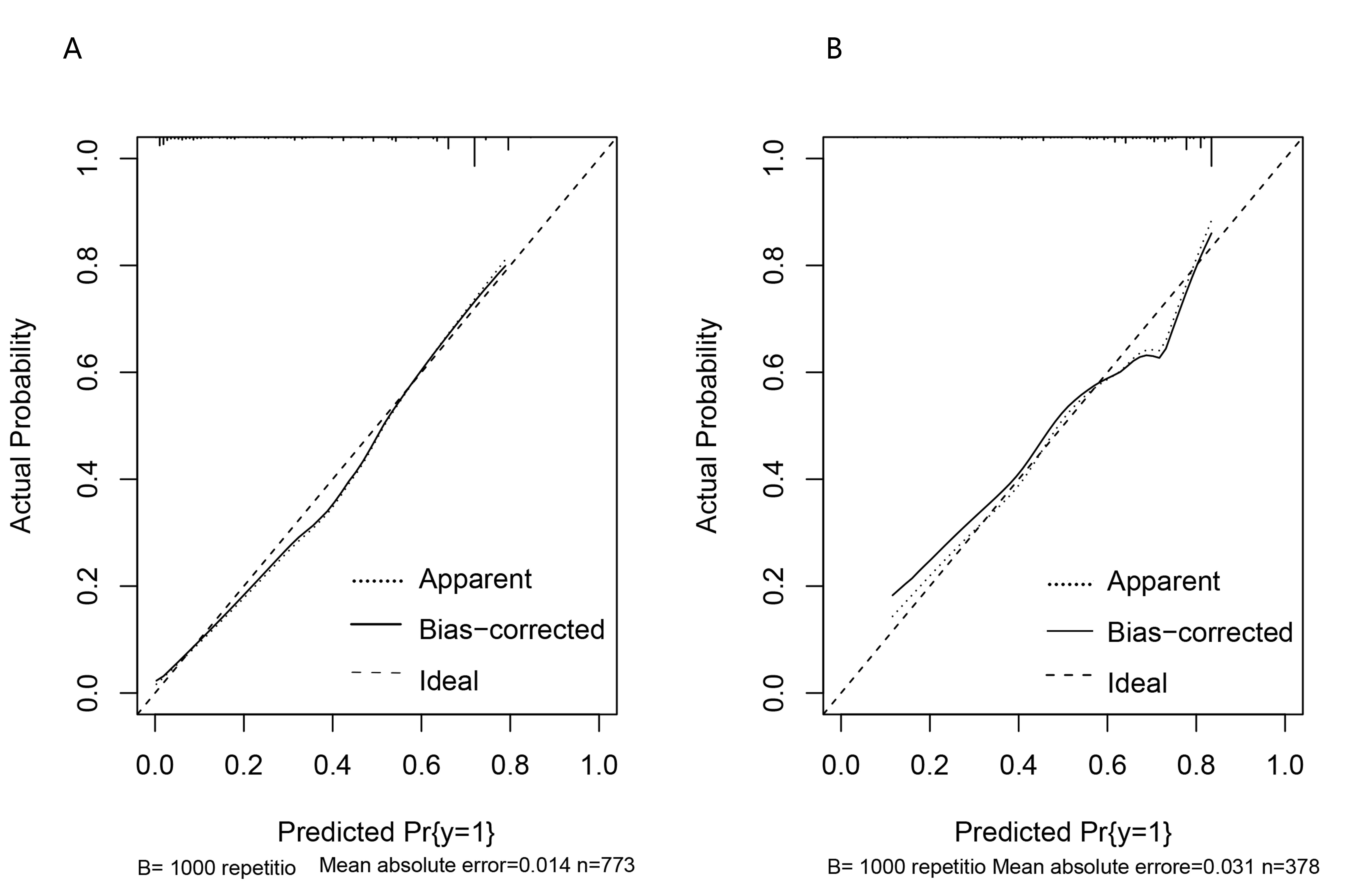
The calibration curve shows the consistency between the probability of a good prognosis for the patient predicted by the model and the actual result. The calibration curve showed good calibration (Fig. 2A,B).
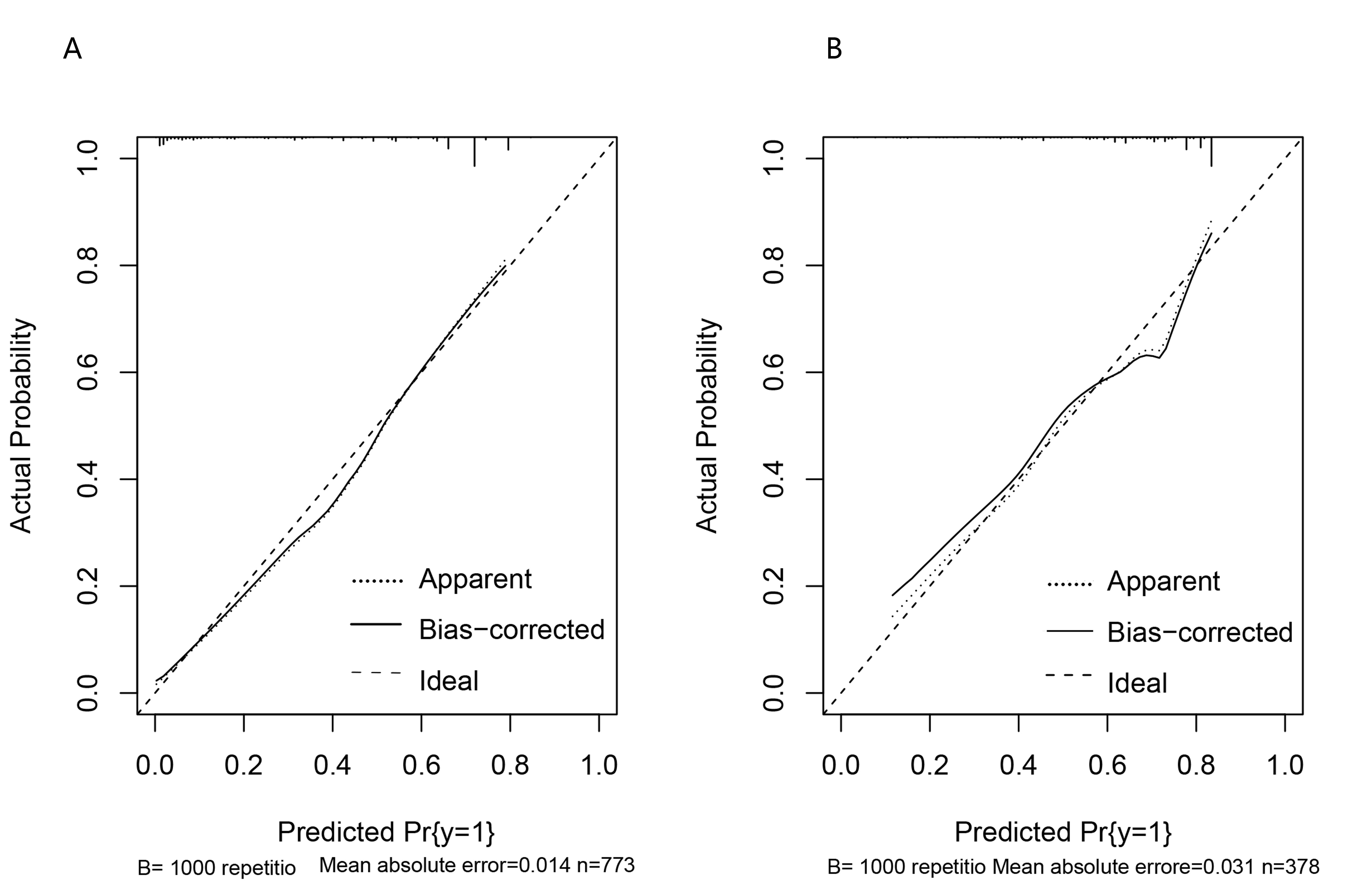 Fig. 2.
Fig. 2.Calibration curve. (A) The calibration curve for the training curve. (B) The calibration curve for the verification curve.
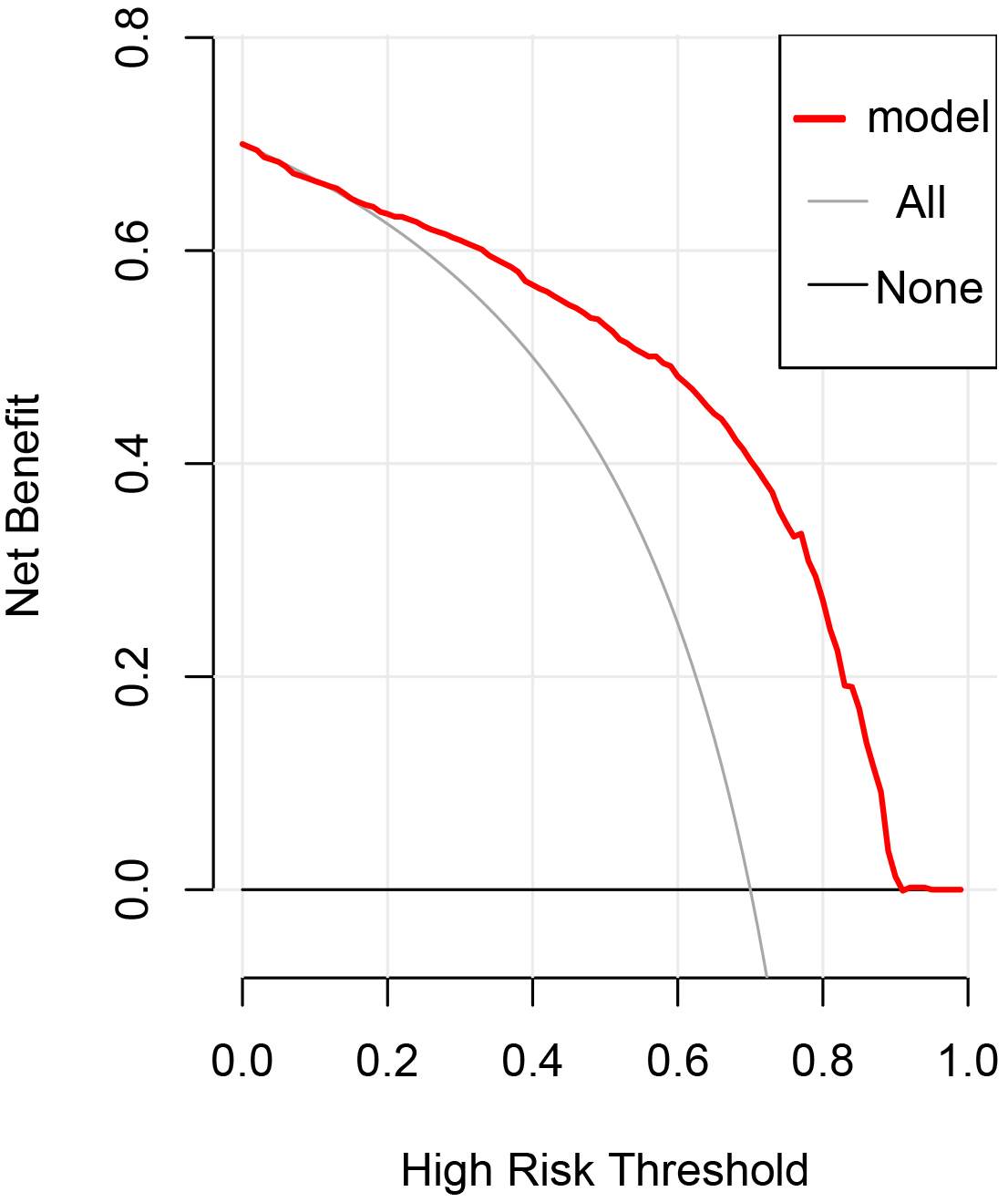
The DCA (decision curve analysis) curve of this model is shown in Fig. 3. The
threshold probability was
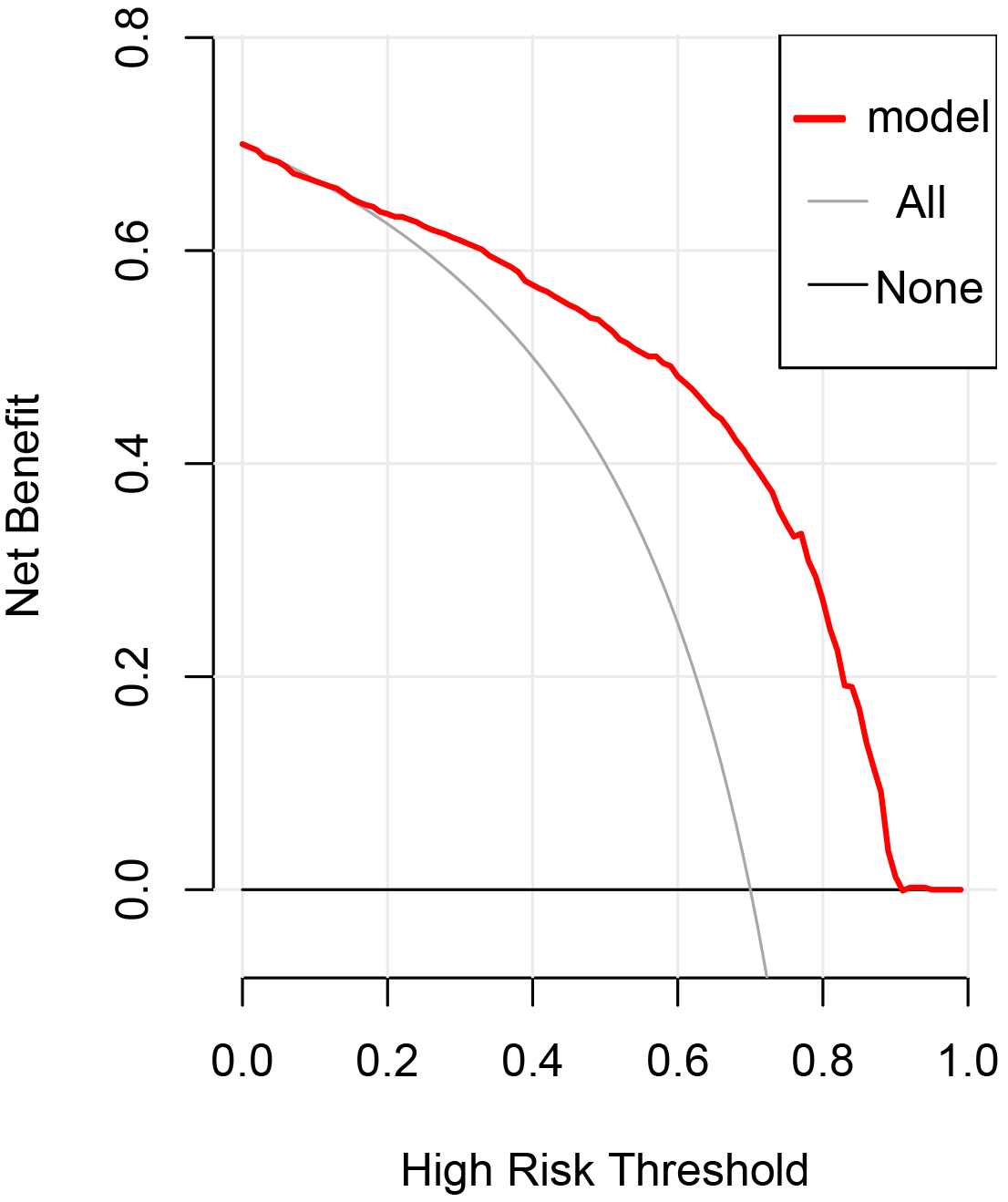 Fig. 3.
Fig. 3.Decision curve analysis of the prediction model in the
validation cohort. The dashed line represents the model, the thick solid line
represents the assumption that all subjects are actively treated, and the thin
solid line indicates that all patients are not treated. Decision curve analysis
(DCA) shows that when the threshold probability
Despite the development of many advanced surgical approaches and standard medical treatments, it has been reported that only 12–39% of hemorrhage patients acquire the ability to live independently [14]. It is critical to accurately assess the prognosis of hemorrhage patients to assist clinicians in formulating the best poststroke care program. Here, we used several indicators to construct a convenient clinical prevention model. Our results showed that the ICH score, GCS score, irregular shape, uneven density, IVH relation and surgery were related to the outcomes of intracranial hemorrhage patients. Some scholars have reported that intracranial hemorrhage patient prognosis is related to age, GCS score, blood pressure, hematoma location and volume [15, 16], intraventricular hemorrhage, use of anticoagulation drugs, hematoma expansion [17] and some inflammatory factors [18], which is consistent with our results.
Hemphill’s ICH score is a widely used scoring system that incorporates admission GCS score, age, hematoma volume, IVH relation, and infratentorial/supratentorial location [19, 20]. A meta-analysis conducted by Mattishent et al. [19] showed that the Hemphill-ICH score had the most validation queues (9 studies involving 3819 patients), and the area under the curve (AUC) was 0.80. The GCS score assesses the consciousness of patients by eye-opening response, verbal response, and motor response. Shah’s results showed that the GCS score was independently associated with functional outcomes at three months after traumatic intracranial hemorrhage [16]. Wang’s [21] results showed that both the GCS score and ICH score independently predicted 30-day mortality in ICH patients. Similarly, our results showed that the ICH score and GCS score at admission were independent predictors of 6-month prognosis in patients with spontaneous ICH. The ICH score and GCS score objectively reflect the state of ICH patients, which is potentially related to hematoma volume and other indicators and is likely to predict prognosis.
The irregular shape of the intracranial hemorrhage indicates multiple sites of hemorrhage, while the uneven density indicates active hemorrhage [22]. Therefore, some scholars speculate that these two imaging features can predict the prognosis of ICH patients [23]. Barras’ results showed that ICH patients with irregular shapes had larger bleeding volumes and were more likely to experience hematoma enlargement than patients with regular shapes, and uneven density was an independent predictor of hematoma enlargement [22]. Delcourt’s results showed that irregular shape was an independent predictor of death and severe disability in ICH patients, but uneven density was not a significant predictor of prognosis [24]. Masotti’s results showed that ICH patients with irregularly shaped hematomas were more likely to require observation in the intensive care unit (ICU) ward [25]. Wang’s results showed that irregular shape was independently associated with 30-day mortality in ICH patients [21]. Combined with our study, irregular shape and uneven density are important indicators for predicting the prognosis of ICH patients. More attention should be given to ICH patients with the abovementioned imaging characteristics to obtain a good prognosis.
According to previous reports, intracranial hemorrhage rupture into the
ventricle is a predictor of poor prognosis [26]. Our univariate analysis and
multivariate analysis showed that the IVH relationship was an independent
predictor of prognosis. A retrospective study by Nishikawa et al. [27]
showed that older age, IVH volume, acute hydrocephalus, and poor initial level of
consciousness were independent predictors of poor prognosis of spontaneous
intracranial hemorrhage. Some scholars found that enlarged ventricle hemorrhage
in patients with spontaneous intracranial hemorrhage was also associated with
poor prognosis [28, 29, 30]. Li et al. [30] found that increased ventricular
hemorrhage (newly bleeding ventricular hemorrhage or an increase
In some emergency situations, surgical treatment is an emergency measure to save
the lives of ICH patients. The surgical methods include craniotomy, minimally
invasive surgery and decompression surgery. Whether surgery improves the outcome
of ICH patients compared with conservative treatment has not been determined. The
Surgical Trial in Intracerebral Haemorrhage (STICH) study found that early craniotomy hematoma removal did not improve the
prognosis of ICH patients and may be beneficial for patients with hematoma
locations
A predictive model of patients with hypertensive intracranial hemorrhage
established by Ding et al. [35] found that a GCS score
There are several nomogram models for predicting the prognosis of patients with intracranial hemorrhage. Han et al. [1] established a nomogram model to predict 30-day mortality in patients with spontaneous intracranial hemorrhage, incorporating the GCS score, hematoma location, hematoma volume, white blood cell count, and D-dimer indicators. Similarly, the GCS score, hematoma location, hematoma volume, and primary intraventricular hemorrhage were included to construct a nomogram for predicting death within 2 days in intracranial hemorrhage patients [45]. In this nomogram model established by Song et al. [46] to predict the functional status (good: mRS score 0–3, poor: mRS score 4–6) of spontaneous ICH patients at the 3-month follow-up, midline shift, noncontrast computed tomography (NCCT) time from sICH onset, GCS score, serum glucose levels, uric acid levels, and Radiomics Score (Rad-score) were included. Comparing these results with the results of the current study, the short-term prognosis (2-day mortality) of patients with intracranial hemorrhage was mainly related to the characteristics of intracranial hemorrhage (GCS score, hematoma location, hematoma volume and IVH). After gradual stabilization (30-day mortality, mRS score at 3 months, mRS score at 6 months), prognosis may be related to other factors (D-dimer level, serum glucose level, uric acid level, white blood cell count and long-term blood pressure control).
As mentioned earlier, we used the ICH score, GCS score, irregular shape, uneven density, IVH relation and surgery to construct this clinical predictive model. According to the C statistic (0.840), this prediction model has good discriminability. The calibration curve shows that the model has good calibration in the training cohort and similar results in the validation cohort. In future clinical work, the use of the abovementioned convenient and simple indicators can accurately assess the possibility of intracranial hemorrhage patients living independently six months later and provide important information for the formulation of rehabilitation programs.
There are several limitations in our study. First, this is a single-center study, and the problem of selection bias cannot be completely avoided. Second, this was a retrospective study, and errors were inevitable in data collection. Third, the sample size of our data was small, so it was difficult to avoid statistical errors in the process of data analysis. Therefore, in some cases, the application of the prediction model should be combined with clinical findings. In the future, the results of prospective studies with large samples may increase the reliability and generalization of the prediction model.
We established a nomogram model to predict the prognosis of patients with intracranial hemorrhage that included the indicators of ICH score, GCS score, irregular shape rate, uneven density, IVH relation and surgery. The model needs to be confirmed in more large clinical trials.
GCS, Glasgow Coma Scale; ICH, intracranial hemorrhage; IVH, intraventricular hemorrhage; LDL, low density lipoprotein; HDL, high-density lipoprotein; HB, hemoglobin; WBC, white blood cell; NBC, neutrophil blood cell; LBC, lymphocyte blood cell; NLR, neutrophil lymphocyte ratio; DVT, deep venous thrombosis; PE, pulmonary embolism; CT, computerized tomography; SAH, subarachnoid hemorrhage; AVM, arteriovenous malformation; APTT, activated partial thromboplastin time; PT, prothrombin time; INR, international normalized ratio; AST, aspartate aminotransferase; RDW, red blood cell distribution width; PDW, platelet distribution width; PLT, platelet; mRS, modified Rankin Scale; OR, odds ratio; AUC, area under the curve; ICU, intensive care unit; NCCT, noncontrast computed tomography.
Research program formulation and data collection—YL, XL, JW CP; paper writing—YL; language polishing, paper review and editing—CP, ZT.
This study was approved by the Ethics Committee of Tongji Hospital of the Huazhong University of Science and Technology (TJ-IRB20220118). Informed consents were obtained from patients.
We are very grateful to Gaigai Li for his help in improving the language of this article.
This work was supported by grant 81901219 and 82071330 from National Natural Science Foundation of China.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.