1 Centro de Investigación Nebrija en Cognición (CINC), Universidad Nebrija, 28015 Madrid, Spain
2 Unidad Sueño, Hospital Universitario de la Ribera-FISABIO, 46600 Valencia, Spain
3 Facultad de Medicina, Universidad Católica de Valencia, 46001 Valencia, Spain
4 AcqVA Aurora Center, The Arctic University of Norway, 9019 Tromsø, Norway
Abstract
Background: Sleep disturbances represent a major health burden today, affecting up to one-third of the population worldwide. Computerized cognitive stimulation has been proven as an effective approach in diminishing negative symptomatology and improving the quality of life in a range of medical conditions. Given its nature in enhancing neural networks, such as those involved in stimulus monitoring and inhibitory processes, computerized cognitive stimulation is arising as a potential tool to overcome underlying cognitive deficits found among patients suffering from insomnia. In the current study, we report the results of Phase 1 and Phase 2 clinical trials of a home-based computerized cognitive stimulation program. Methods: The cognitive stimulation intervention followed a home-based approach with online supervision by a psychologist. The training activities were gamified cognitive tasks that had been designed to improve executive functions, with a focus on inhibition skills. The Insomnia Severity Index and the Pittsburgh Sleep Quality Index scales were used as the main assessment measures. Data from the Behavior Rating Inventory of Executive Function, the Beck Depression Inventory, the State-Trait Anxiety Inventory, and the Penn State Worry Questionnaire were also recorded before and after the intervention. During 15 consecutive days, participants performed on alternate days a total of 7 training sessions (each lasting 45 minutes). Results: Twelve patients with clinical insomnia were administered the home-based online cognitive stimulation program. After seven training sessions, mean changes in sleep quality, depressive and anxiety symptoms, worry thoughts, and everyday function were found, with significant improvements in these domains in the full absence of safety issues. Conclusions: In patients with insomnia, cognitive stimulation demonstrated improvements in sleep quality, mood, and cognitive performance over a 15-day protocol. No relevant side effects were reported. The long-term effectiveness of the intervention is still unknown. Clinical Trial Registration: The study protocol has been reviewed and published in ClinicalTrials.gov, assigning it the code NCT05050292 https://clinicaltrials.gov/ct2/show/NCT05050292?term=NCT05050292&draw=2&rank=1.
Keywords
- sleep disorder
- insomnia
- cognitive stimulation
- computerized intervention
- cognitive training
- sleep quality
- cognitive performance
Around 1.2 billion people are estimated to meet insomnia criteria worldwide [1, 2]. This pathology is characterized by the dissatisfaction with sleep quantity or quality derived from the difficulty initiating or maintaining sleep, or the difficulty returning to sleep after early-morning awakening [3]. The main consequences of sleep disruption include fatigue, reduced cognitive ability, mood disturbances, and reduced performance of daily tasks [4, 5]. Cognitive models of insomnia concur that cortical hyperactivity is one of the main precipitating and maintaining factors of insomnia [6]. The increased activity stems from a general state of anxiety and worries, as well as constant monitoring of both internal (i.e., thoughts) and external (i.e., noises) non-relevant stimuli [7]. This could be interpreted as a deficit in executive functions, specifically regarding inhibitory and attentional processes. As a result, individuals may suffer impairment in discerning which stimuli to pay attention to and which to ignore or inhibit. Neuroimaging studies have corroborated these findings, revealing changes in the frontal lobes and hippocampus [8] and decreased connectivity among brain regions involved in executive control and attention [9]. At the same time, research conducted with evoked potentials coincides with the presence of hypervigilance and difficulties in cognitive inhibition processes in patients with insomnia [10, 11, 12]. In the current study, we investigated the feasibility, safety, and efficacy of non-pharmacological treatment for the cognitive dysfluency often associated with insomnia based on a computerized cognitive training protocol aimed to strengthen inhibitory and attention skills.
The high negative effects associated with pharmacology (mainly related to the addictive power) [13, 14] have led to the exploration of treatment alternatives in patients with sleep disturbances, and cognitive interventions have acquired a great deal of attention in recent years. Cognitive stimulation—also referred to as cognitive training—is conceptualized as the enhancement of neural connections through the repeated execution of activities aimed at improving or limiting the deterioration of cognitive function. In the field of sleep disturbances, cognitive stimulation is expected to improve cognitive inhibition ability and thus reduce alertness and monitoring of disruptive stimuli during sleep. At the same time, reasoning capacity is strengthened, and the presence of arousing, anxious, worrying, or distressing thoughts is reduced [15, 16, 17, 18, 19].
There is a unanimous consensus in the medical community that cognitive interventions are to be the first-choice treatment for insomnia, and pharmacology (if needed) should be administered as an adjunct to cognitive treatment. This way, most major medical guidelines recommend physicians provide cognitive behavior therapy (CBT) to their patients [20, 21, 22, 23]. CBT is defined as a psychological intervention aimed at assisting the patient in managing and overcoming specific difficulties through a change in thinking and behavior brought about by the use of discourse. The main objective of CBT is that the individual becomes aware of his or her thoughts, visualizes and analyzes the situation, and responds to it more effectively or adaptively. Although there is no established protocol and implementations of CBT-I (for the insomnia CBT program) tend to vary in content, methodology, and time of application [24, 25], there are certain components shared by most of the CBT-I programs: sleep hygiene education, sleep restriction, stimulus control, cognitive restructuring, and relaxation training [26, 27]. In addition to the main components mentioned above, some CBT-I programs incorporate, along with cognitive restructuring, the training of cognitive functions such as memory, attention, visuospatial skills, and executive functions, in other words, cognitive stimulation [28].
Despite five decades of research has shown that either a full CBT-I program or the administration of its isolated components has an acceptable efficacy [29], the evidence reveals that physicians continue prescribing medication (e.g., Z-meds, Trazodone, and Benzodiazepines) in their clinical setting [30], and only an approximated 1% of patients diagnosed with insomnia are provided with a CBT-I intervention [31, 32]. The lack of knowledge of the healthcare personnel, the need for trained professionals, or insufficient resources may limit the implementation of CBT-I [33]. Furthermore, some of the CBT-I components present certain contraindications. For instance, sleep restriction usually leads to daytime sleepiness, fatigue, tiredness, headache, and irritability during the first weeks of application, which affects motivation and adherence [34]. Besides, stimulus control involves getting out of bed and moving to another room, which may increase the risk of falls [35]. And relaxation techniques have been shown to generate paradoxical anxiety in certain patients [36]. These facts highlight the need for further research into plausible cognitive intervention alternatives for patients with insomnia.
The remarkable technological progress of the last decades has enabled the integration of electronic devices into clinical care. Ranging from data monitoring to treatment provision, Digital Therapeutics covers a wide range of processes that facilitate patients’ access to intervention and reduce practitioners’ and healthcare providers’ workloads [37, 38, 39]. In this line, computerized cognitive training (CCT) programs—construed as online cognitive stimulation activities—have been shown to generate significant benefits in various clinical pathologies [40, 41, 42, 43]. CCT training activities are designed as gamified exercises where the positive reinforcers inherent to the games allow for a high cognitive demand to complete the tasks, without negatively affecting the participant’s motivation toward the intervention. In contrast, CBT programs mostly include storytelling, reading, and performing tasks that are not always pleasurable (i.e., restricting sleep time or getting out of bed). Likewise, CBT is founded on a patient’s behavioral and cognitive modification that requires a substantial intrinsic effort that is not always feasible. To date, few studies have addressed the potential benefits of CCT in insomnia, but the existing data suggest improvements in sleep quality after only an eight-week training [44].
Thus, given that cognitive stimulation targets cognitive mechanisms underlying sleep problems (i.e., attentional control and inhibitory processes), the lack of anticipated contraindications, and the expected high adherence, CTT may be considered an accessible alternative intervention to pharmacological treatment and an ideal ally for other cognitive-based treatments like CBT-I. The noninvasive nature of CCT suggests that it should have no side effects, but still there is limited evidence to determine that CCT-based cognitive intervention programs could be implemented at no risk for insomnia patients [23]. Therefore, here we propose to evaluate the optimal training time and safety of a CTT for insomnia, to provide continuity to the implementation of the program over a longer period. In the current study, we present the results of Phase I/II of the clinical trial proposed by Tapia and colleagues [45].
Participants were recruited from the Sleep Unit of the Hospital Universitario de la Ribera (Spain). All were patients under medical follow-up, aged between 25 and 55 years of age, and diagnosed with insomnia disorder [307.42 (F51.01)]. Diagnostic criteria included (1) complaints concerning sleep quality or quantity characterized by difficulty falling asleep, difficulty maintaining sleep throughout the night, and/or early awakenings; (2) sleep difficulties occurring three or more times per week over at least three months; (3) the sleep difficulties involving clinically significant distress, and do not deriving from poor environmental conditions for sleep, or being explained by another pathology.
After being enlisted by their physician, a trained clinician interviewed potential participants for suitability. The exclusion criteria were the following ones: the existence of another sleep-wake disorder or medical/psychological relevant pathology; the use of non-prescribed medication with stimulant action; alcohol, caffeine, or drug abuse or dependence; and the existence of significant visual or motor impairment. The final sample included 12 patients with a mean age of 44 years, three of whom were females. Eight patients were married or in a relationship, two were divorced, and two were single. In terms of education, six had graduated from college, five had finished higher education, and one had completed compulsory education. All participants lived in a rural area. The study was approved by the Ethics Committee of La Ribera Health Department - Generalitat Valenciana, and the Ethics Committee - CEI of the Universidad Nebrija. The medical team revied medical records and face-to-face interviews were performed. All patients were briefed on the study and signed the informed consent before participation. Note that in no case was the medical treatment they were receiving modified.
Intervention. Before the intervention, all participants were registered in the training platform and had installed the CogniFit mobile application [46] on their smartphones. The program started with a complete cognitive evaluation done using the Cognitive Assessment Battery (CAB)™ PRO (CogniFit Inc., San Francisco, CA, USA; https://www.cognifit.com/cab), which then created the individual cognitive profiles (namely, cognitive strengths and weaknesses) that were used by a patented Individualized Training System™ (ITS) software (v.2022.1, CogniFit Inc., San Francisco, CA, USA) to tailor the stimulation program to the specific characteristics of each patient. The training activities were gamified and designed to stimulate executive functions, attention, and reasoning skills. Each training activity lasted for about 5 minutes and its difficulty was automatically adjusted according to the user’s performance, always requiring maximum cognitive effort. All activities had real-time feedback, as well as a final score at the end of the game. Before starting each activity, the instructions and cognitive skills to be trained were indicated on-screen. The first time each game was launched, a practice level was shown. If the practice level was succeeded, the software assumed that the instructions have been understood and the game would automatically start. If at any point the software detected that the instructions were not being followed (i.e., inconsistent answers or high error rates), the practice level would be restarted. The game could be paused at any time. Instructions about the ongoing game were available in the pause menu. The whole list of activities and games employed can be found in Appendix 1.
Assessment. The Phase I/II trial aimed at rejecting the possibility of the appearance of adverse effects because of the use of the CCT. To this end, the main outcome measure was the set of responses to a safety questionnaire. Safety outcome measures included: a Likert scale fatigue rating from 0 (No fatigue) to 10 (Very fatigued); a binary Yes/No question about the feeling of any side effect caused by the training; and a binary Yes/No question about having felt any undesirable experience associated to the training. There was a complementary structured interview in case any side effects or undesirable experiences were reported (see Appendix 2). Together with this, a critical set of additional outcome measures was obtained to assess the efficacy of the CCT, evaluating the differences before and after the intervention in the quality of sleep, cognitive functioning, and emotional state. Sleep outcome measures included the Insomnia Severity Index (ISI), which addresses both daytime and nighttime insomnia components [47], and the Pittsburgh Sleep Quality Index (PSQI), for evaluating general sleep quality [48]. The secondary outcome measures for measuring general cognitive and emotional state included the Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) for assessing everyday behavioral aspects of executive functions [49], the Beck Depression Inventory-II (BDI-II) for depressive symptomatology [50], the State-Trait Anxiety Inventory (STAI) to assess anxiety patterns and anxious symptomatology [51], and the Penn State Worry Questionnaire (PSWQ) for evaluating thoughts of concern about future events [52]. The procedure section details the administration sequence of the assessment instruments in each Phase.
Phase I consisted of a dose-escalation 3+3 design for establishing the maximum tolerated training time per session. This Phase was carried out individually at the hospital facilities, supervised by a psychologist. After 15 minutes of cognitive training using a lab-property smartphone (including 3 cognitive tasks or activities of approximately 5 minutes each), the safety protocol was administered, which included an evaluation of the level of fatigue, an exploration of the occurrence of adverse effects, and the question regarding the feeling of undesirable experiences (see Assessment section). If no potential side effects associated with the clinical process were identified, the process was continued with another round of 15 minutes of training, followed again by the safety assessment. Participation concluded when extreme fatigue (characterized as a score equal to or higher than 8 out of 10 on the fatigue Likert scale) or the presence of side effects was reported. In the presence of side effects, the clinician appraised the reported effects following the structured interview adapted from existing patient-reported questionnaires on adverse effects (Appendix 2). The maximum tolerated dose of CCT would be set according to the safety protocol responses [53]. If 2/3 of the patients reported adverse effects, the previous training-block time would be established for the intervention. If 1/3 of the patients reported adverse effects, the protocol would start again with three additional patients. If none of them reported adverse effects, the dose would be escalated. If one of them reported adverse effects, the training time would be set to that of the previous training block.
Phase II consisted of a CCT intervention that included a series of safety questions to ensure no undesirable side effects occur because of training, and two critical complete evaluation moments (at pre-test and post-test) to assess the feasibility and possible effectiveness of the training. With an estimated time of 1 hour of dedication per training day, participants engaged in a protocol lasting for 15 consecutive days. During this period, participants had to follow the instructions provided by the application on their smartphones. The protocol started (day 1) and ended (day 15) with a complete sleep quality, cognitive and emotional assessment (using the ISI, PSQI, BRIEF-A, BDI-II, STAI, and PSWQ). On even days (day 2, day 4, day 6, day 8, day 10, day 12, and day 14) the training was performed, reaching this way a total of 7 training sessions that each included 9 cognitively demanding tasks in the form of games of 5 minutes each. The duration of the training sessions was set to 45 minutes given the results obtained in Phase I (see Results section). Immediately after finishing the last task of the training, the safety protocol was conducted. The complete sleep quality, cognitive and emotional assessment performed on the pre-test (day 1) and post-test (day 15) lasted approximately 40 minutes. The presentation of the training activities was randomized for each participant each day according to a patented algorithm that tailors the selection of activities to the specific cognitive profile of each individual. The entire process was carried out individually by each participant at home with daily supervision via videoconference by a clinical psychologist of the team.
From a total of 51 eligible potential participants, 24 voluntarily enrolled in the study after meeting the inclusion criteria. Out of these 24, 12 participants withdrew from the study for various reasons (7 did not complete all training or evaluation sessions, 2 felt it was too time-demanding, 2 presented other medical complications that precluded them from adhering to the protocol, and 1 was decided to quit for personal reasons). Thus, the final sample was composed of 12 patients (75% males), with a mean age of 44 years (range 25–55).
Phase I. The first cohort of three participants reported extreme fatigue (namely, a score of 8 or above on a Likert-like ordinal scale from 1–10) after finishing the fourth training block, after approximately 60 minutes of training (see Table 1). None of the participants reported any side effects or undesirable experiences associated with the training. These results determined that the maximum tolerated training time was 45 minutes, and this was the training time set for the CCT in Phase II.
| Patient number | 1st training block | 2nd training block | 3rd training block | 4th training block |
|---|---|---|---|---|
| 1 | 2 | 4 | 6 | 9 |
| 2 | 2 | 2 | 5 | 9 |
| 3 | 2 | 4 | 6 | 9 |
Responses of each participant to the fatigue question posed after each training block. Range of responses from 1–10.
Phase II. In no training session were extreme fatigue (i.e., scores of 8 or above on the fatigue scale) or undesirable side effects reported. In the full absence of safety-related complications, the measures obtained in the assessment tests showed an improvement in the different areas evaluated, as reflected by a decrease in the direct scores obtained when comparing the pre-test (day 1) and post-test (day 15) of the different assessment instruments (Fig. 1).
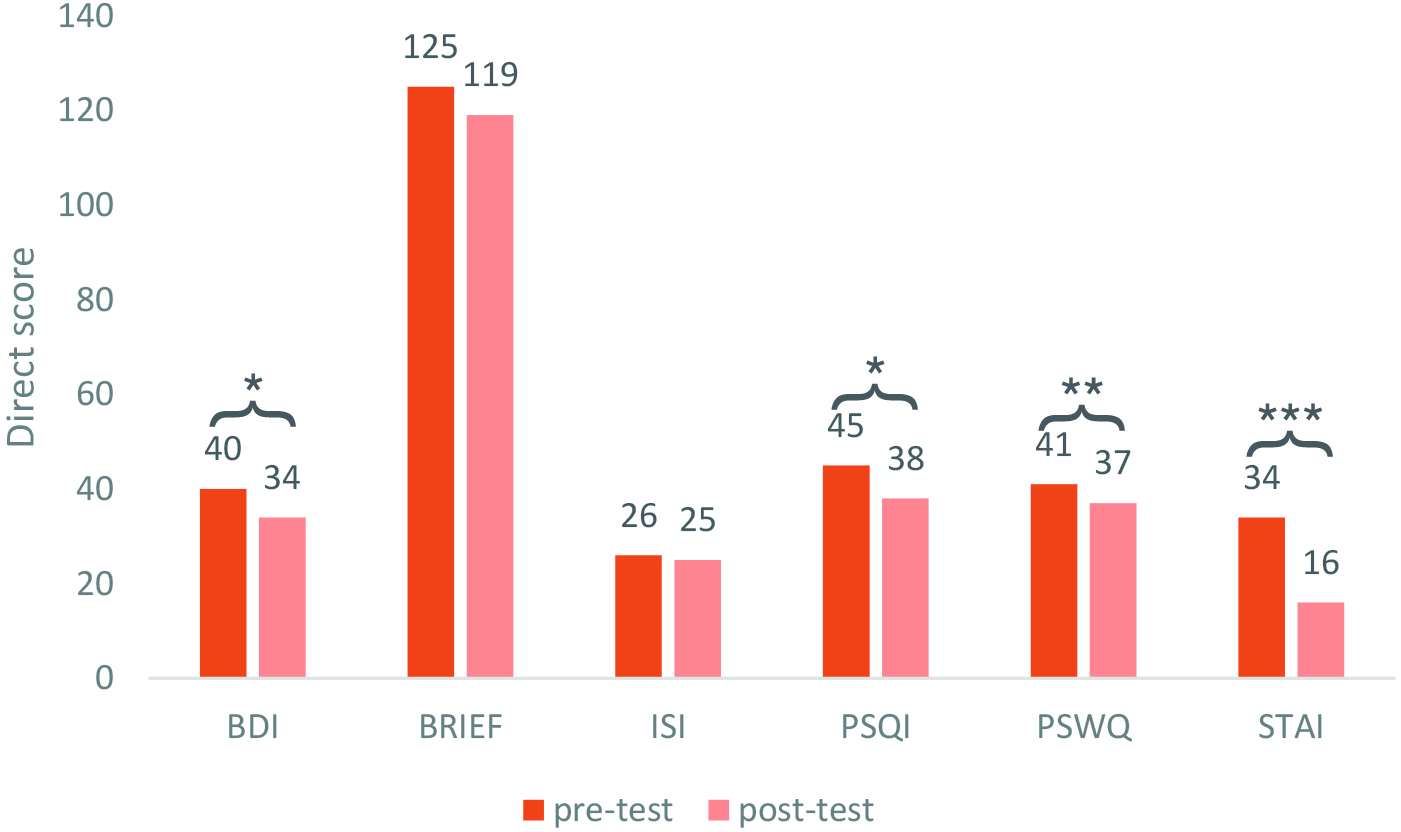 Fig. 1.
Fig. 1. Comparison of direct scores at pre- and post-evaluation.
Representation of the mean scores of all participants obtained in each evaluation
measure on day 1 (pre-test) and day 15 (post-test). Scores are rounded to the
nearest integer. *p
Pre- and post-intervention (day 1 vs day 15) scores were analyzed using paired
t-tests. Mean BDI-II scores significantly from day 1 (Mean (M) = 39.75, Standard Deviation (SD) =
10.96) to day 15 (M = 33.58, SD = 10.76), t(11) = 2.87, p = 0.015, Mean Difference (MDiff)
= –6.17, with a large effect size (d = 0.83), showing that the depressive
symptomatology of the sample was reduced at post-test. Results from the BRIEF-A
inventory that specifically assessed differences in self-reported executive
functioning did not show any significant difference between day 1 (M = 125.33, SD
= 25.37) and day 15 (M = 119, SD = 26.45), t(11) = 1.43, p = 0.182,
MDiff = –6.33, with small-medium effect size (d = 0.41). Since normality
assumptions were violated in the distribution of the results of the ISI,
Wilcoxon’s correction was applied and results indicated that there were no
differences in the indices of severity of insomnia when comparing the scores
before and after the CCT, with the median at day 1 and day 15 being similar (Median (Mdn)
= 25), t(11) = 45, p = 0.364, with medium effect size, r
In Spain, the country in which this Phase I/II clinical trial was developed and implemented, there are 4.7 million people diagnosed with insomnia among the population of 47 million inhabitants, reaching over 10 million people who present some of the symptoms or sleep disturbances (nearly one person in every five) [54, 55]. In this sense, computerized cognitive training (CCT) could offer an accessible alternative for a large part of the population, reducing the workload of professional medical assistance, especially considering that a very limited proportion of patients with insomnia have access to first-choice treatment (CBT-I). Thus, in line with the current results, we propose that CCT is a feasible and safe non-pharmacological intervention that could be used on its own or together with those CBT programs that lack this component. CCT stands as a promising treatment that could be administered independently when CBT is not available, in cases of milder insomnia, or when some symptoms are present but not meeting the diagnostic criteria for insomnia (e.g., in pre-clinical stages or in persons at risk of developing insomnia). In this line, CCT could also be considered a preventive tool for populations with a high predisposition to suffer sleep disturbances by improving the underlying cognitive mechanisms of insomnia.
The present study aimed to evaluate the feasibility of administering a computerized cognitive stimulation intervention for patients with clinical insomnia, exploring possible noticeable side effects and the extent to which the protocol could result effective. The proposed CCT intervention targeted inhibition and stimuli monitoring, being these cognitive skills that reduce cognitive arousal and contribute to modulating many sleep mechanisms. Secondary endpoints included sleep quality, executive functions, mood disturbances, and every day worries. Adverse events were assessed by a Likert scale and two binary response items.
The results from the Phase I study showed that a 45-minute gamified training does not present any negative effects (measured by a Likert scale exploring fatigue and two binary-response questions regarding feelings of adverse effects or undesirable experiences), setting the maximum tolerated training time to an intervention based on sessions of this length as a safe protocol. In the Phase II study, and again in the full absence of negative effects associated with the intervention, we found that sleep quality improved with a mere 7-session training of 45 minutes each (namely, with a total of 5 hours and 15 minutes of training). The components of sleep quality assessed included sleep latency, total sleep time, presence of sleep disturbances (not being able to fall asleep, night or morning awakenings, getting up to go to the toilet, feeling cold or hot, having nightmares, etc.), need for medication to sleep, and presence of daytime sleepiness, and participants reported higher sleep quality after the CCT than before it. The Phase II study also showed improvements in the following cognitive-emotional domains: sadness, pessimism, concentration problems, sleeping habits, loss of interest, loss of pleasure, disconformity, loss of energy, suicidal thoughts, calmness, feeling of security, overexcitement, worries, negative repetitive thoughts, restlessness, feeling of control, self-confidence, anguish, nervousness, and feeling of satisfaction. Notwithstanding these findings, it is yet to be explored what the minimum desirable training time needed to obtain significant benefits is, and what would be the minimum number of sessions necessary to maintain and generalize the benefits.
In sum, we found no unwanted or undesirable effects related to the CCT intervention. Adherence was acceptable, with a 50% drop-out rate [56]. Furthermore, symptoms of insomnia and mood disturbances showed a tendency to improve. Although not all differences were significant, note that some of the questionnaires we used are not sensitive to the short timeframe of the intervention (i.e., ISI, BRIEF-A). For instance, contrary to emotional states, changes in behavioral patterns might require time to settle in. Likewise, questionnaires usually include items addressing general matters, or concerning the last month or weeks (i.e., “During the past month, how often has each of the following behaviors been a problem?”).
As would be expected in a clinical trial such as the present one that closely resembles a case study approach, several factors such as the small sample size, the lack of a control group, and the unknown future outcome limit the generalizability of our findings. Another limitation of the study was the clinicians’ daily monitoring of the intervention process. Although a Phase I/II study requires such a level of supervision, it remains to be determined whether patients would achieve comparable results in an unsupervised self-administered setting. Future studies could shed light on the generalizability of these results over time and different subtypes of persons with insomnia with randomized control trials along the lines worked in preceding studies [41].
To the best of our knowledge, this study represents the first attempt to obtain quantitative data regarding the safety and potential efficacy of a CCT in insomnia, opening doors to the development of digital tools to alleviate the symptoms of a highly widespread problem. Our findings pave the way to novel, cost-effective and accessible interventions based on digital therapeutics for sleep-related disorders. Computerized home-based cognitive stimulation programs might be a promising intervention tool for treating insomnia.
CBT, cognitive behavioral therapy; CBT-I, cognitive behavioral therapy for insomnia; CCT, computerized cognitive training; ISI, insomnia severity index; PSQI, Pittsburgh sleep quality index; CAB, Cognitive Assessment Battery (CAB)™ PRO; BRIEF-A, Behavior Rating Inventory of Executive Function-Adult; BDI-II, Beck depression inventory-II.
The datasets supporting the results of this study are not publicly available due to data protection legislation of the national health system. Data are however available from the corresponding author upon reasonable request and with permission of the Hospital Universitario de la Ribera.
JLT and JAD conceived and designed the research study; JLT, FJP, and JAD created the experimental materials; JLT and FJP collected the data; JLT and JAD analyzed the data. All authors contributed to manuscript redaction and approved it for publication.
This study has been approved by the Research Commission of La Ribera Health Department - Generalitat Valenciana, and by the Research Ethics Committee - CEI of the Nebrija University and complies with the ethical requirements and regulations agreed upon in the Declaration of Helsinki, thus granting it the approval code UNNE-2021-006.
The authors thank CogniFit Inc. for the technical support.
This research was partially funded by the State Plan for Scientific and Technical Research and Innovation of the Government of Spain with grant numbers FPU19/02239 and PID2021-126884NB-I00, and by the BBVA Foundation with the grant ISERIE.
The authors declare no conflict of interest.
Appendix 1
List of Computerized Cognitive Training activities for Insomnia. Please note that the order established here does not correspond to the order in which the cognitive activities were presented, given that the intervention was individualized using the patented algorithm that creates a tailored training for each patient.
Note. All links were accessible in November 2022.
Appendix 2
Structured interview protocol to be followed in case an adverse event or a side effect was reported by any participant at any stage of the clinical trial. The protocol is an adaptation of the Patient-Reported Adverse Drug Event Questionnaire [57].
1. When did you first experience this side effect of the intervention?
2. How much does this side effect bother you?
3. How much influence does this side effect have on your daily functioning?
4. How satisfied are you with the intervention when you consider both this particular side effect and the effect of the intervention?
5. Why do you think this symptom was caused by the intervention?
6. How sure are you that this side effect is caused by this intervention?
7. Do you think there are other reasons for your experiencing this side effect other than the intervention?
8. Have you experienced this side effect in the past in combination with other interventions?
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

