1 Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, 705-717 Daegu, Republic of Korea
2 Department of Neurosurgery, College of Medicine, Yeungnam University, 705-717 Daegu, Republic of Korea
Abstract
Introduction: This study investigated the relationship between
Coma Recovery Scale-Revised (CRS-R) and the neural networks between the medial
prefrontal cortex (mPFC) and precuneus (PCun)/posterior cingulate cortex (PCC) in
disorders of consciousness (DOC) patients with a traumatic brain injury (TBI)
using diffusion tensor tractography (DTT). Measures: Twenty-five
consecutive patients with TBI admitted to the rehabilitation department of a
university hospital were enrolled in this study. The Coma Recovery Scale-Revised
(CRS-R) was used to evaluate the consciousness state. The pathway of the neural
networks between the mPFC and the PCun (mPFC-PCun DMN)/PCC (mPFC-PCC DMN) were
reconstructed using DTT. Fractional anisotropy (FA) and the tract volume (TV)
were obtained to assess the diffusion tensor imaging parameters.
Results: The CRS-R score had strong positive correlations with the FA
value and TV of the mPFC-PCun DMN (p
Keywords
- traumatic brain injury
- Coma Recovery Scale-Revised
- default mode network
- diffusion tensor tractography
- disorders of consciousness
Traumatic brain injury (TBI) is a major cause of neurological disability in adults [1, 2]. Disorders of consciousness (DOC) is a common and serious sequela after TBI; approximately half of patients in a vegetative state at one month after TBI remain vegetative until one year after onset [1, 2]. Therefore, the elucidation of neural correlates for consciousness is clinically important for managing patients with DOC after TBI because this information can be useful for predicting the prognosis prediction and guideline development for neurorehabilitation. In particular, recently developed non-invasive brain stimulation therapies, such as transcranial direct current stimulation or repetitive transcranial magnetic stimulation, can be applied to specific neural structures to promote the recovery of DOC [3, 4].
Consciousness consists of arousal and awareness of oneself and the environment [5, 6]. The neural network for the control of consciousness is not understood completely, but it was controlled by a complicated series of complex actions involving various neural structures, including the default mode network (DMN), frontoparietal network, frontostriatal network, thalamocortical network, and ascending reticular activating system [7, 8, 9, 10]. The DMN is a specific brain network that is preferentially active when individuals are not focused on the external environment [11]. Four areas of the DMN are considered important for consciousness: the medial prefrontal cortex (mPFC), temporoparietal junction, precuneus (PCun), and posterior cingulate cortex (PCC) [12, 13, 14, 15, 16]. The mPFC has been reported to be an important neural area for recovering DOC, and PCun and PCC play a pivotal role in consciousness [17, 18]. As a result, the networks between the mPFC and PCun/PCC (the mPFC-PCun/PCC DMN) play an important role in consciousness [13, 14, 15, 16, 19].
Several studies have reported the relationship between the consciousness state and functional connectivity of the mPFC-PCun/PCC DMN in patients with DOC following various brain pathologies, including TBI using resting-state functional magnetic resonance imaging (fMRI) and positron emission tomography [20, 21]. By contrast, the relationship between the consciousness state and structural connectivity of the mPFC-PCun/PCC DMN is unclear in patients with DOC [15]. This study hypothesized that the consciousness state would be correlated with the structural connectivity of the mPFC- PCun/PCC DMN in DOC patients with TBI. Furthermore, the correlation with the consciousness state would be different between the structural connectivity of the mPFC-PCun DMN and mPFC-PCC DMN because the functions of the PCC and PCun are different. PCun is involved in conscious information processing and self-consciousness [17, 22, 23]. By contrast, PCC was reported to be involved in autonomic arousal and awareness, monitoring for behaviorally relevant stimuli and environmental changes [24, 25, 26].
In this study, we investigated the relationship between the consciousness state and the mPFC-PCun/PCC DMN in DOC patients with TBI using diffusion tensor tractography (DTT).
Twenty-five consecutive patients (nineteen males, six females; mean age 47.92
The Coma Recovery Scale-Revised (CRS-R) was used to evaluate the consciousness state on the day of DTT scanning [27, 28]. This is a standardized assessment scale consisting of six subscales: auditory, visual, motor, oromotor/verbal, communication and arousal functions [27, 28]. The CRS-R is commonly used because it is the most comprehensive, evidence-based behavioral assessment for detecting the signs of consciousness in patients recovering from coma [27, 29]. The validity and reliability of CRS-R are well established [3].
The DTT data were acquired at an average of 11.52
The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (https://www.fmrib.ox.ac.uk/fsl) was used to analyze the DTT data. Affine multi-scale two-dimensional registration was used to correct the head motion effects and image distortion due to the eddy current. FMRIB Diffusion Software with the routines option (0.5 mm step lengths, 5000 streamline samples, and curvature thresholds = 0.2) was used for fiber tracking.
The pathway of the neural networks between the mPFC and the PCun/PCC was determined by the selection of fibers passing through seed regions of interest (ROI) and the target ROI. The seed ROI was placed on the mPFC (Brodmann’s areas 14, 24, 25, and 32)—the superior boundary: the cingulate sulcus, the medial boundary: the midline between the right and left hemispheres, and lateral boundary: a line 11.25 mm lateral from the midline [1]. The target ROIs were placed on the PCun and the PCC [2]. Of the 5000 samples generated from the seed voxel, the results were visualized at the threshold of two streamline through each voxel for analysis. The fractional anisotropy (FA) values and tract volumes (TV) for the neural networks between the mPFC and the PCun/PCC were determined in both hemispheres (Fig. 1).
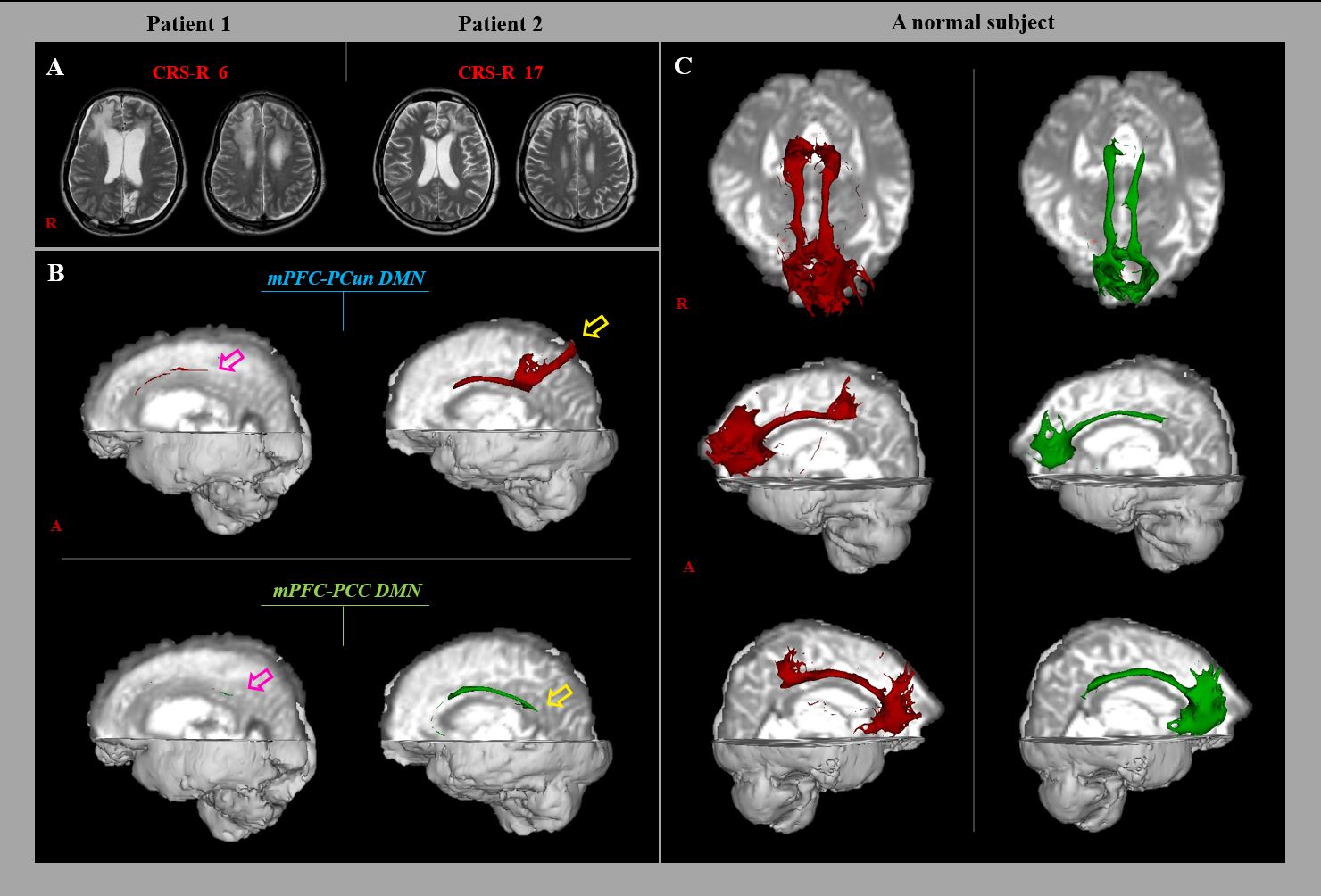
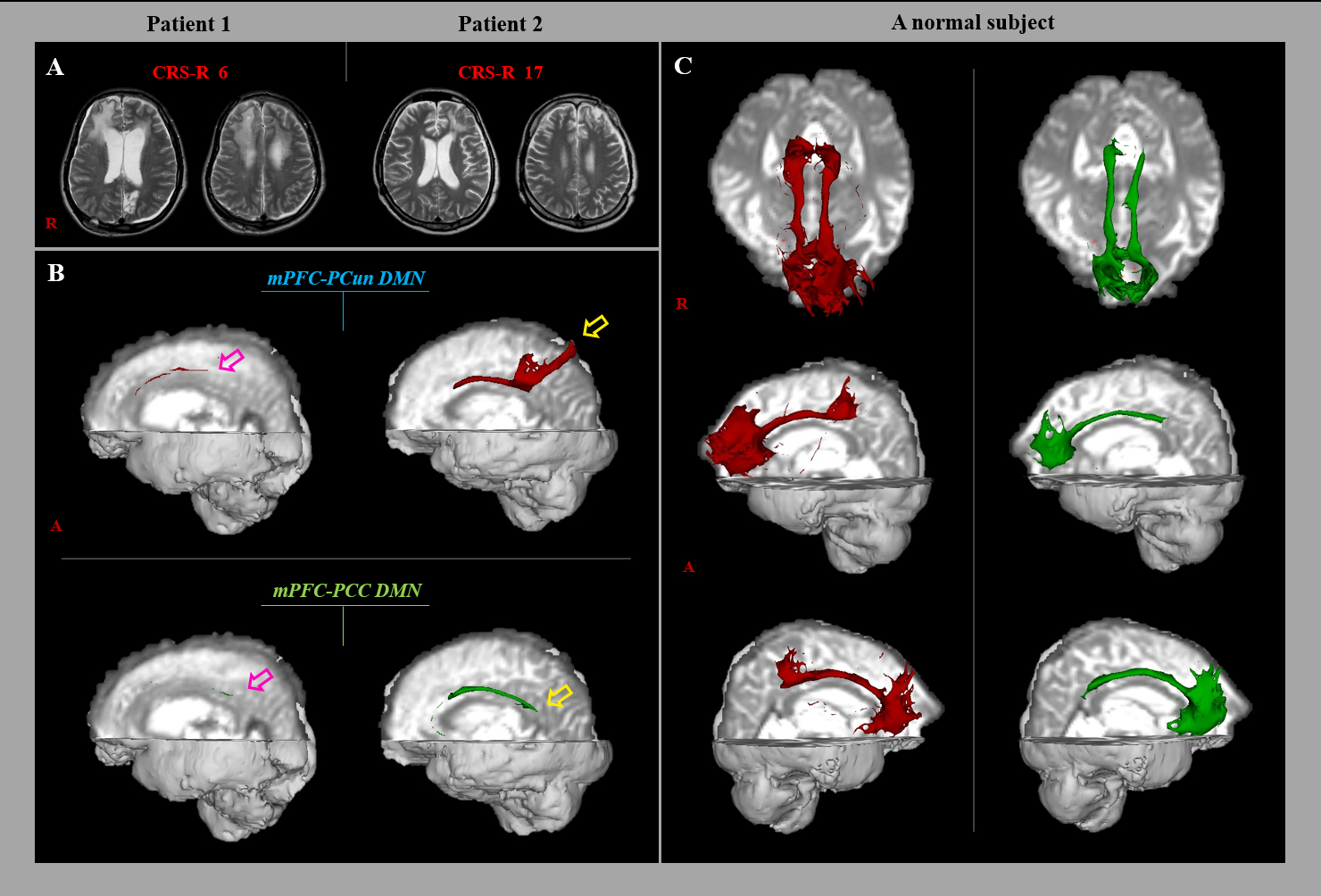 Fig. 1.
Fig. 1.Results of diffusion tensor tractography (DTT) of the neural networks between the medial prefrontal cortex (mPFC) and precuneus (PCun)/posterior cingulate cortex (PCC) in a representative subjects (patient 1: 26-year old male, patient 2: 41-year old male) and normal subject. (A) T2-weighted brain magnetic resonance images were obtained at the time of the diffusion tensor imaging. (B) Results of DTT of the mPFC-PCun/PCC DMN. The mPFC-PCun/PCC DMN in patient 1 (pink arrow) had a lower tract volume than that in patient 2 (yellow arrow). (C) Results of DTT of the mPFC-PCun/PCC DMN in a normal subject.
Statistical analysis was performed using SPSS 21.0 for Windows software (SPSS,
Chicago, IL, USA) and SAS 9.4 (SAS institute, Cary, NC, USA). Spearman
correlation analysis was used to detect the correlation between the CRS-R scores
and DTT parameters (FA value and TV) of the neural networks between the mPFC and
the PCun/PCC. A p-value was
Table 1 lists the correlations between the CRS-R score and DTT parameters for
the mPFC and the PCun/PCC DMN. The CRS-R score had a strong positive correlations
with the FA value and TV of the mPFC-PCun DMN (FA: r = 0.725, p
| mPFC-PCun | mPFC-PCC | ||||
| FA | TV | FA | TV | ||
| Total | r | 0.725 | 0.577 | 0.396 | 0.447 |
| p | 0.05 | 0.03* | |||
| CRS-R, Coma Recovery Scale-Revised; mPFC, medial prefrontal cortex; PCun,
precuneus; PCC, posterior cingulate cortex; FA, fractional anisotropy; TV, tract
volume.
*Significant correlation between the Coma Recovery Scale-Revised scores and diffusion tensor tractography parameters, p | |||||
Table 2 lists robust regression results of the variables contributing to the
CRS-R score. Robust regression analysis for the CRS-R score showed that the
regression model was statistically significant (p
| CRS-R | Variables (DTT parameters) | Estimate | Standard error | 95% Confidence Intervals | p-values | R-square | |
|---|---|---|---|---|---|---|---|
| Minimum | Maximum | ||||||
| Total | Intercept | 5.888 | 3.510 | –0.992 | 12.768 | 0.094 | 0.561 |
| FA value of mPFC-PCun | 41.238 | 12.1686 | 17.3879 | 65.088 | 0.001* | ||
| TV value of mPFC-PCum | 0.002 | 0.0013 | –0.0006 | 0.005 | 0.129 | ||
| FA value of mPFC-PCC | –11.6189 | 12.6237 | –36.361 | 13.123 | 0.357 | ||
| TV value of mPFC-PCC | 0.0042 | 0.0035 | –0.0027 | 0.011 | 0.231 | ||
| CRS-R, Coma Recovery Scale-Revised; DTT, diffusion tensor tractography; VIF,
variance inflation factor; SE, standard error; FA, fractional anisotropy; mPFC,
medial prefrontal cortex; PCun, precuneus.
*: significant result analyzed by multiple linear regression, p | |||||||
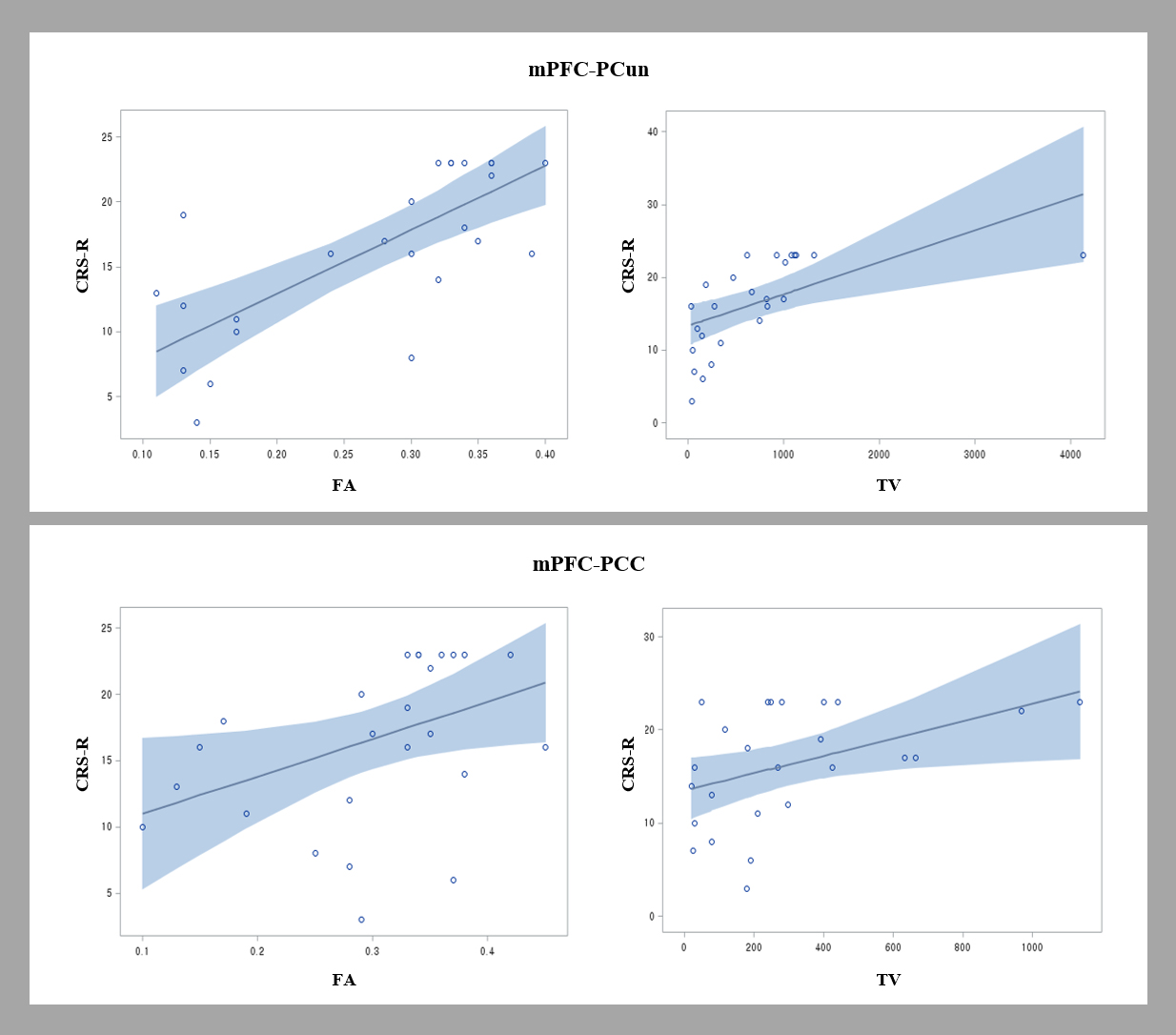
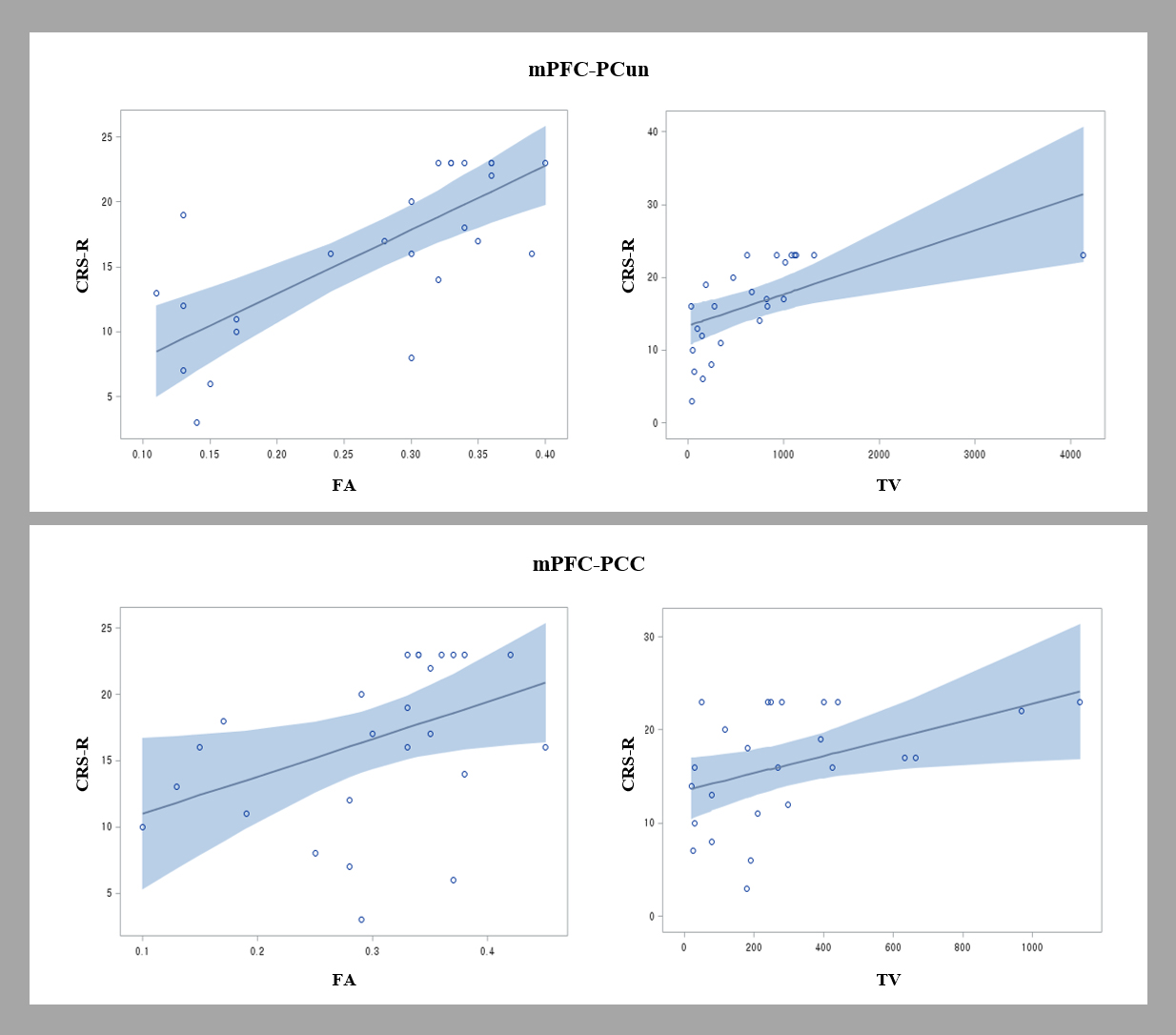 Fig. 2.
Fig. 2.Scatter plots showing the correlation of the Coma Recovery Scale-Revised (CRS-R) scores and diffusion tensor tractography (DTT) parameters of the neural networks between the medial prefrontal cortex (mPFC) and the precuneus (PCun)/posterior cingulate cortex (PCC) [fractional anisotropy (FA) and tract volume (TV)]. The CRS-R score showed a positive correlation with the FA value of the mPFC-PCun DMN.
In this study, DTT was performed to examine the relationship between the CRS-R score and the mPFC-PCun/PCC DMN in DOC patients with TBI. The following results were obtained. (1) The CRS-R score had close correlations with the DTT parameters of the mPFC-PCun/PCC DMN (FA of mPFC-PCun DMN; strong [r = 0.725], TV of mPFC-PCun DMN; strong [r = 0.577], and TV of mPFC-PCC DMN; moderate [r = 0.447]). (2) The regression between the CRS-R score and the DTT parameters of the DMN: FA value of the mPFC-PCun DMN was a predictor describing the variability of the CRS-R score. On the other hand, the parameters of the mPFC-PCC DMN did not explain the variability of the CRS-R score.
Among the various DTT parameters, the FA value and TV are used to evaluate the status of neural tracts in patients with TBI [31]. FA value indicates the degree of directionality of water diffusion and the integrity of white matter microstructures, such as axons, myelin, and microtubules. This reflects the fiber density, axonal diameter, and myelination of the white matter [32, 33]. TV represents the total number of voxels within a neural tract, which is deemed representative of the number of neural fibers within the neural tract [32, 33]. Consequently, the CRS-R score correlated with the DTT parameters of the mPFC-PCun/PCC DMN (FA of and TV of mPFC-PCun DMN; strong, TV of mPFC-PCC DMN; moderate) suggested that the microstructural integrity and fiber numbers of mPFC-PCun DMN, and the microstructural integrity of mPFC-PCC DMN were closely correlated with the CRS-R score of the patients. On the other hand, the mPFC-PCun DMN was correlated more closely with the CRS-R score than the mPFC-PCC DMN. Robust analyses of the DTT parameters of the mPFC-PCun DMN for the consciousness state showed that the FA value of the mPFC-PCun DMN was a predictor for describing the variability of the CRS-R scores, suggesting that that the microstructural integrity of the mPFC-PCun DMN influenced the CRS-R scores.
Several studies have reported the relationship between the CRS-R and the functional connectivity of the DMN in patients with DOC [20, 21]. Athena Demertzi et al. [21] reported a significant correlation between the CRS-R score and mPFC-PCC DMN in acute/chronic patients with DOC after severe brain injury (TBI and hypoxic brain injury, using resting-state fMRI and positron emission tomography). In 2016, Lant et al. [20] using resting-state fMRI, reported that the CRS-R score correlated with the functional connectivity of the mPFC Pcun/PCC DMN in chronic DOC patients with various brain pathologies (TBI, hypoxic brain injury, stroke, and seizure). Regarding the structural connectivity, this study examined the relationship between the CRS-R and mPFC PCun and reported no significant correlation in DOC patients with brain injury (TBI and hypoxic brain injury) [15]. On the other hand, the number of patients included was too small (eight patients). To the best of the authors’ knowledge, this is the first study to demonstrate the relationship of the CRS-R with mPFC PCun and PCC in DOC patients with TBI using DTT.
This study had some limitations. First, DTT analysis is operator-dependent and
can induce false-positive and false-negative results due primarily to crossing
fibers or the partial volume effect [34]. Second, a relatively small number of
subjects. Third, only the CRS-R score was used to evaluate the consciousness
state because this study was conducted retrospectively. Fourth, heterogenous
duration between onset and DTI scanning (11.52
This study observed a close correlation of the consciousness state with the mPFC-PCun DMN and mPFC-PCC DMN in DOC patients with TBI. On the other hand, the mPFC-PCun DMN was correlated more closely with the consciousness state than the mPFC-PCC DMN. These results suggest that the mPFC-PCun DMN and mPFC-PCC DMN could be important neural correlates for consciousness in DOC patients with TBI. In addition, these results could be useful for neurorehabilitation for DOC. Recently developed neuro-stimulation techniques, such as repetitive transcranial magnetic stimulation and transcranial direct current stimulation, could be applied to the mPFC-PCun DMN (primary target) and mPFC-PCC DMN (second target) for the recovery of DOC. Further studies on this topic will be needed.
The data sets supporting the conclusion of this study are included in this article [and its supplementary information files].
SHJ—study concept and design, manuscript development, writing, funding, and critical revision of manuscript for intellectual content. SHK—study concept and design, manuscript development. MKC—study concept, design, writing, acquisition and analysis of data, and critical revision of manuscript for intellectual content.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval was obtained from the ethics committee of Yeungnam University (YUMC-2019-06-032). Informed consent was obtained from all individual participants included in the study.
Not applicable.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (No. 2021R1A2B5B01001386).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.