1 Science and Technology Innovation Center, Guangzhou University of Chinese Medicine, 510405 Guangzhou, Guangdong, China
2 Clinical Medical College of Acupuncture Moxibustion and Rehabilitation, Guangzhou University of Chinese Medicine, 510405 Guangzhou, Guangdong, China
Abstract
Background: Fo-Shou-San (FSS) is a traditional Chinese medicine (TCM) decoction that can effectively treat vascular dementia (VD). In the face of unclear pharmacological mechanisms, we set out to validate that FSS treats chronic cerebral hypoperfusion (CCH)-induced cognitive impairment in mice. Methods: CCH animal model caused by permanent right unilateral common carotid arteries occlusion (rUCCAO) was established to verify that FSS could treat subcortical ischemic vascular dementia (SIVD). We performed novel object recognition test and Morris water maze test, observed morphological changes via HE and Nissl staining, and detected hippocampus apoptosis by TUNEL staining and oxidative stress by biochemical assays. Ferroptosis-related markers and NRF2/HO-1 signaling-related expressions were examined via qPCR and immunofluorescence staining. Results: We found that FSS ameliorated cognitive disorders, and lessened oxidative stress by decreasing MDA and GSH-PX while increasing the reduced glutathione (GSH)/oxidized glutathione disulfide (GSSG) ratio, which are associated with ferroptosis. Additionally, FSS reduced expression of SLC7A11, GPX4, ROX and 4HNE, as vital markers of ferroptosis. Further, FSS regulated NRF2/HO-1 signaling by downregulating NRF2 and HO-1. Conclusions: Our study suggests that FSS may ameliorate chronic cerebral hypoperfusion-induced cognitive deficits through regulation of the NRF2/HO-1 pathway against ferroptosis. Taken together, our study highlights the neuroprotective efficacy of FSS.
Keywords
- Fo-Shou-San
- vascular dementia
- cognitive impairment
- ferroptosis
Vascular dementia (VD), the second most prevalent form of dementia, leads to cognitive impairment among elderly people [1]. Clinically, chronic cerebral hypoperfusion frequently causes subcortical ischemic vascular dementia (SIVD), a subtype of vascular dementia [2]. Pathological changes of SIVD include white matter injury, microglial activation, oligodendrocyte apoptosis and progressive demyelination [3, 4, 5]. The permanent right unilateral common carotid arteries occlusion (rUCCAO) mice model was performed as a chronic cerebral hypoperfusion (CCH) animal model mimicking pathophysiological characteristics of SIVD patients [6]. At present, there are no effective treatments for SIVD [7]. Thus, it is imperative for novel drugs to restrain the pathogenetic progress of SIVD.
Primary features of Traditional Chinese medicine (TCM) are synergistic and complementary effects and multi-target action [8]. Fo-Shou-San (FSS) is a traditional Chinese herbal decoction first published in Puji Benshi Fang in the Song Dynasty (AD 1132) by Xu Shuwei, containing Angelica sinensis (AS, Danggui, DG) and Ligusticum wallichii (LW, Chuanxiong, CX) in a weight ratio of 3:2. Initially, FSS was intended for obstetrical and gynecological diseases, such as dystocia and intrauterine fetal death [9]. According to TCM theory and previous research, FSS enhances immune, circulatory, and hematopoietic systems, whereby DG dispels blood stasis and CX nourishes blood circulation [10]. In recent years, FSS has been widely applied to various diseases, especially cerebral vascular impairment [11, 12, 13]. Accordingly, we set out to determine whether FSS could treat vascular dementia in mice, underlying its specific mechanism.
There is a rising focus on the impact of ferroptosis on neurological diseases [14]. Studies have revealed that inhibitors of ferroptosis lessen neuronal deterioration and improve neurological impairment induced by intracerebral hemorrhage and ischemic stroke [15, 16, 17]. Ferroptosis depends on the accumulation of iron ions, leading to non-apoptotic oxidation and lipid super oxidation including reactive oxygen species accumulation and polyunsaturated fatty acid consumption [18]. Three key components represent the main molecular mechanism of ferroptosis: reduced glutathione (GSH) utilized by glutathione peroxidase 4 (GPX4), cystine uptake by glutamate transporter solute carrier family 7 membrane 11 (SLC7A11), and existence of iron ions and lipid reactive oxygen species (ROS) [19].
Research has demonstrated a close relationship between vascular cognitive impairment (VCI) and ferroptosis [20]. In CCH-induced cognitive damage, iron deposition leads to oxidative stress wherein the most severe neuronal damage occurs in the CA1 region where iron most accumulates [21, 22]. Cognitive impairment is associated with abnormal iron accumulation in SIVD patients’ cortical zones [23]. Moreover, researchers found that enlarged nuclear factor erythroid 2-related factor 2 (NRF2) expression ameliorates the cognitive impairment caused by CCH [24].
Recently, it has been found that NRF2 performs a nuclear transcription factor to regulate cellular antioxidant response. Once activated, NRF2 binds with antioxidant response elements (ARE) to initiate a series of genes against oxidative stress [25, 26]. There are two primary types of antioxidant substances related to NRF2 in the nervous system: GSH-related regulatory enzymes, and Heme oxidation system, in particular, heme oxygenase-1 (HO-1) [27, 28]. Research has demonstrated that activation of NRF2 and its downstream target genes in vascular dementia models clarifies neuroprotective influence of NRF2 on ischemic injury and oxidative stress [29, 30, 31, 32, 33].
Herein, we set out to explore a potential therapeutic for SVID. First, we applied FSS treatment in rUCCAO-induced cognitive impairment mice. We then investigated the underlying pharmacological mechanisms whereby FSS regulates the NRF2/HO-1 pathway against ferroptosis.
FSS comprises Angelica sinensis (Lot No. G01201124-06) and Ligusticum wallichii (Lot No. B21051401-01), which were obtained from the Guangzhou Medicine Company in accordance with the standard in Pharmacopoeia of People’s Republic of China. The herbs were separated into small fragments in a weight ratio of 3:2, soaked in 10 volumes of purified water for 2 h, and boiled in the volatile oil extraction device for 6h. Residues were immersed in eight volumes of distilled water overnight. The extract was boiled three times for one hour. The filtrate was then concentrated by rotary evaporator. Lastly, the crude water-extracted drugs combined with the volatile oil were stored at –20 °C [9, 34, 35, 36].
Male C57BL/6 mice, 12 weeks old and 25–28 g, were acquired from Experimental
Animal Center of Guangzhou University of Chinese Medicine (License:
SCXK2018-0034). All procedures under principles for animal experiments were
approved by Guangzhou University of Chinese Medicine Animal Ethics Committee
(approval ID: 20210615006). Moreover, nimodipine (N837938, Macklin, China), a
dihydropyridine Ca
The rUCCAO mice were placed under general anesthesia through a facemask with 5% isoflurane for anesthesia induction and 1.5% isoflurane for anesthesia maintenance. Exposed by a middle cervical cut, the right common carotid artery was double ligated with 6-0 silk distal and proximal to heart. The same process without carotid ligation was operated on the sham group. Body temperature was sustained until mice were woken from anesthesia [39, 40, 41, 42, 43].
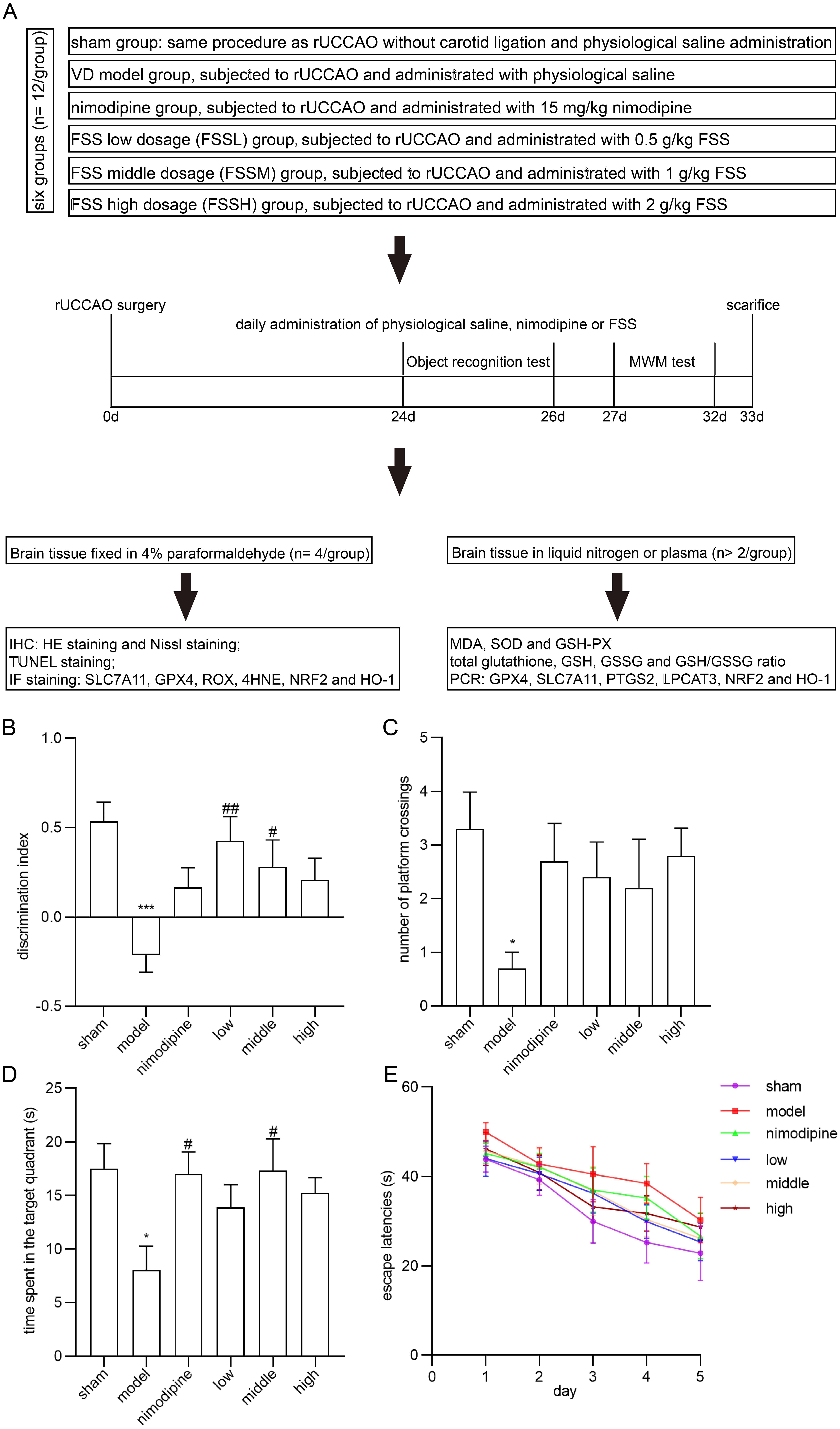
The mice were separated into six groups (n = 12/group): sham group (same procedure as rUCCAO without carotid ligation and physiological saline administration), VD model group (subjected to rUCCAO and administrated with physiological saline), nimodipine group (subjected to rUCCAO and administrated with 15 mg/kg nimodipine), FSS low dosage (FSSL) group (subjected to rUCCAO and administrated with 0.5 g/kg FSS), FSS middle dosage (FSSM) group (subjected to rUCCAO and administrated with 1 g/kg FSS), and FSS high dosage (FSSH) group (subjected to rUCCAO and administrated with 2 g/kg FSS). Intragastrical administration followed rUCCAO once daily from the time of surgery (day 0) for 32 days. Following behavioral tests performed from day 24 to day 32, mice were sacrificed to store brain samples for further analysis (Fig. 1A).
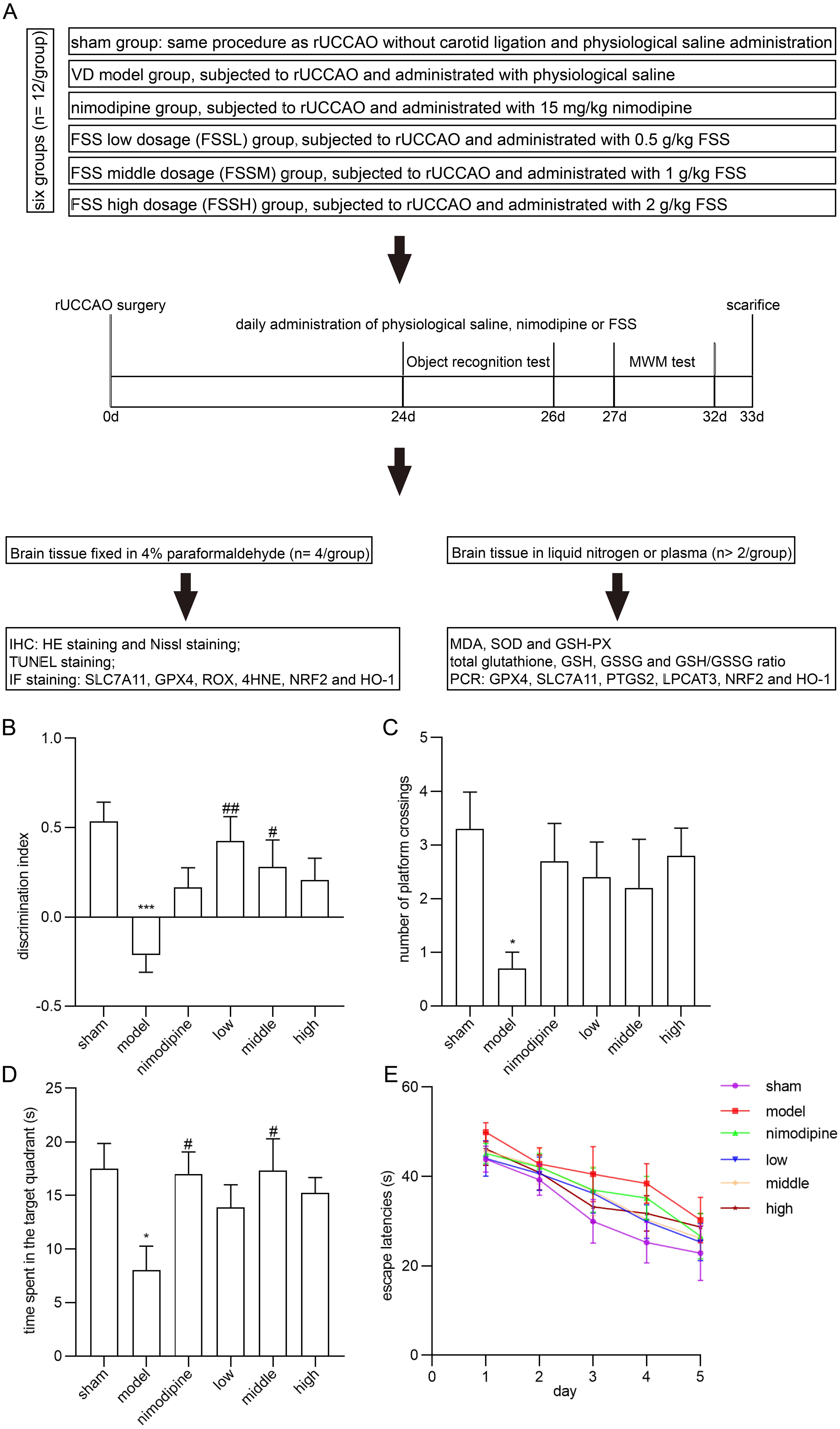 Fig. 1.
Fig. 1.FSS improved cognitive functions after chronic cerebral
hypoperfusion. (A) Experimental schedule in vivo. (B) Object recognition
test. (C–E) Morris water maze test. n = 10 mice per group. ***p
The experiment site was kept bright and quiet. On day 24, mice were placed into
a white experimental box (40 cm
Procedures were performed as described in a previous study [45]. In a five-day learning period (days 27–31), mice were randomly trained to discover a hidden platform at the center of one of the four equal quadrants of a pool in order to calculate the average escape latency. On the sixth day of testing (day 32), swimming parameters were recorded of mice exploring the pool without the platform to assess cognitive memory.
On day 33 following rUCCAO surgery, cervical dislocation was performed followed by intracardiac injection of phosphate buffer solution (PBS). Brain tissue was collected and divided for liquid nitrogen storage at –80 °C for biochemical analysis, as well as for morphological and immunohistochemistry analysis as IHC, IF and TUNEL fixed in 4% paraformaldehyde.
The brains of four mice from each group were embedded in paraffin, cut 5
The frozen sections of brain (30
TUNEL detection employed the one-step TUNEL kit (Beyotime, Shanghai, China);
brain slices were saturated in 4% paraformaldehyde and incubated in
immunostaining strong permeable solution at room temperature. Then, slices were
saturated with TUNEL reaction mixture, PBS-washed three times and stained with
DAPI (1.0
RNA was isolated by RNAiso Plus (Takara, Beijing, China) from brain tissue.
PrimeScript™RT Master Mix (Takara, Beijing, China) compounded
first-strand cDNA under the corresponding protocol. The cDNA was enlarged by TB
Green® Premix Ex Taq™ II (Takara, Beijing, China)
in a 20
| mRNA | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| Lpcat3 | GACGGGGACATGGGAGAGA | GTAAAACAGAGCCAACGGGTAG |
| PTGS2 | TGAGCAACTATTCCAAACCAGC | GCACGTAGTCTTCGATCACTATC |
| NFE2L2 | TTTGTAGATGACCATGAGTCGC | GCCAAACTTGCTCCATGTCC |
| Hmox1 | GTCCCAGGATTTGTCCGAGG | GGAGGCCATCACCAGCTTAAA |
| SLC7A11 | GAAGCATTCCCAGGGGCTAA | AGGTGTCTCACCAAGGATGTC |
| GPX4 | CTGCCGTGCTATCTCTAGCC | TATTCCCACAAGGCAGCCAA |
The mice eyeballs from each group were removed to collect blood. Blood samples were centrifuged at 3500 rpm for 15 min to separate serum. Serum levels of Malondialdehyde (MDA) (Beyotime, Shanghai, China), Superoxide Dismutase (SOD) (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) and Glutathione peroxidase (GSH-PX) (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) were performed by matching assay kits according to instructions [49, 50, 51]. Glutathione (GSH) activity, oxidized glutathione disulfide (GSSG) activity, the ratio of GSH/GSSG and total glutathione in corpus striatum were tested by the GSH and GSSG Assay Kit (Beyotime, Shanghai, China) [52].
Data were analyzed by GraphPad Prism 8.0 software (GraphPad Inc., La Jolla, CA,
USA), and results shown as mean
Some reports have found that chronic cerebral hypoperfusion triggers cognitive damage [53, 54]. To assess whether FSS could improve cognitive functions, we conducted a series of behavioral tests. FSS made a considerable improvement in discrimination skills for the novel object recognition test (Fig. 1B). In the probe test of Morris water maze test, results indicated that the number of platform crossings (Fig. 1C) and time spent in target quadrant (Fig. 1D) increased in mice treated with FSS and nimodipine compared to model group. As for the learning period, administration of nimodipine and FSS in mice raised their spatial learning ability to locate a find hidden platform as shown by less average escape latency versus the rUCCAO mice (Fig. 1E). This is a clear indication that FSS could improve cognitive functions after chronic cerebral hypoperfusion.
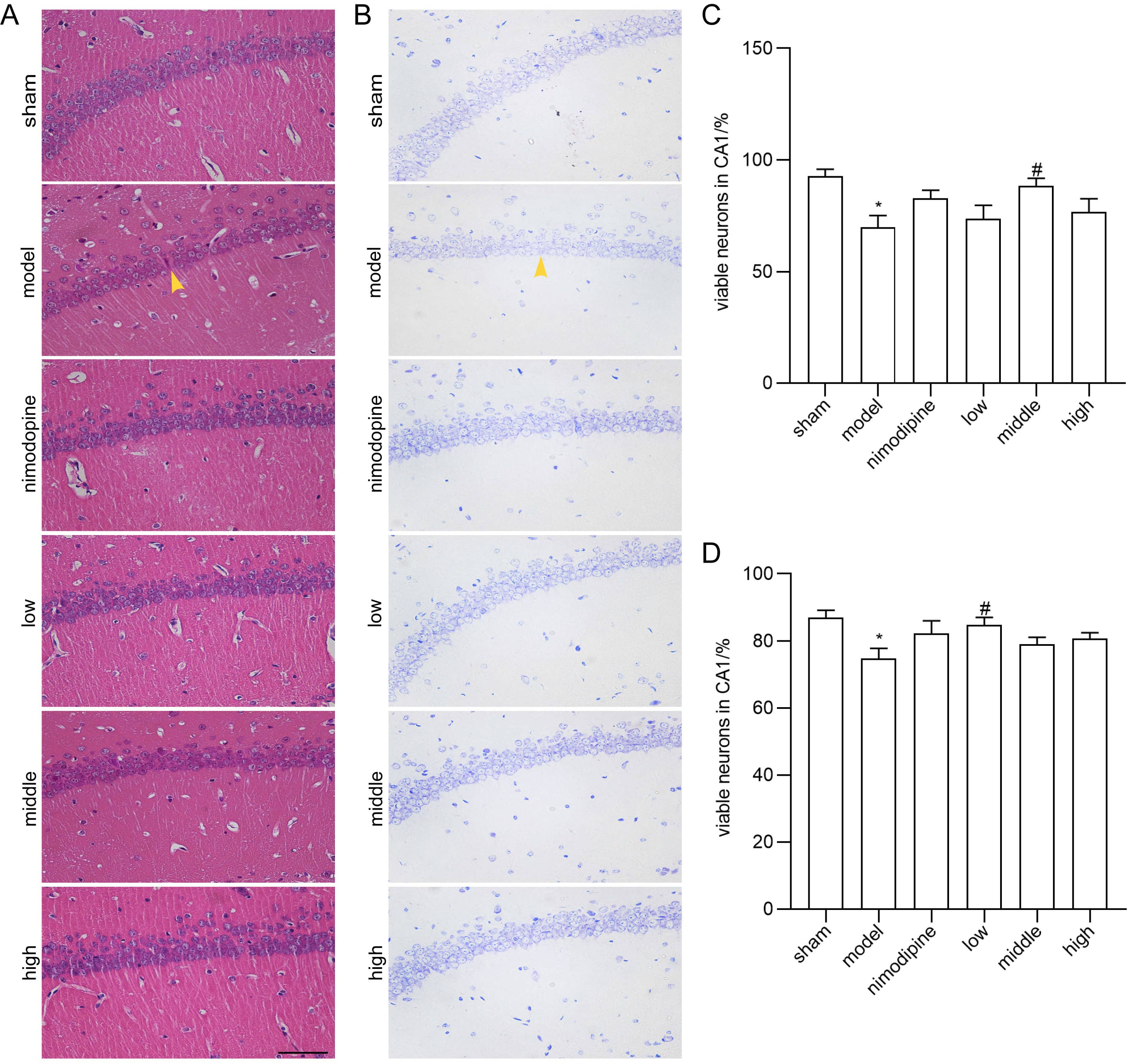
We applied HE and Nissl staining to examine neuron death in the CA1 area of hippocampus damaged by rUCCAO. In HE staining, FSS and nimodipine treatment attenuated the morphological changes of VD mice, while neurons in the sham group had round or oval nuclei (Fig. 2A and C). Similarly in Nissl staining, FSS and nimodipine treatment prohibited neuron loss (Fig. 2B and D). Together, these data indicate that FSS could prevent hippocampal neuron loss through chronic cerebral hypoperfusion.
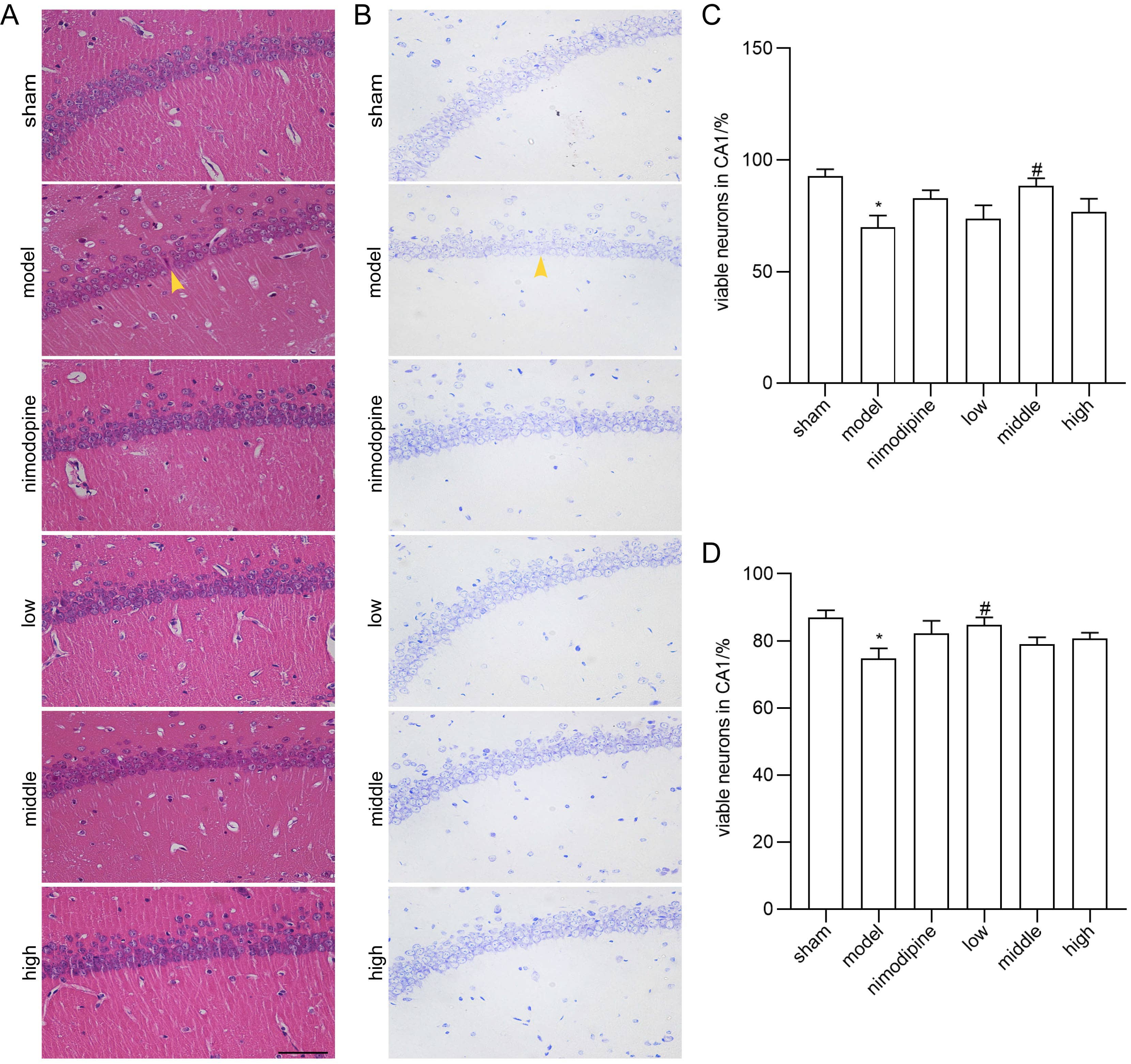 Fig. 2.
Fig. 2.FSS prevented neuron loss after chronic cerebral hypoperfusion.
Representative photomicrographs of the histopathological changes in the area CA1
of left hippocampus in mice. (A) HE staining. (B) Nissl staining. Quantification
of numbers of (C) HE-stained and (D) Nissl-stained survival neurons. n = 4.
Magnification = 400
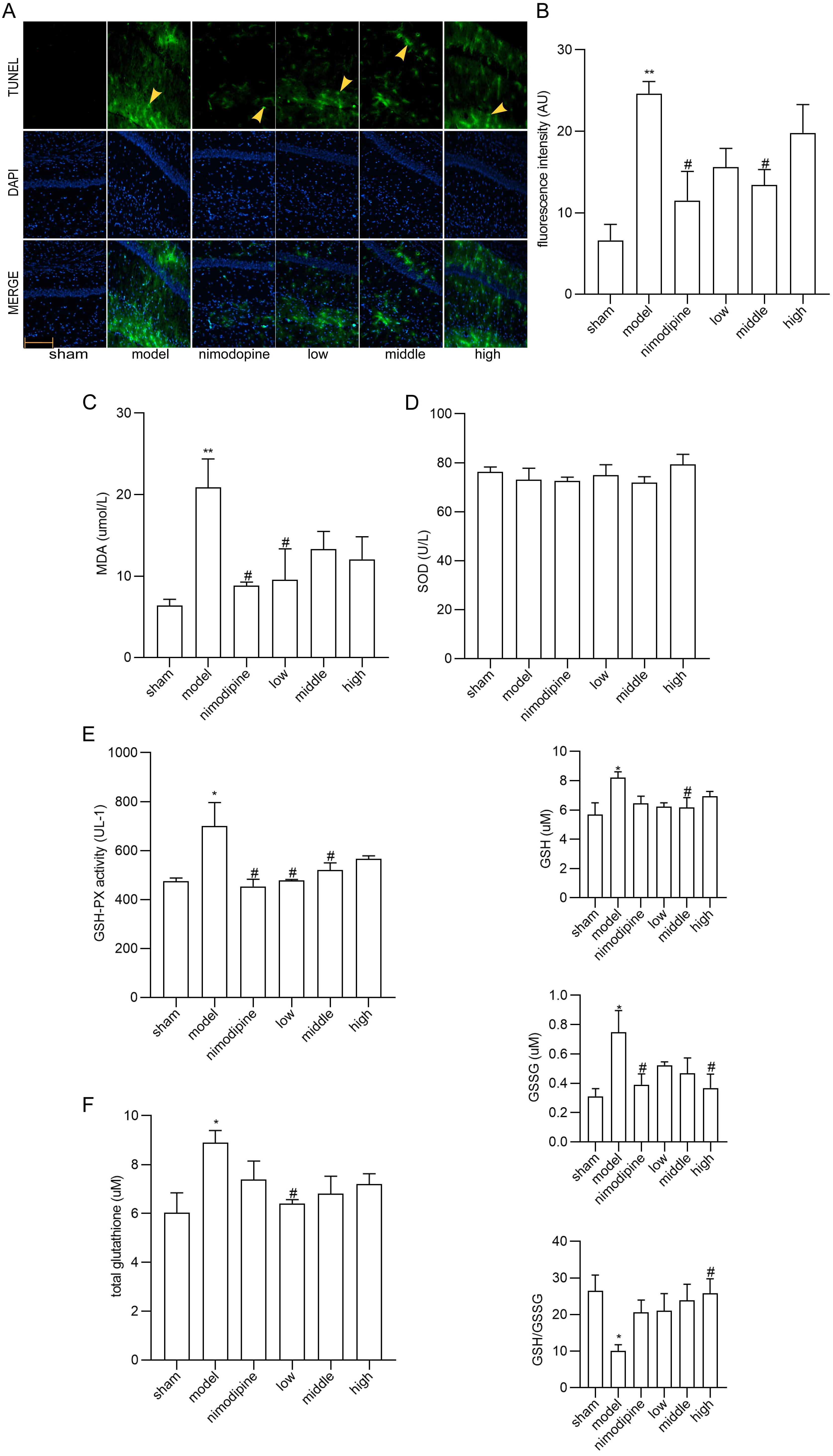
TUNEL staining showed that neurons in the CA1 area of hippocampus displayed apoptosis ameliorated by FSS and nimodipine (Fig. 3A,B). Moreover, as for the MDA, SOD and GSH-PX content in serum (Fig. 3C,D and E), MDA and GSH-PX were decreased by FSS and nimodipine versus the model group, while FSS showed no impact on SOD. Compared with model group, total glutathione, GSH and GSSG levels in corpus striatum decreased in the FSS and nimodipine treatment group. Accordingly, FSS and nimodipine could improve GSH/GSSG ratio in contrast with the model group (Fig. 3F). All data indicate that FSS could attenuate apoptosis and oxidative stress after chronic cerebral hypoperfusion.
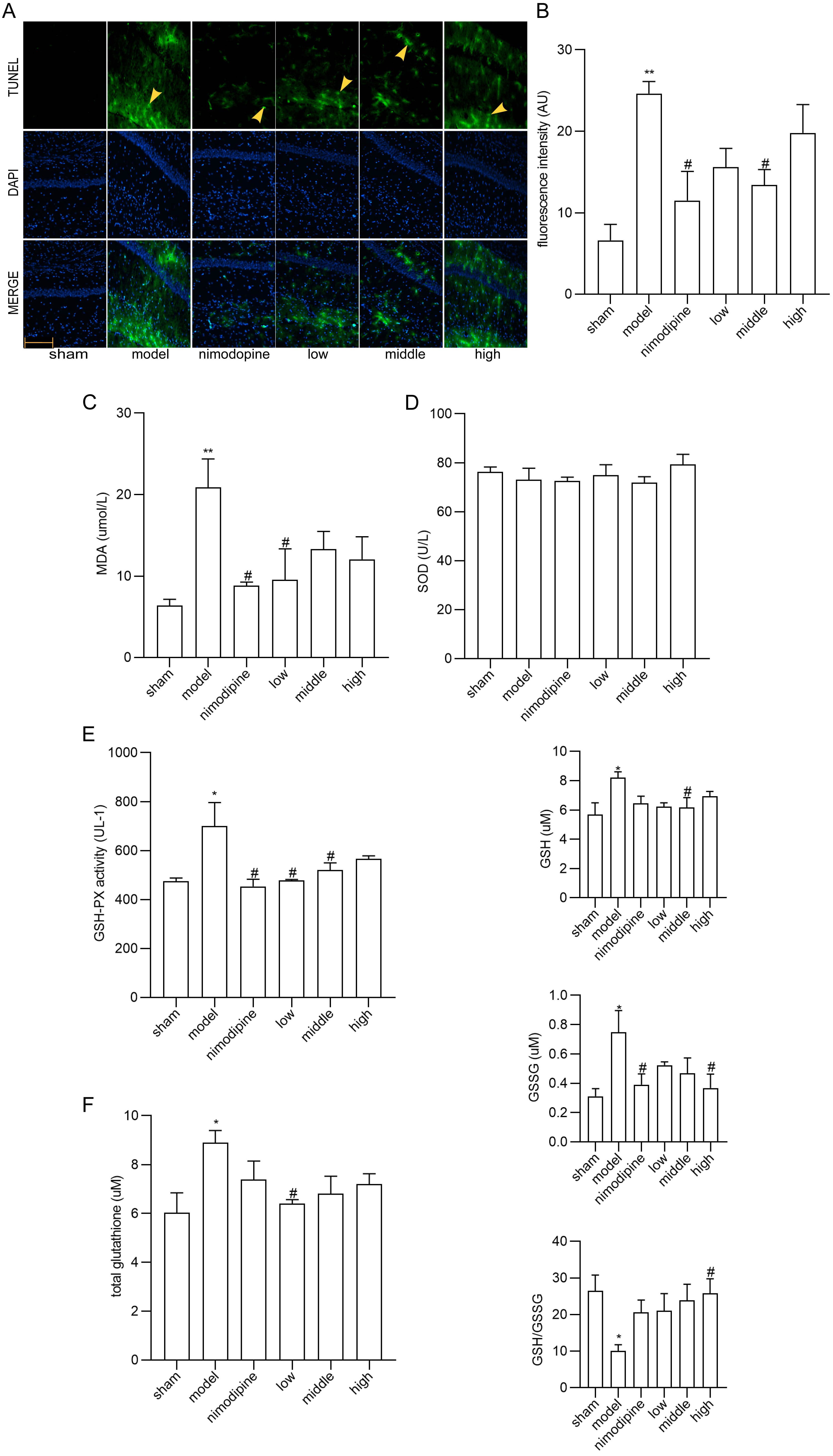 Fig. 3.
Fig. 3.FSS attenuated apoptosis and oxidative stress in hippocampus
after chronic cerebral hypoperfusion. (A) TUNEL staining in the area CA1 of left
hippocampus. Magnification = 200
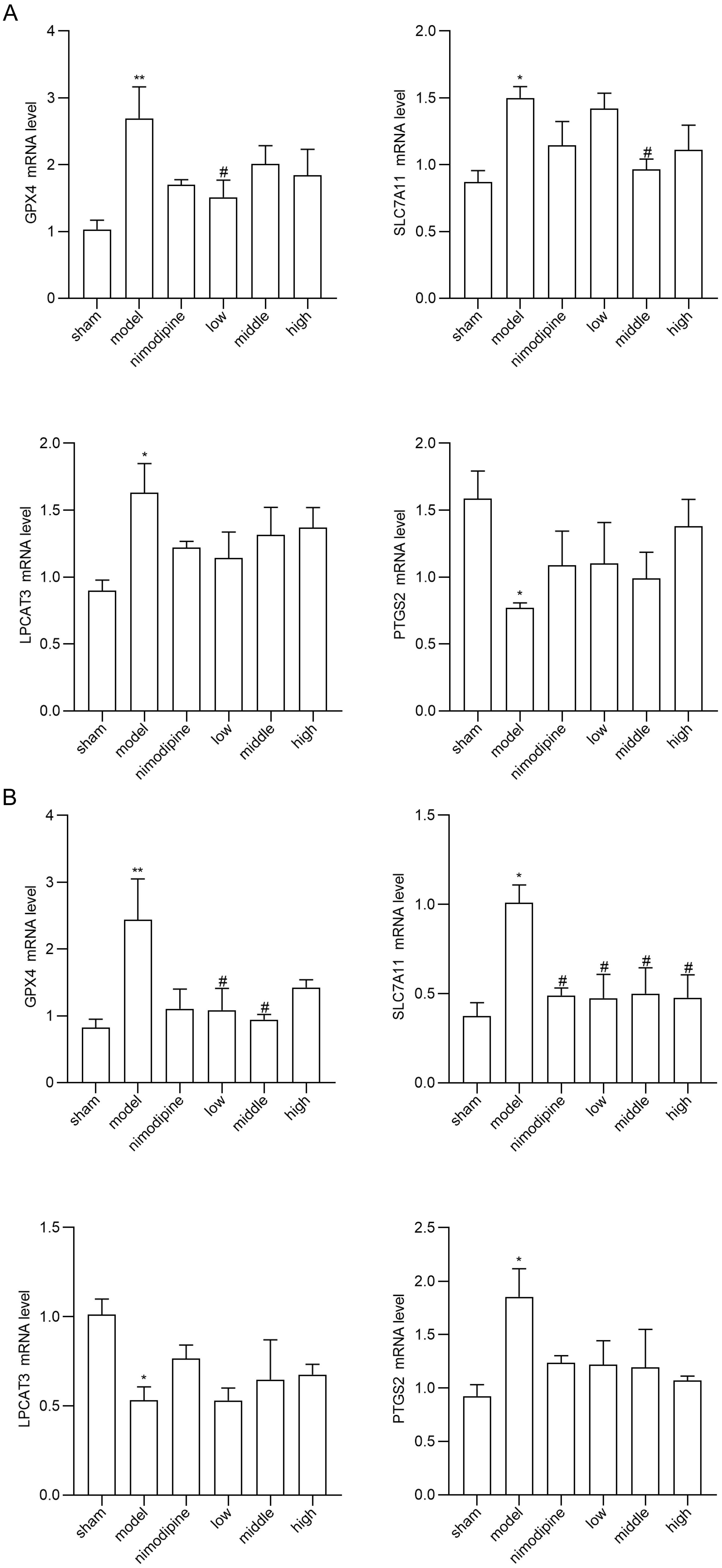
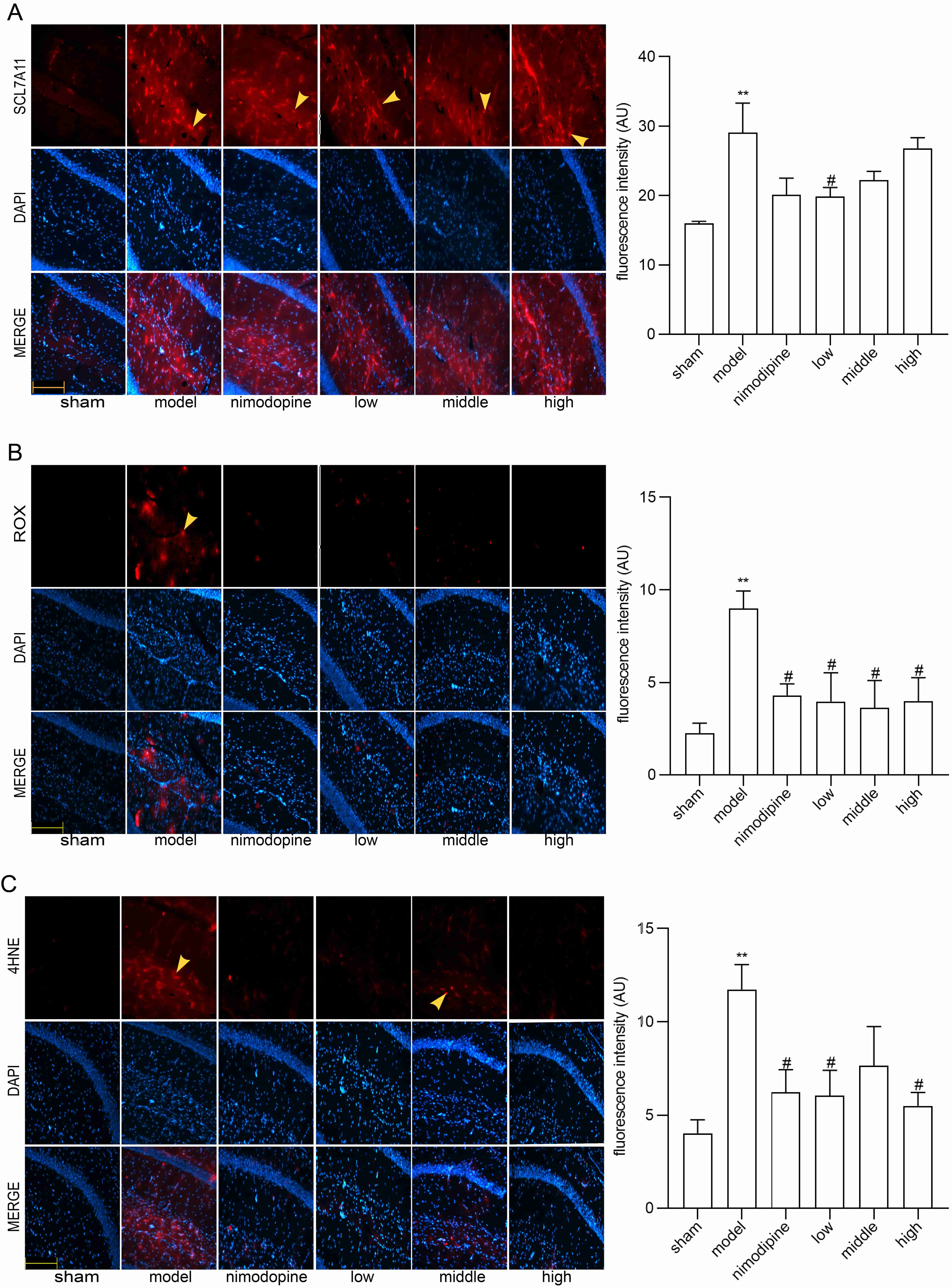
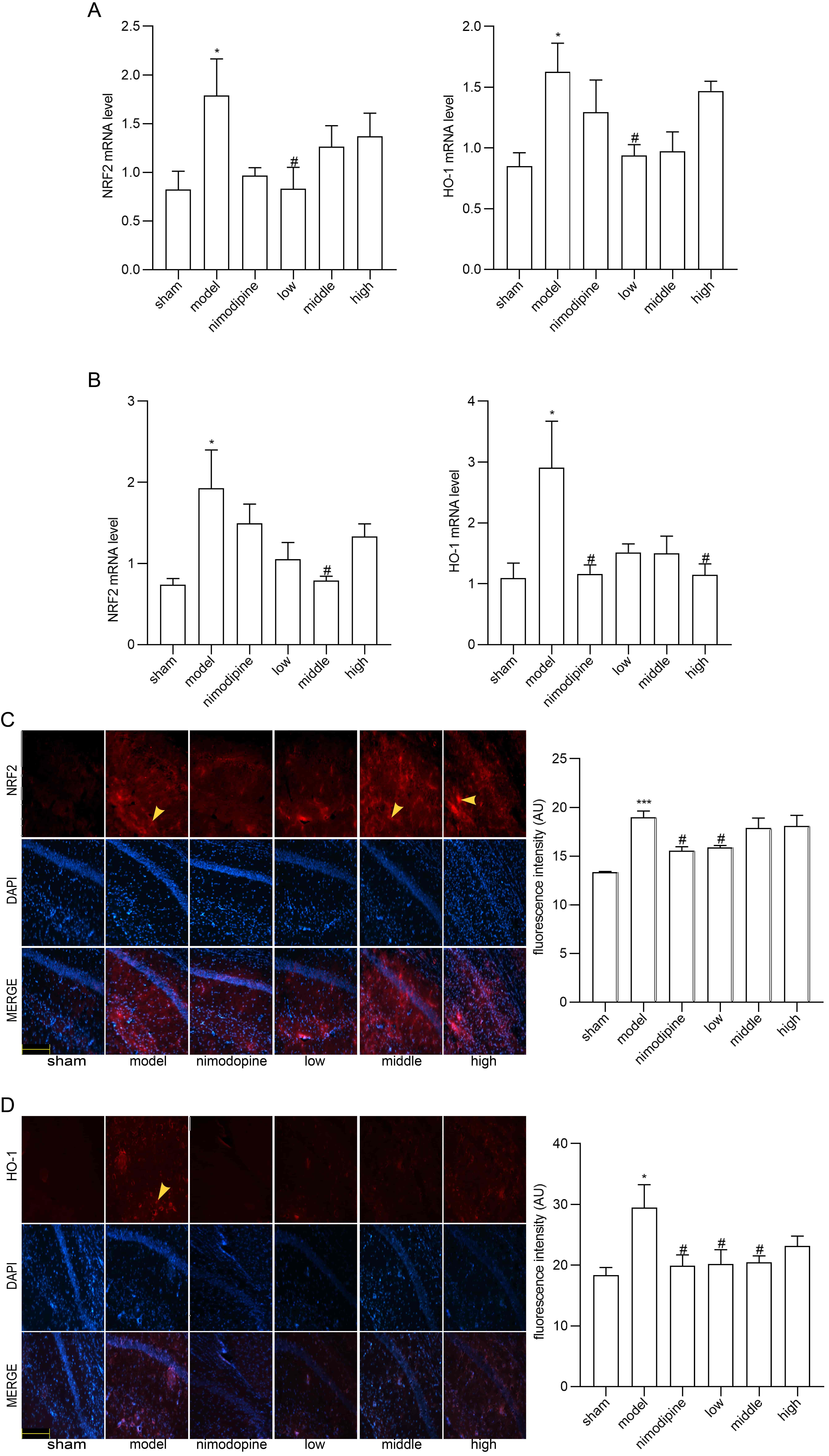
To explore FSS against rUCCAO-induced ferroptosis, we revealed transcriptional mRNA measured by RT-qPCR and at post-transcriptional level by immunofluorescence staining. The FSS and nimodipine treatment group presented lower expression of GPX4 and SLC7A11, and different level of PTGS2 and LPCAT3 compared to the VD model group in cerebral cortex (Fig. 4A) and hippocampus (Fig. 4B). Further, FSS lessened expression level of SLC7A11, ROX and 4HNE in the CA1 area of hippocampus (Fig. 5A,B and C), indicating that FSS could decrease ferroptosis through System Xc−. These results demonstrate that FSS could suppress ferroptosis after chronic cerebral hypoperfusion.
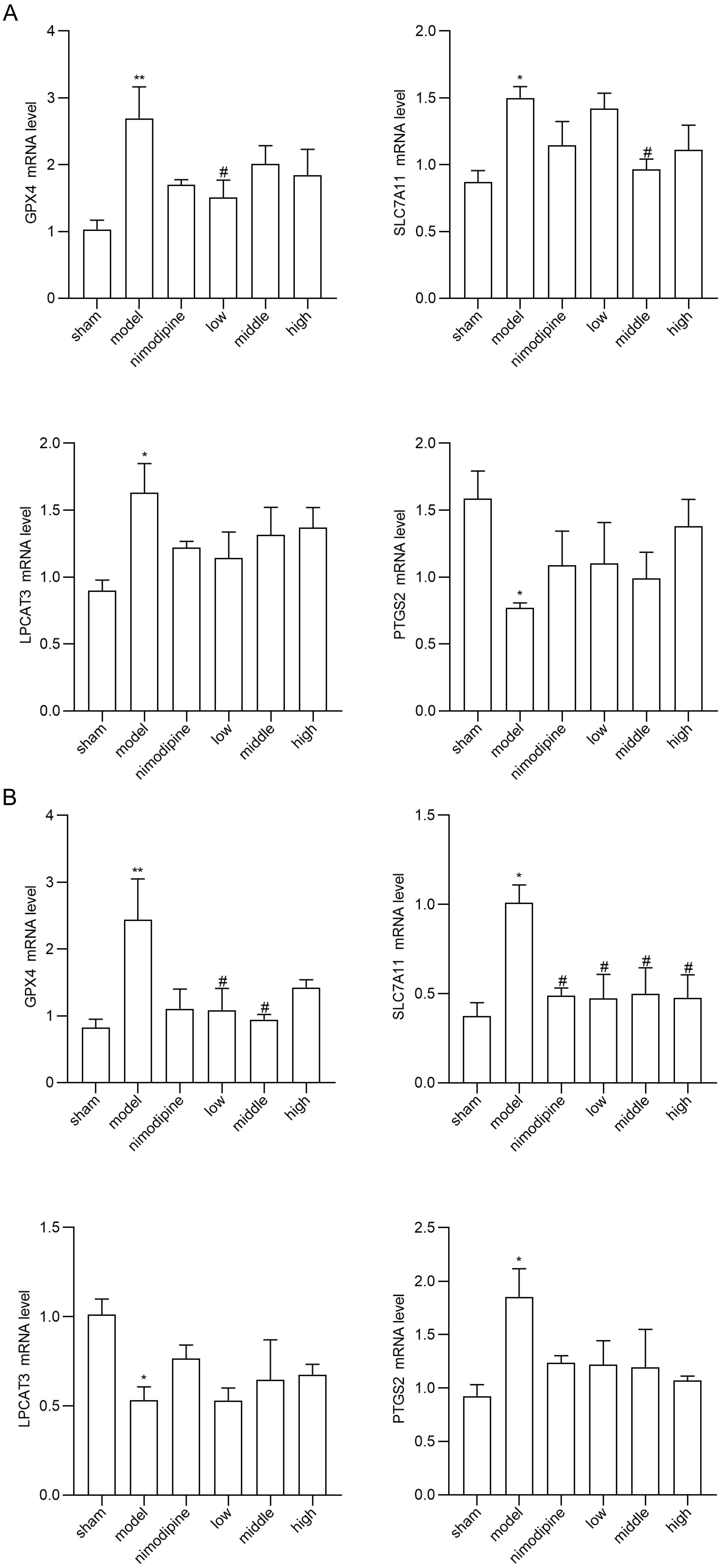 Fig. 4.
Fig. 4.FSS suppresses ferroptosis after chronic cerebral hypoperfusion.
GPX4, SLC7A11, PTGS2 and LPCAT3 mRNA expression levels in (A) cerebral cortex and
in (B) hippocampus. n = 4. **p
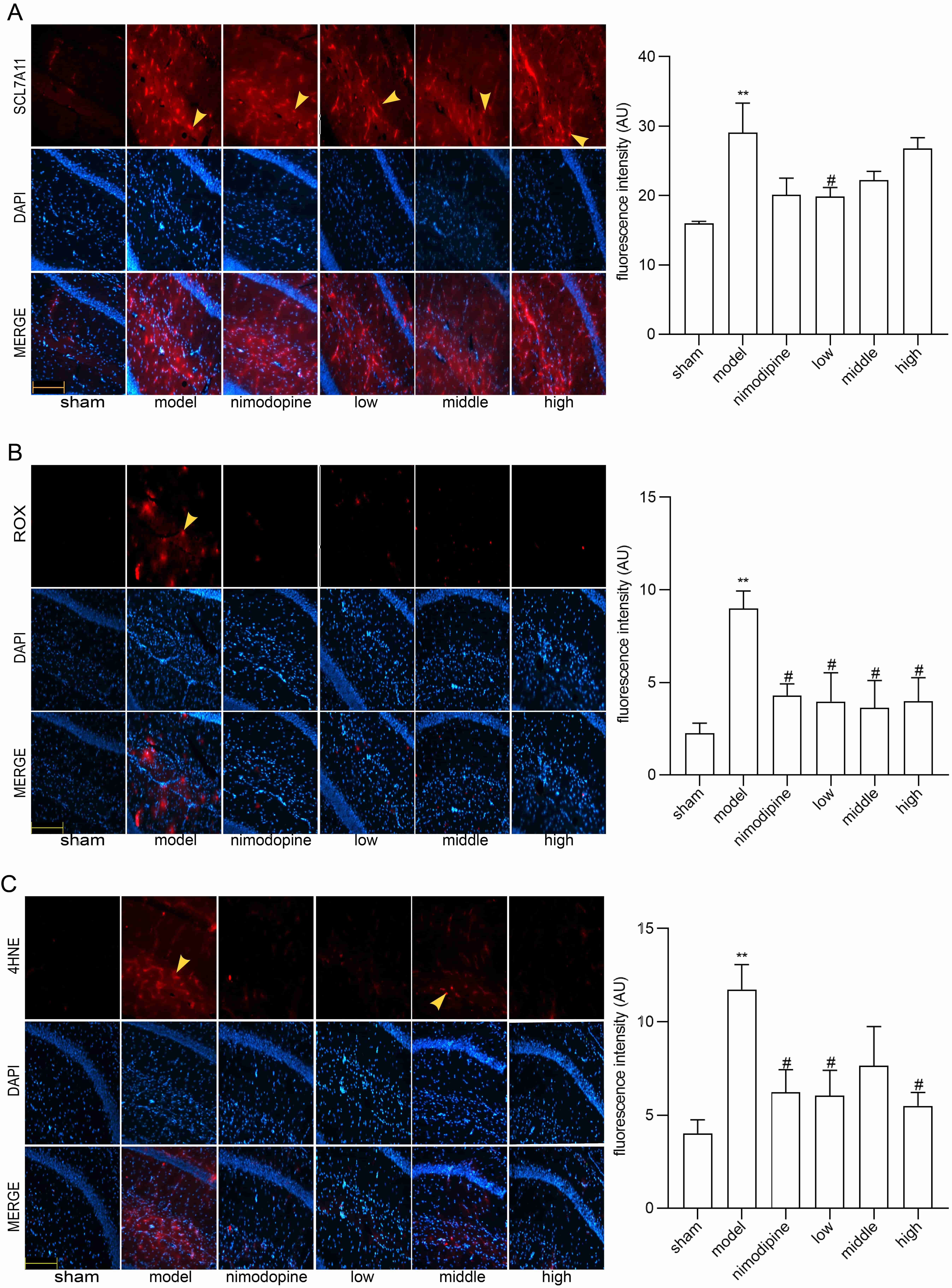 Fig. 5.
Fig. 5.FSS decreased ferroptosis in the area CA1 of hippocampus.
Immunofluorescence was utilized to observe the positive stain of and relative
fluorescence intensity statistics of the expression of (A) SLC7A11, (B) ROX and
(C) 4HNE. Magnification = 200
NRF2/HO-1 signaling can lessen ferroptosis by reducing oxidative stress [55]. As shown in Fig. 6A and B, NRF2 and HO-1 mRNA were decreased by FSS and nimodipine in cerebral cortex and hippocampus versus the model group. The immunofluorescence staining in the CA1 area of hippocampus similarly indicate that FSS and nimodipine decreased NRF2 and HO-1 expressions compared with the model group (Fig. 6C and D). This suggests that FSS could regulate NRF2/HO-1 pathway after chronic cerebral hypoperfusion.
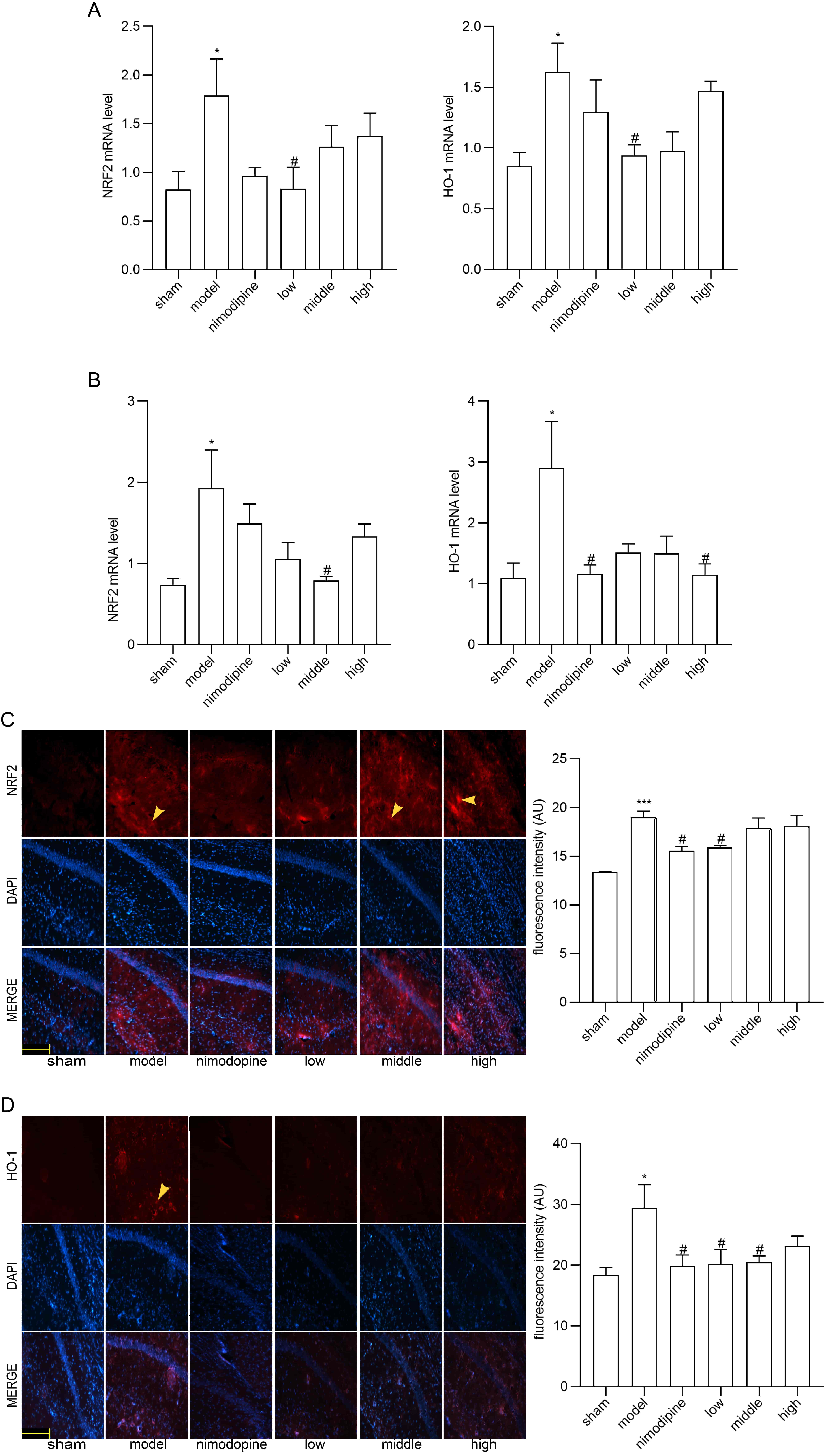 Fig. 6.
Fig. 6.FSS regulated NRF2/HO-1 pathway in mice. NRF2 and HO-1 mRNA
expression levels in (A) cerebral cortex and (B) hippocampus. Immunofluorescence
was utilized to observe the positive stain of and relative fluorescence intensity
statistics of the expression of (C) NRF2 and (D) HO-1 in the area CA1 of
hippocampus. Magnification = 200
The decline of cerebral blood flow perfusion contributes to cognitive malfunction [56]. As an approach of permanent occlusion of major brain vessel in mice, UCCAO leads to a drop by 35–55% in the ipsilateral cortical perfusion to induce CCH [57]. The animal model rUCCAO was established to imitate the disease conditions of SIVD in order to investigate the impact of CCH on the VD pathophysiological mechanism and identify new treatment options [58].
In terms of syndrome differentiation and treatment of TCM, FSS has been extensively applied for cerebrovascular diseases to promote and nourish blood flow [59, 60]. Herein, we verified that FSS could improve rUCCAO-induced cognitive deficiency regulated by NRF2/HO-1 pathway against ferroptosis.
We found that FSS treatment alleviated cognitive behavior disorders as well as hippocampal neuron damage after chronic cerebral hypoperfusion. FSS lessened impaired learning and memory abilities with higher discrimination index, more platform crossings, increased time spent in target quadrant and quicker escape latencies in the FSS treatment group compared with the VD model. Moreover, as evidenced by HE and Nissl staining, FSS improved neuronal morphological impairment in the VD group whereby hippocampal neurons disappeared. Chronic cerebral ischemia affects the hippocampus site connected with learning and memory skill [61]. Hence, the results of behavioral and immunohistochemical tests imply that FSS helps offset cognitive deficits.
Recently, apoptosis, oxidative stress, cholinergic nerve malfunction and energy metabolism syndromes have been associated with the intricate pathogenesis of VD [62, 63]. According to our study, FSS treatment moderated the apoptosis, as shown via TUNEL staining. Similarly, the antioxidant effects of FSS have been validated by MDA and GSH-PX content in serum, and by GSH/GSSG ratio in corpus striatum after chronic cerebral hypoperfusion. MDA was observed as the final product of peroxidation reaction between free radicals and lipids, while antioxidant enzymes such as SOD and GSH-PX can maintain cellular redox homeostasis [64, 65, 66]. Cystine synthesizes and affects GSH, which disturbs the balance between lipid peroxidation and antioxidant stress [67]. Thus, one of the neuroprotective features of FSS is an antioxidant system that lessens lipid peroxidation injury.
Ferroptosis is a regulated cell death characterized by iron-mediated oxidative damage [18]. Canonical ferroptosis deactivates the main membrane mechanisms against peroxidation damage [68]. System Xc− plays an essential role in regulating lipid peroxidation. SLC7A11 is a cystine antiporter that sustains redox balance of GSH synthesis [69]. GPX4 is a selenoenzyme that converts antioxidant GSH to oxidized GSSG, thereby protecting cells from ferroptosis by restraining cytotoxic lipid peroxidation such as esterified lipid hydroperoxide [70]. Inhibition of GPX4 or system Xc− induces ferroptosis by dropping GSH while accumulating ROS and lipid peroxidation [71]. Brains that are replete with phospholipids are more vulnerable to lipid peroxidation damage [72]. Notably, ferroptosis has be found as a vital factor of VCI [73]. Herein, we found that SLC7A11 and GPX4 mRNA were increased in VD models in the cerebral cortex and hippocampus, and were reduced by FSS. Compared to the VD model group, the FSS treatment group showed different mRNA levels of PTGS2 and LPCAT3, the main ferroptosis targets to deactivate GPX4 [74]. Further, our quantitative analysis of immunofluorescence staining revealed that FSS decreased the products of ROS as SLC7A11, ROX and 4HNE in the hippocampus CA1 area of mice, suggesting that FSS could decrease ferroptosis by enhancing System Xc− activity [75, 76]. These data indicate that FSS could have protective effects against ferroptosis of the SLC7A11/GPX4 axis dysfunction after chronic cerebral hypoperfusion.
NRF2, a transcription factor, plays a role as a critical endogenous regulator of ferroptosis involved in genes of antioxidant and iron metabolism [77, 78]. NRF2 stays inactive with Kelch-like ECH-associated protein 1 (Keap1) in cytosol [79]. Activated NRF2 is isolated from Keap1 and transferred to the nucleus, combining with enhancer sequences named ARE that target genes including HO-1 [80, 81]. HO-1 has a protective effect against oxidative injury, apoptosis and inflammation, while abnormal HO-1 levels are related to the pathogenesis of neurodegeneration [82, 83]. Studies have also verified that activation of NRF2 signaling shows neuroprotective potential to treat neurodegenerative diseases by inhibiting ferroptosis [28, 84, 85]. Moreover, NRF2 dysfunction causes oxidative damage in CCH-induced rats [86]. Thus, inactivated NRF2/HO-1 axis could be a promising treatment target in preventing ferroptosis. In our study, NRF2 and HO-1 mRNA in cerebral cortex and hippocampus, and NRF2 and HO-1 fluorescence intensity in the hippocampus CA1 region, indicate that FSS decreased these expressions compared with the model group. Overall, we propose that FSS could inhibit CCH-caused ferroptosis by regulating NRF2/HO-1 pathway.
Our study reveals that FSS likely ameliorates chronic cerebral hypoperfusion-induced cognitive impairment in vivo, and is potentially neuroprotective against ferroptosis by regulating the NRF2/HO-1 pathway. Additional studies are warranted to identify the fine pharmacological mechanisms of FSS.
ARE, antioxidant response elements; CCH, chronic cerebral hypoperfusion; CX, Chuanxiong (Ligusticum wallichii, LW); DG, Danggui (Angelica sinensis, AS); FSS, Fo-Shou-San; GPX4, glutathione peroxidase 4; GSH, reduced glutathione; GSH-PX, glutathione peroxidase; GSSG, oxidized glutathione disulfide; HE, hematoxylin-eosin; HO-1, heme oxygenase-1; Keap1, Kelch-like ECH-associated protein 1; LPCAT3, lysophosphatidylcholine acyltransferase 3; MDA, Malondialdehyde; NRF2, nuclear factor erythroid 2-related factor 2; PBS, phosphate buffer; PTGS2, prostaglandin-endoperoxide synthase 2; ROS, reactive oxygen species; rUCCAO, permanent right unilateral common carotid arteries occlusion; SIVD, subcortical ischemic vascular dementia; SLC7A11, solute carrier family 7 membrane 11; SOD, Superoxide Dismutase; SPF, specific Pathogen-Free; TCM, Traditional Chinese medicine; VCI, vascular cognitive impairment; VD, vascular dementia.
The data used and analyzed in the study are available from the corresponding author upon reasonable request.
JYW and QW designed the study. JYW, JHS, YFX and GCC implemented the experiments. WZ and QW provided reagents and materials. CY and LND analyzed the data. JYW, JHS, YFX and GCC edited the manuscript. CY, LND, WZ and QW revised and supervised the paper.
All procedures under principles for animal experiments were approved by Guangzhou University of Chinese Medicine Animal Ethics Committee (approval ID: 20210615006).
We thank all members who were involved in this research.
This research was supported by Guangdong province science and technology plan international cooperation project (No. 2020A0505100052).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.