1 The Third Clinical Medical College, Zhejiang Chinese Medical University, 310053 Hangzhou, Zhejiang, China
2 The Third Affiliated Hospital of Zhejiang Chinese Medical University, 310013 Hangzhou, Zhejiang, China
3 Department of Geriatric Rehabilitation Center, Zhejiang Rehabilitation Medical Center, 310053 Hangzhou, Zhejiang, China
4 Department of Hangzhou Innovation Institute, Beihang University, 310053 Hangzhou, Zhejiang, China
5 Department of Neurology, The Third Affiliated Hospital of Zhejiang Chinese Medical University, 310013 Hangzhou, Zhejiang, China
6 Department of Center for Rehabilitation Assessment and Therapy, Zhejiang Rehabilitation Medical Center, 310053 Hangzhou, Zhejiang, China
7 Department of Encephalopathy Rehabilitation Center, Zhejiang Rehabilitation Medical Center, 310053 Hangzhou, Zhejiang, China
8 Integrated Medicine Research Center for Neurological Rehabilitation, College of Medicine, Jiaxing University, 314001 Jiaxing, Zhejiang, China
9 Department of Pharmacy, College of Medicine, Jiaxing University, 314001 Jiaxing, Zhejiang, China
†These authors contributed equally.
Academic Editor: Jesús Pastor
Abstract
Background: The efficacy of intermittent theta-burst stimulation (iTBS) and transcranial direct current stimulation (tDCS) combined with cognitive training in the treatment of post-stroke cognitive impairment (PSCI) requires further investigation. Methods: We randomly assigned 60 patients with PSCI to receive iTBS (n = 21), tDCS (n = 19), or cognitive training alone (n = 20). Cognitive function was evaluated by the Loewenstein Occupational Therapy Cognitive Assessment (LOTCA), and the performance of activities of daily living (ADL) was assessed with the modified Barthel Index (MBI). Of these patients, 14 participated in the functional near-infrared spectroscopy (fNIRS) measurement. Results: After six weeks of treatment, cognitive function improved in all three groups of PSCI patients. Compared with patients receiving only cognitive training, the cognitive function of patients in the iTBS combined with cognitive training (p = 0.003) and tDCS combined with cognitive training groups (p = 0.006) showed greater improvement. The cognitive improvement from tDCS was related to the activation of the frontopolar cortex (FPC), while the improvement of cognition by iTBS was based on the activation of the stimulation site (the dorsolateral prefrontal cortex) and some distant regions. Conclusions: Both iTBS and tDCS in addition to cognitive training appear to improve cognitive function and quality of life of patients with PSCI, compared to cognitive training alone. tDCS improved cognitive function by improving the patient’s valuation, motivation, and decision-making substructures, while iTBS improved patients’ assessment and decision-making abilities, improving cognitive control and, ultimately, overall cognitive function.
Keywords
- post-stroke cognitive impairment
- intermittent theta-burst stimulation
- transcranial direct current stimulation
- functional near-infrared spectroscopy
Stroke, also known as cerebrovascular accident, is the leading cause of death and disability in the world, affecting 15 million people every year [1]. Post-stroke cognitive impairment (PSCI) refers to a common complication after stroke, a series of syndromes that appear within three months of the clinical event of stroke and must last for at least six months to meet the diagnostic criteria. More than half of stroke patients will have impairment in one or more cognitive domains, while the core clinical symptom is impaired executive function [2]. This can lead to serious insufficiency in or loss of patients’ self-care, social participation, and work skills, which brings heavy life and economic burdens to their family and society [3].
Theoretically, drug treatments such as acetylcholinesterase inhibitors may improve PSCI. However, studies have shown that these drugs have only short-term benefits, and all have side effects of varying degrees [4, 5]. Cognitive training is a commonly used method to treat cognitive impairment in clinic that can delay or improve cognitive decline through repeated training. However, its efficacy varies from person to person, and to a certain extent depends on the patient’s self-investment and cooperation [6]. Therefore, in recent years, it has often been combined with other, more technological treatments to ensure better clinical effects. Non-invasive brain stimulation (NIBS) is a non-invasive and effective treatment method for PSCI that can be used to gradually improve cognition.
Repetitive transcranial magnetic stimulation (rTMS) refers to the generation of a high-intensity instantaneous magnetic field that acts on brain tissue and induces electrical currents to stimulate the corresponding neurons and change the cortical excitability [7]. Theta-burst stimulation (TBS) is a special stimulation mode of rTMS that can more effectively improve cortical excitability by matching the theta rhythm of natural discharge in the brain [8, 9]. Intermittent TBS (iTBS) is a stimulation mode that contains a 2-second TBS sequence (10 TBS bursts) that is transmitted every 10 seconds (the burst interval between columns is 8 seconds) and lasts a total of 200 seconds (600 pulses). In clinical applications, it has the advantages of short time requirement, low intensity, and strong effect.
Studies have shown that, compared with high-frequency rTMS, iTBS can produce greater and longer-lasting excitability of the motor cortex [10], lasting for up to 60 minutes after stimulation [9]. Use of iTBS over the left dorsolateral prefrontal cortex (DLPFC) is a novel stimulation mode. In recent years, studies have confirmed the clinical efficacy of this stimulus program in the treatment of mental diseases such as depression and autism [11, 12, 13]. One study showed that three-day iTBS stimulation improved overall cognition, attention, and visuospatial ability in patients with Parkinson’s disease [14]. Although studies have confirmed the effectiveness of iTBS in improving Parkinson’s cognitive impairment, the efficacy of iTBS in patients with PSCI has not been determined.
Transcranial direct current stimulation (tDCS) refers to the regulation of specific areas of the brain by the placement of electrodes on the scalp to affect the resting membrane potential and change the excitability of the cortex [15]. Anode tDCS stimulation can depolarize the membrane potential in the affected area and increase the firing frequency of neurons to produce a lasting after-effect [16]. This procedure has been widely used in stroke patients to treat motor dysfunction, aphasia, and dysphagia [17, 18, 19]. Although the use of tDCS in the treatment of cognitive disorders such as Alzheimer’s disease and Parkinson’s disease has attracted some attention [20, 21], only a few small-scale studies have investigated the efficacy of tDCS in the treatment of PSCI. Therefore, the ameliorative effect of tDCS on PSCI remains unclear. Considering the two neuromodulation technologies, iTBS and tDCS, there are few studies on the application of iTBS in cognitive function, and the two intervention methods seem to have different effects on cognitive function [22, 23]. The advantages and disadvantages of the two intervention methods on overall cognition and its various sub-domains (attention, thinking operation, orientation, etc.) are not yet known, showing the need for further research.
Few studies have explored the role of neuroplasticity in the use of iTBS and tDCS to improve cognitive function, and the mechanism remains unclear [24]. Functional near-infrared spectroscopy (fNIRS) is a non-invasive optical imaging technology that relies on the principle of neurovascular coupling. It uses the attenuation of near-infrared light to quantify changes in the concentration of cerebral cortex oxygenation (HbO) and deoxyhemoglobin (HHb), thus measuring activation of the cerebral cortex [25]. Compared with more mature imaging methods, such as functional magnetic resonance and electroencephalography, fNIRS has the advantages of portability, low cost, and lack of interference from motion artifacts, imposing fewer constraints on subjects during the measurement process [26]. Patients with cognitive impairment showed lower levels of activation in specific brain regions than healthy people [27, 28, 29]. The literature has substantiated that, for both healthy people and patients with cognitive impairment, fNIRS can be used to detect the hemodynamics of the frontal lobe to assess their cognitive status [30]. However, it has rarely been used to investigate the effects of iTBS and tDCS on the neuroplasticity of PSCI. Herein, this study aimed to observe the effects of iTBS and tDCS combined with cognitive training on PSCI.
This was a prospective, randomized, single-blind and controlled study. Ethical approval was granted by the Third Affiliated Hospital of Zhejiang Chinese Medical University Ethics Committee, and written consent was obtained from all participants and their families.
Patients diagnosed with PSCI in the Third Affiliated Hospital of Zhejiang Chinese Medical University from March 2021 to October 2021 were recruited. Sixty-six patients were enrolled and divided equally to the iTBS, tDCS, and control group according to random number table. Among them, two patients dropped out the study for unwillingness to continue treatment and evaluation and another four patients were excluded out the analysis for transferring or returning home. Finally, 21 cases in the iTBS group, 19 cases in the tDCS group, and 20 cases in the control group were included into the statistical analysis (Fig. 1). The inclusion criteria were as follows: patients diagnosed with stroke based on the diagnostic criteria of the Guidelines for Diagnosis and Treatment of Cerebral Hemorrhage in China (2019) or the Guidelines for Diagnosis and Treatment of Acute Ischemic Stroke in China (2018), cerebral hemorrhage or cerebral ischemia accompanied by cognitive impairment within six months as defined by a Mini–Mental State Examination (MMSE) score below 26, no serious visual or hearing impairment, and stable vital signs with no progression of neurological symptoms. The exclusion criteria were as follows: patients with metallic devices, pacemakers or skull defects; history of epilepsy or risk of seizures; intracranial occupying lesion, including arteriovenous malformation or brain tumor, according to imaging results; cognitive dysfunction caused by other reasons (such as Alzheimer’s disease); and depression and other mental disorders. Prior to inclusion in the study, all patients underwent a neuropsychological evaluation to screen for the inclusion/exclusion criteria. For each participant in the corresponding group, stimulation or no stimulation was applied over the left DLPFC in 30 sessions over 30 consecutive weekdays.
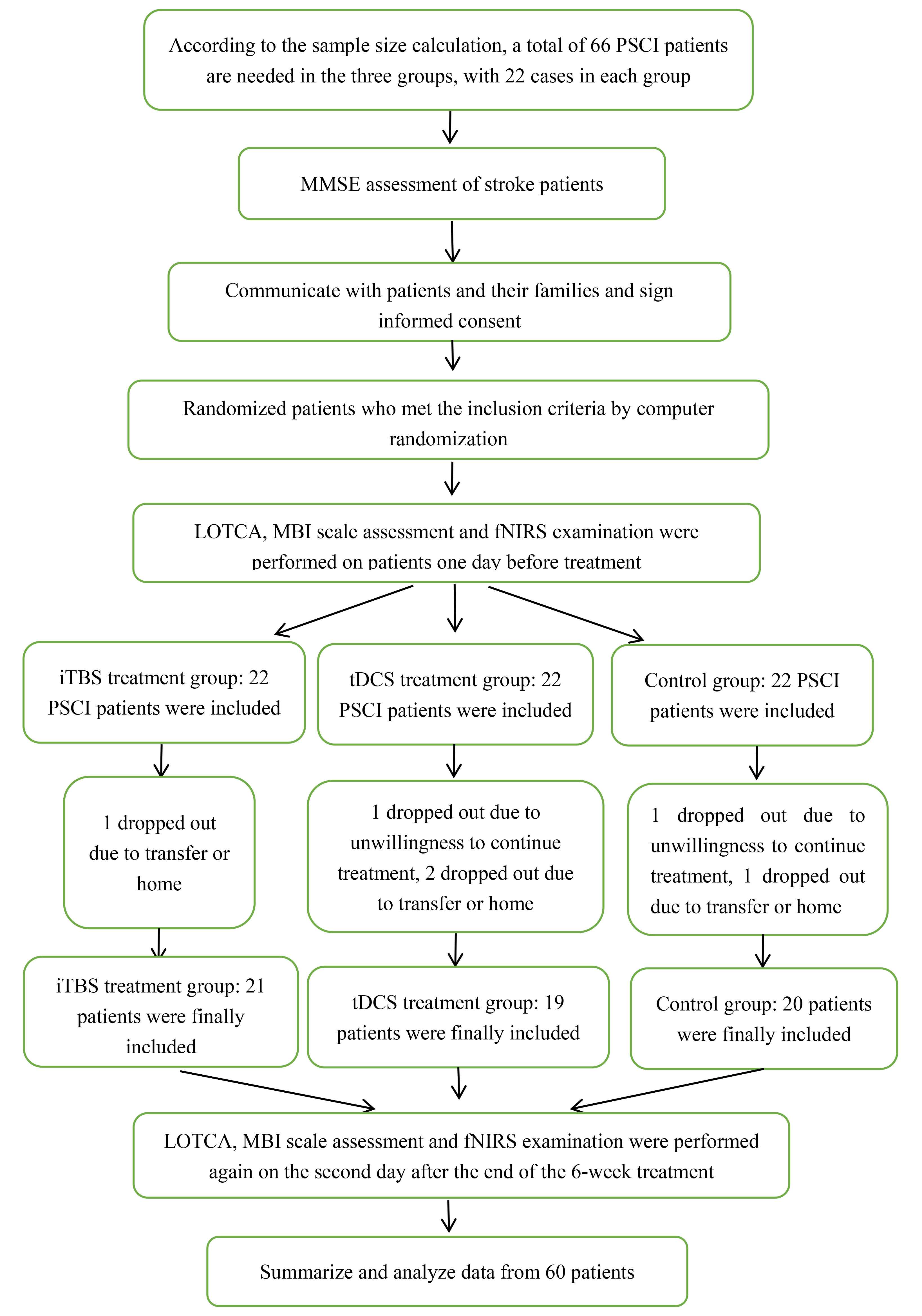 Fig. 1.
Fig. 1.Patient Inclusion Flowchart.
2.3.1.1 iTBS Protocol
The iTBS procedure used a CCY-I–type stimulator with an “8”-shaped coil (Wuhan Yiruide Medical Equipment New Technology Co., Ltd., China). The left DLPFC was selected as the stimulation site, and the cranial landmarks were located by the international 10–20 EEG method. The body surface was positioned at F3, and the center point of the coil was tangent to the surface of the patient’s scalp. Meta-analysis indicated that the DLPFC is the best stimulation site for improving cognitive function [31, 32]. Stimulation intensity was set to 70% of the relaxed motor threshold, triplet 50 Hz bursts, repeated at 5 Hz, 2 s on and 8 s off; this was repeated 20 times, with 600 pulses per session, for a total duration of 3 min 20 s. The course of treatment was comprised of 30 sessions in total: once-daily sessions on weekdays, with five sessions per week over six weeks.
2.3.1.2 Cognitive Training
After each iTBS treatment, the therapist conducted computer-assisted cognitive rehabilitation with the patient, including attention training, executive function, memory, calculation, and reasoning ability training. The total number of cognitive training sessions was 30, each for 30 min, occurring five times a week over six weeks.
2.3.2.1 tDCS Protocol
The tDCS procedure adopted a transcranial direct current rehabilitation therapy device (TES-02, Harbin Aobo Medical Equipment Co., Ltd., Heilongjiang, China). The stimulating electrode was a circular conductive rubber electrode with a diameter of 25 mm; the anode was placed on the patient’s left DLPFC using the international 10–20 EEG method to locate the head mark, and the body surface was positioned at F3. The cathode was placed on the opposite shoulder. The stimulation intensity was 2.0 mA, and it lasted for 20 min. The course of treatment was comprised of 30 sessions in total: once-daily sessions on weekdays, with five sessions per week for six weeks.
2.3.2.2 Cognitive Training
The protocol for cognitive training was the same as that of the iTBS group.
The protocol for cognitive training was the same as that of the iTBS group.
At baseline and the day after the end of six weeks of treatment, patients were assessed for cognitive function and activities of daily living using the following scales.
The primary outcome measure was the Loewenstein Occupational Therapy Cognitive Assessment (LOTCA): research shows that, compared with the MMSE and the Montreal Cognitive Assessment (MoCA), the LOTCA can comprehensively assess the executive function of patients and predict the degree of overall functional improvement after stroke [33]. This study used the first version of the LOTCA (the Chinese version). The total possible LOTCA score is 91, including orientation (from 2 to 8 points), visual perception and spatial perception (from 6 to 24 points), visuo-motor organization (from 7 to 28 points), thinking operation (from 6 to 27 points), and attention (from 1 to 4 points).
The secondary outcome measure, the modified Barthel Index (MBI), is a scale commonly used to clinically to assess patients’ activities of daily living (ADL), including 10 points for eating, 5 points for bathing, 5 points for grooming, 10 points for dressing, 20 points for controlling urine and bowel, 10 points for toileting, 15 points for transferring between bed and chair, 15 points for walking on flat ground, 10 points for going up and down stairs, and 5 points for using a wheelchair, a total of 100 points. The higher the score, the better the independence of the patient.
We used the verbal fluency test (VFT) during data collection, as it has been
reported that the brain activity of patients with cognitive impairment decreases
during administration of the VFT [34]. The VFT has been widely used to
investigate prefrontal brain function and is also a valid measure of executive
function [35, 36, 37]. We performed fNIRS measurements on a subset of patients (7 in
the iTBS group and 7 in the tDCS group, 14 in total). The fNIRS measurements were
conducted using an ETG-4000 Optical Topography System (Hitachi Medical Co., Tokyo,
Japan) to measure the concentration changes of HbO and HHb. We used the “3
The VFT is divided into four parts. During the early stage of the task, the subject sits quietly and looks at the “cross” icon 1 m in front of them for 10 s. Then there is a waiting period of 30 s, and the patient is required to count from 1 to 5 and look at the cross icon. During the stimulation time, every 20 s the patient is given a Chinese character, a total of 3 Chinese characters every 60 s, and told to group as many words as possible. During the last task, the patient is asked to continue counting from 1 to 5 and to look at the cross icon for 70 s. The test is carried out in a quiet and dark environment. During the entire detection process, efforts are made to keep the patient quiet and relaxed, not engaged in active thinking activities, and free from outside interference.
Many studies have confirmed that HbO is positively correlated with local cerebral blood flow [39]. Compared with individual changes in HHb [40], HbO can better reflect changes in local cerebral blood flow [41]. Therefore, in this study, we chose to analyze only the HbO concentration.
The NIRS_KIT software package, implemented in Matlab2020b (Mathworks Inc., Natick, MA, USA), was used to preprocess the original light intensity data. First, a polynomial regression model was used to estimate and remove any linear or non-linear trend in the signal for drift removal. Next, time derivative distribution repair was used to eliminate the head motion artifacts [42]. A 0.01–0.8 Hz band-pass filter was used to remove common noises, including physiological noises caused by heartbeat, respiration, and Mayer waves. Finally, based on the modified Beers–Lambert law, the filtered optical density data was converted into HbO concentration changes.
After removing the artifacts, the 30 s waiting period was used as the pre-task baseline. The baseline HbO was subtracted from the HbO during the task and divided by the standard deviation of the baseline period. Then a single-sample t-test and paired t-test were used to investigate the brain activation of each channel under each condition and generate a t-value heat map. Finally, the xjview toolbox (http://www.alivelearn.net/xjview8/) and BrainNet Viewer toolbox (http://www.nitrc.org/projects/bnv/) were used to project the heat map onto the 3D brain model [43].
Statistical analyses were performed with the Statistical Packages for Social
Sciences (SPSS) (SPSS-25.0, IBM, Chicago, IL, USA). The Shapiro–Wilk tests were used
to examine the normal distribution. One-way ANOVA analysis (for the LOTCA test
and the MBI scale) and the Kruskal–Wallis rank sum test (for LOTCA sub-items)
were used to evaluate the clinical efficacy of the treatments in the three
groups. Two-sample t-tests and non-parametric Mann–Whitney tests were
used to compare two groups of continuous variables. Spearman correlation was used
to measure the association between primary and secondary outcomes. Normally
distributed data were expressed as the mean (SD), and non-normal data were
expressed as the median (interquartile range). The level of significance was set
at p
This is a prospective, randomized, single-blind and controlled study. A total of 60 PSCI patients were recruited and divided into the iTBS group (n = 21), tDCS group (n = 19) and control group (n = 20). Of these, 7 patients in each of the iTBS and tDCS groups completed fNIRS measurements. Demographic and clinical characteristics are presented in Tables 1,2,3; there was no difference between the groups. As there were no obvious adverse reactions during the experiment, the safety and tolerance are assumed to be good.
| Characteristics | iTBS group (n = 21) | tDCS group (n = 19) | Control group (n = 20) | p value |
| Age (years) | 57.24 |
61.58 |
66.75 |
0.087 |
| Education (years) | 9.00 |
9.00 |
9.00 |
0.073 |
| Onset time (months) | 4.00 |
2.00 |
6.00 |
0.073 |
| Male/Female | 18/3 | 14/5 | 13/7 | 0.306 |
| Injury site (left/right) | 12/9 | 12/7 | 13/7 | 0.863 |
| Hemorrhage/Infarction | 8/13 | 5/14 | 8/12 | 0.626 |
| MMSE | 13.81 |
13.89 |
12.40 |
0.502 |
| Severity (mild/moderate/severe) | 3/12/6 | 1/15/3 | 1/13/6 | 0.549 |
| LOTCA | 50.48 |
51.79 |
45.00 |
0.305 |
| orientation | 4.00 |
4.74 |
4.50 |
0.789 |
| visual perception and spatial perception | 20.00 |
20.00 |
19.00 |
0.361 |
| visuo-motor organization | 13.86 |
14.63 |
12.25 |
0.386 |
| thinking operation | 11.43 |
11.74 |
9.50 |
0.351 |
| attention | 3.00 |
2.00 |
1.50 |
0.355 |
| MBI | 58.33 |
42.00 |
45.10 |
0.181 |
| Characteristics | iTBS group (n = 7) | tDCS group (n = 7) | p value |
| Age (years) | 56.14 |
53.86 |
0.813 |
| Education (years) | 9.14 |
15.00 |
0.159 |
| Onset time (months) | 2.86 |
2.57 |
0.781 |
| Male/Female | 6/1 | 7/0 | 0.299 |
| Injury site (left/right) | 6/1 | 3/4 | 0.266 |
| Hemorrhage/Infarction | 2/5 | 1/6 | 1.000 |
| MMSE | 15.14 |
13.71 |
0.453 |
| Severity (mild/moderate/severe) | 1/5/1 | 0/7/0 | 0.311 |
| LOTCA | 53.00 |
55.14 |
0.740 |
| orientation | 4.14 |
4.43 |
0.726 |
| visual perception and spatial perception | 18.71 |
20.14 |
0.553 |
| visuo-motor organization | 15.29 |
15.43 |
0.959 |
| thinking operation | 12.00 |
12.57 |
0.804 |
| attention | 2.71 |
2.57 |
0.786 |
| MBI | 53.14 |
52.86 |
0.984 |
| Values denote means Abbreviations: iTBS, intermittent Theta Burst Stimulation; tDCS, transcranial direct current stimulation; MMSE, Mini-mental State Examination; LOTCA, Loewenstein Occupational Therapy Cognitive Assessment; MBI (Activity of Daily Living), Modified Barthel index; F, female; M, male. | |||
| Characteristics | patients with fNIRS (n = 14) | patients without fNIRS (n = 46) | p value |
| Age (years) | 55.00 |
63.85 |
0.035* |
| Education (years) | 10.86 |
9.00 |
0.420 |
| Onset time (months) | 2.00 |
4.00 |
0.138 |
| Male/Female | 13/1 | 32/14 | 0.155 |
| Injury site (left/right) | 9/5 | 27/19 | 0.765 |
| Hemorrhage/Infarction | 3/11 | 18/28 | 0.340 |
| MMSE | 14.43 |
13.00 |
0.271 |
| Severity(mild/moderate/severe) | 1/12/1 | 4/28/14 | 0.187 |
| LOTCA | 54.07 |
47.54 |
0.144 |
| orientation | 4.50 |
5.00 |
0.619 |
| visual perception and spatial perception | 20.50 |
19.00 |
0.171 |
| visuo-motor organization | 15.36 |
11.00 |
0.091 |
| thinking operation | 12.29 |
10.00 |
0.172 |
| attention | 3.00 |
1.00 |
0.008** |
| MBI | 53.00 |
51.33 |
0.822 |
| Values denote means Abbreviations: iTBS, intermittent Theta Burst Stimulation; tDCS, transcranial direct current stimulation; MMSE, Mini-mental State Examination; LOTCA, Loewenstein Occupational Therapy Cognitive Assessment; MBI (Activity of Daily Living), Modified Barthel index; F, female; M, male. | |||
Inter-group results: after treatment, the total scores of the LOTCA test were analyzed by one-way ANOVA (F = 5.804, df = 2, p = 0.005). Pairwise comparisons showed that, compared with the control group, the iTBS group (p = 0.003) and the tDCS group (p = 0.006) both had a significant increase in the total LOTCA score after stimulation (Fig. 2).
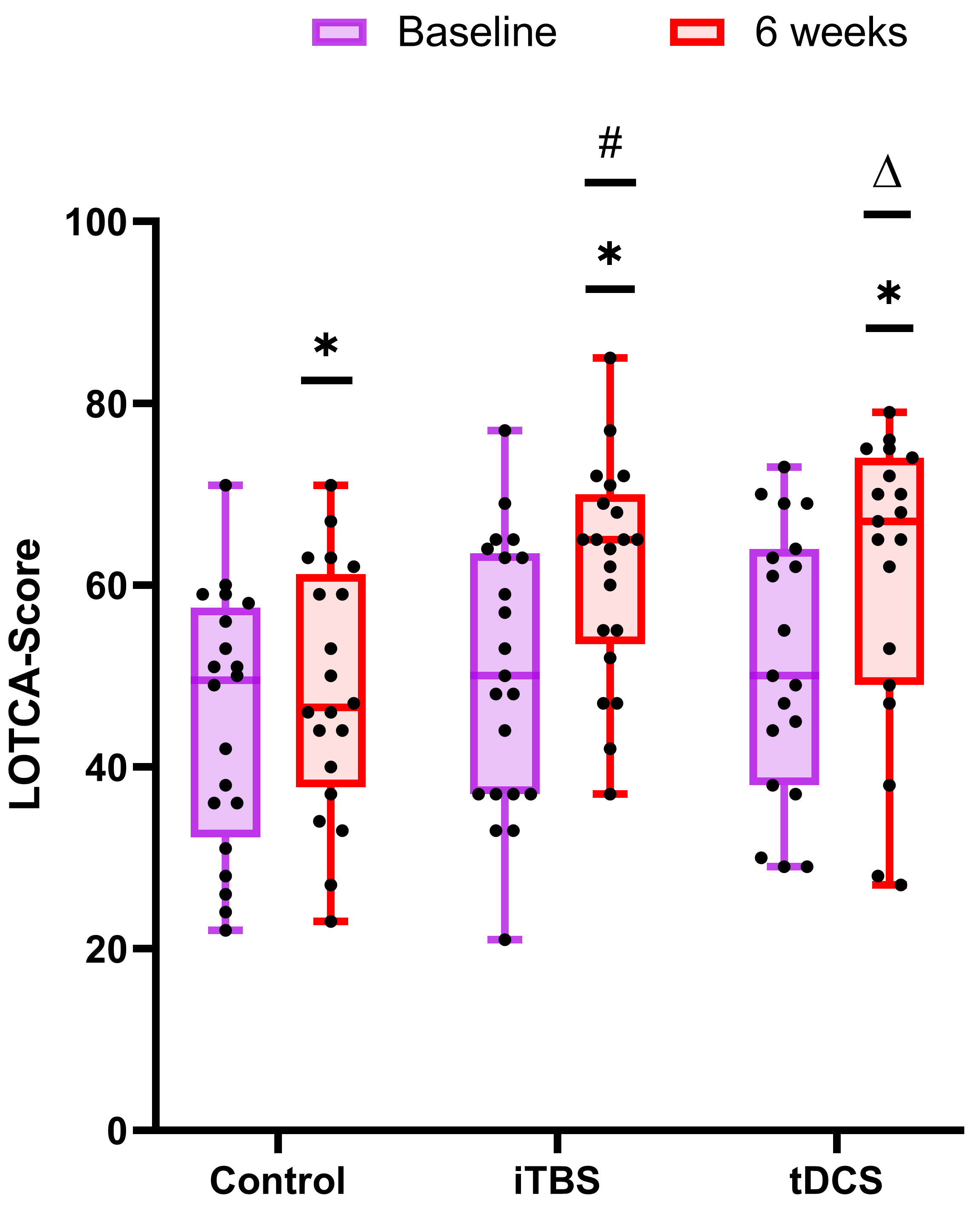 Fig. 2.
Fig. 2.Comparison of the improvement in the neuropsychological
test between the control group, iTBS group, and tDCS group. The baseline is
colored in purple and that at 6 weeks is colored in red. *p
Intra-group results: after the 30 stimulation treatment sessions, paired
comparison showed that the total scores of the LOTCA test in the iTBS group
(p
The value obtained by subtracting the LOTCA before treatment from LOTCA after treatment was compared with 0, and if it was greater than 0, it was recorded as improvement; if it was less than or equal to 0, it was recorded as no improvement. The number of patients who finally improved was 18, 15, and 15 in the iTBS group, tDCS group, and control group, respectively. Using the chi-square test analysis, we can get: p = 0.686, that is, the three groups of intervention methods have the same amount of cognitive improvement in PSCI patients.
One-way ANOVA analysis and the Kruskal–Wallis rank sum test were used to
analyze the five sub-items of the LOTCA test. For the visual-motor organization
(
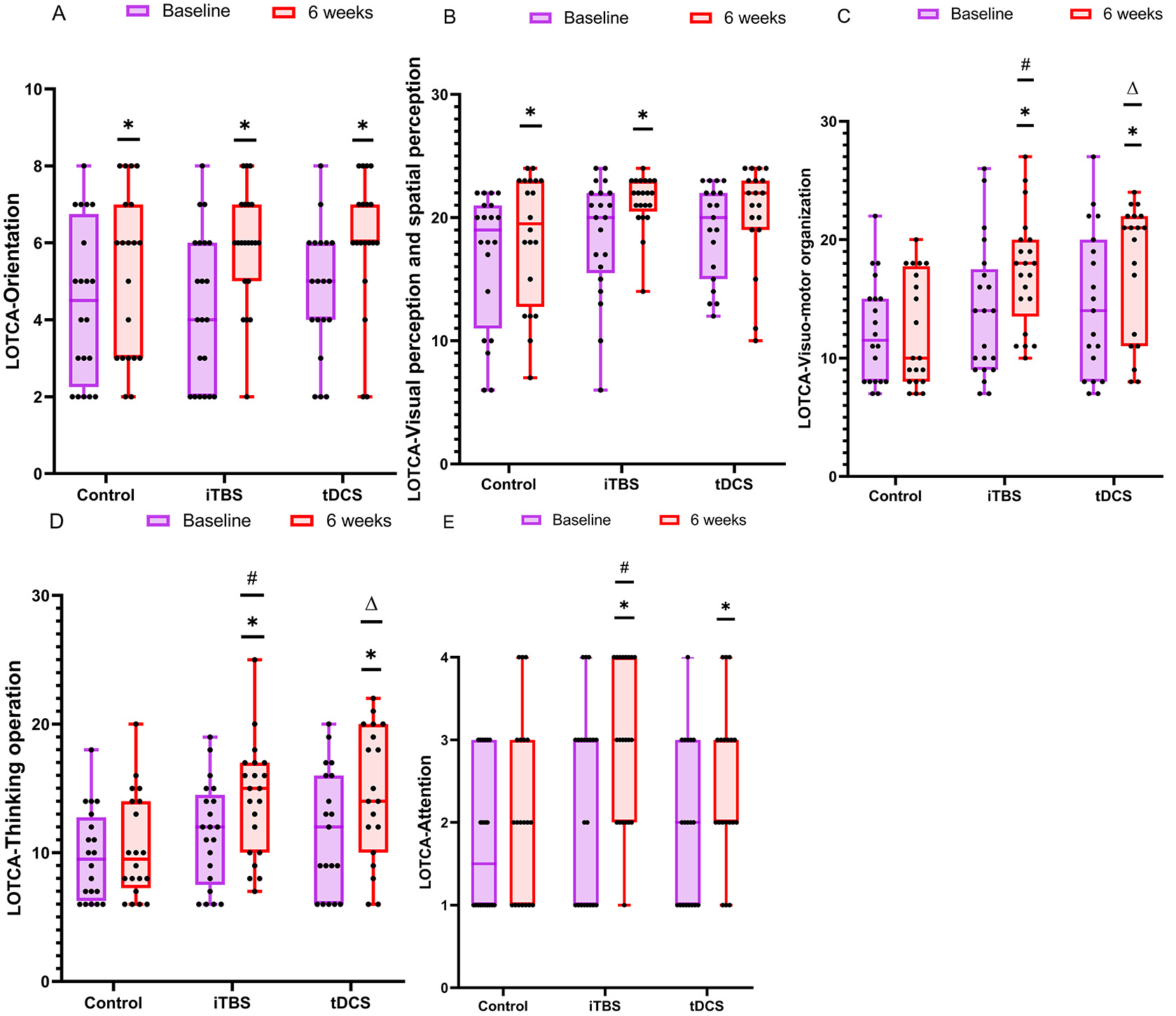 Fig. 3.
Fig. 3.Details of the sub-items of the LOTCA test. The data
were analyzed by the Mann–Whitney U test and the Kruskal–Wallis rank sum test.
*p
Inter-group: the total MBI score after treatment was analyzed by one-way ANOVA (F = 4.617, df = 2, p = 0.014). Pairwise comparison showed that, compared with the control group, the iTBS group (p = 0.010) and the tDCS group (p = 0.012) both had a significant increase in the total MBI score after treatment (Fig. 4).
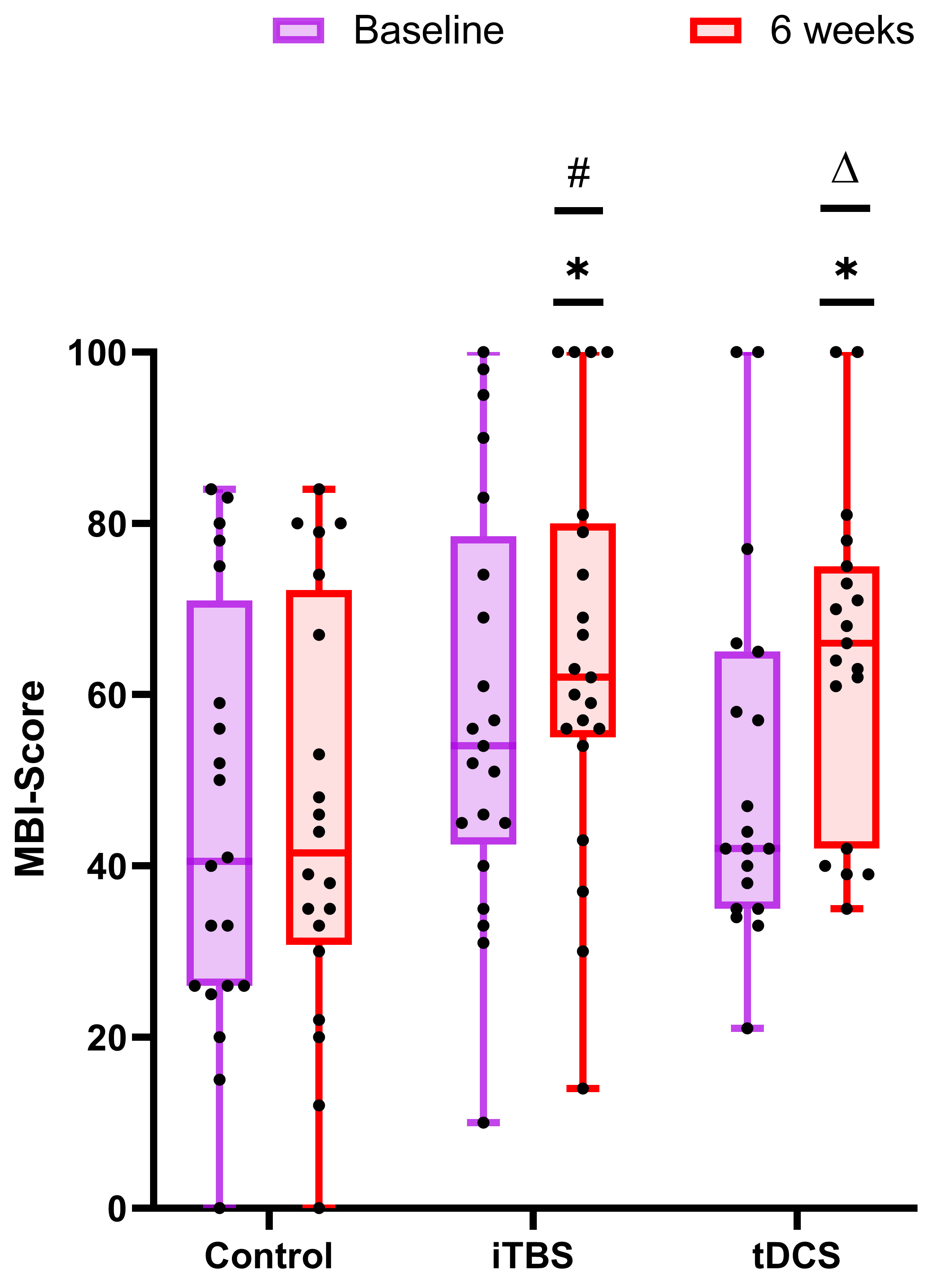 Fig. 4.
Fig. 4.Comparison of the improvement in activities of daily
living in the control group, iTBS group, and tDCS group. The panels
(baseline-tDCS) are non-normally distributed data and were therefore analyzed by
the non-parametric Mann–Whitney U test. The remaining panels are normally
distributed data and were analyzed by the two-sample t-test. The
baseline is purple and 6 weeks is red. *p
Intra-group: after 30 stimulation sessions, compared with the baseline, the paired comparison showed that the total MBI score of the iTBS group (p = 0.009) and the tDCS group (p = 0.002) increased significantly, while there was no statistical difference in the control group (p = 0.367) (Fig. 4).
A multivariate linear regression equation was constructed by including age,
(years), education (years), onset time (months), gender, injury site, type and
MMSE score. The results showed that the influence of different ages (years) on
cognitive function was statistically different (b = 2.57, t = 2.150,
p = 0.036), and the influence of different MMSE scores on cognitive
function was statistically different (b = 1.828, t = 4.736, p
| Variable | b value | Standard Error of the b value | Standardized Coefficients Beta | t value | p value |
| Intercept | 32.822 | 12.435 | - | 2.640 | 0.011* |
| Age (years) | –2.57 | 0.120 | –0.237 | –2.150 | 0.036 |
| Education (years) | 0.639 | 0.430 | 0.168 | 1.485 | 0.144 |
| Onset time (months) | –1.013 | 0.802 | –0.143 | –1.263 | 0.212 |
| Male/Female | –1.373 | 3.715 | –0.040 | –0.370 | 0.713 |
| Injury site (left/right) | 4.964 | 3.432 | 0.161 | 1.447 | 0.154 |
| Hemorrhage/Infarction | 4.669 | 3.540 | 0.150 | 1.319 | 0.193 |
| MMSE | 1.828 | 0.386 | 0.544 | 4.736 | |
| Abbreviations: MMSE, Mini-mental State Examination. *p | |||||
The correlation result showed that the total LOTCA score after treatment was
positively correlated with the MBI score (r = 0.492, p
The activation of the brain was compared before and after treatment in each channel. There was no channel activation in the iTBS group before treatment; channels 29 (t = 2.655, p = 0.038), 37 (t = 2.603, p = 0.040), and 41 (t = 2.865, p = 0.029) were significantly affected after iTBS treatment (Figs. 5,6). In the tDCS group, it was found that channels 36 (t = 2.994, p = 0.024) and 38 (t = 2.654, p = 0.038) were significantly activated after treatment (Figs. 5,7).
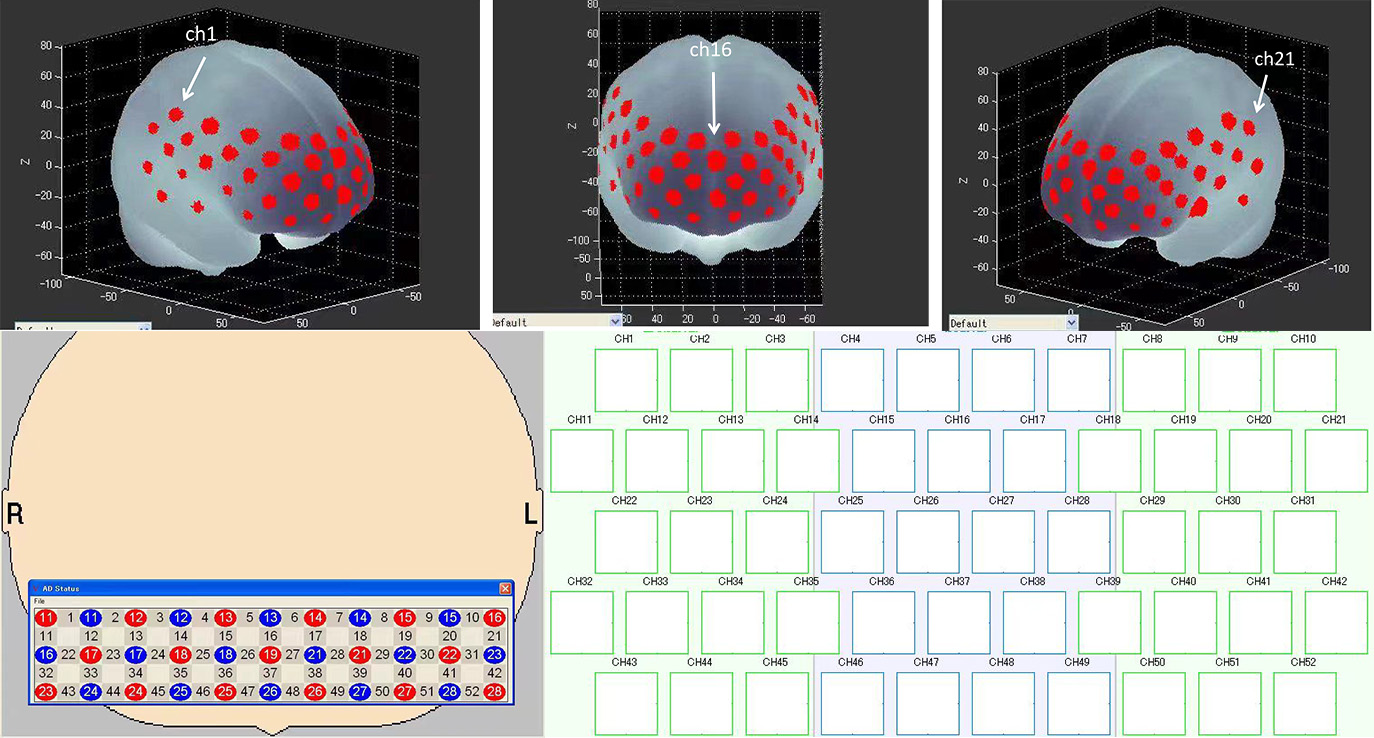 Fig. 5.
Fig. 5.Probe set arrangement with numbers indicating channels. 3D activation map based on the Montreal Neurology Institute (MNI) coordinate system.
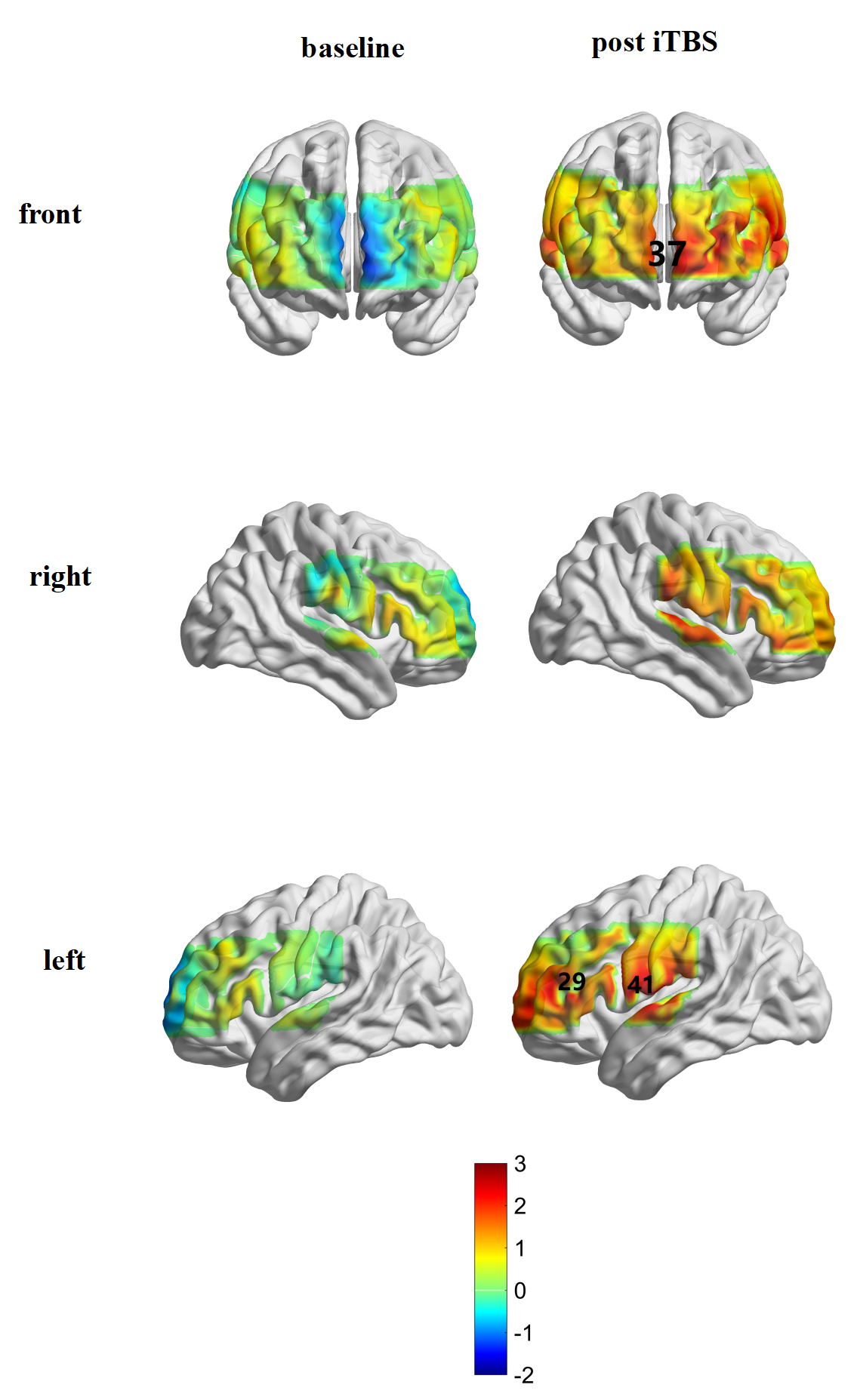 Fig. 6.
Fig. 6.Brain activation in the iTBS group before and after treatment.
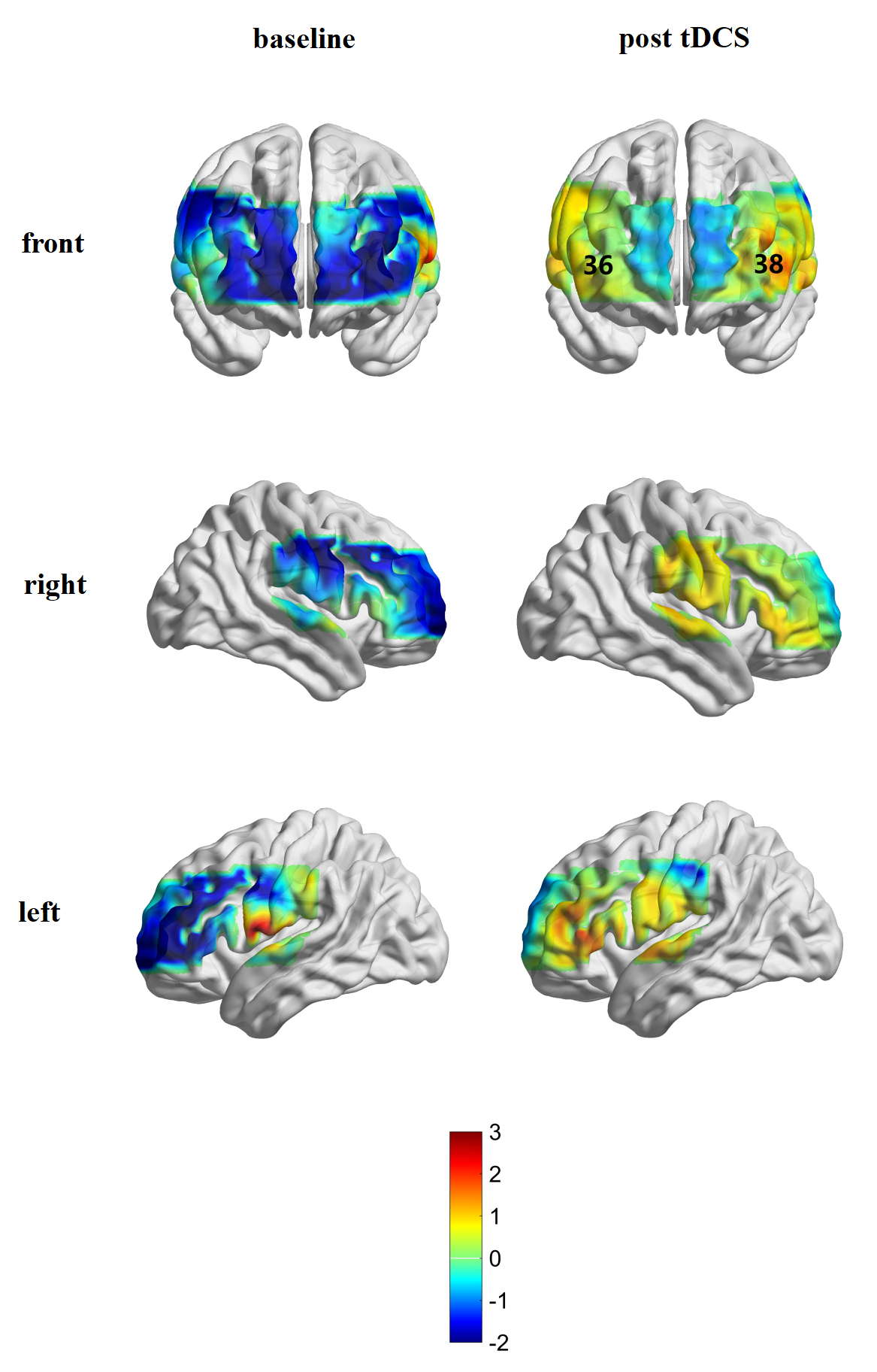 Fig. 7.
Fig. 7.Brain activation in the tDCS group before and after treatment.
This study reports the effects of two treatment protocols, iTBS combined with cognitive training and tDCS combined with cognitive training, on the cognitive function and activities of daily living in patients with PSCI, compared to cognitive training alone. The effectiveness of the two stimulation schemes was found to be accompanied by changes in the activation of different areas of the prefrontal lobe. Results indicated that left DLPFC iTBS or tDCS, combined with cognitive training, are both effective and safe modulation procedures for the treatment of PSCI that can effectively improve cognitive function and quality of life.
Both iTBS and tDCS have a significant impact on executive function (specifically visuo-motor organization and thinking operation, see Figs. 2,3). The results of this study are consistent with previous studies. Yin et al. [44] showed that rTMS on the DLPFC can improve the visuospatial and executive functioning of patients with PSCI. This change was related to the increased connectivity between the DLPFC and other brain regions. Dubreuil-Vall et al. [45] found that, compared with stimulation of the right DLPFC or false stimulation, tDCS stimulation of the left DLPFC can reduce reaction time and improve accuracy, improving the patient’s executive function.
While we found that iTBS combined with cognitive training was more effective than only cognitive training in improving attention, there was no such finding for tDCS. This may be related to the increased connectivity in the PFC after iTBS stimulation [46]. This result is consistent with previous studies. Gan [23] claimed that, compared to tDCS, iTBS has more potential for regulating maintenance and distraction of attention. Simultaneously, MBI scores were significantly improved; cognitive function will affect ADL to a certain extent [47]. Our results showed that higher LOTCA scores after treatment were associated with better MBI scores. The improvement in ADL may be a result of the recovery of executive function, which includes the three core cognitions of response inhibition, working memory, and cognitive flexibility. It also plays a decisive role in constructing high-level cognitive functions such as reasoning, problem solving, and planning [48], which are closely related to quality of life. In addition, this study showed that the age of the patients and the MMSE score at the time of enrollment can have an impact on the cognitive recovery of the patients. The older the patient, the smaller the cognitive function recovery degree within 6 weeks; the higher the MMSE score, the greater the cognitive function recovery degree of the patient within 6 weeks.
When comparing patients with and without fNIRS testing at baseline, those with fNIRS were younger than untested patients and performed better in attention subsection. This is related to the VFT paradigm of fNIRS. The VFT test requires patients to concentrate during the test to avoid the interference of motion and inattention on the results. Therefore, the attention sub-item performance of patients undergoing fNIRS is better. Based on the Montreal Neurology Institute (MNI) template and the Broadmann partition, and with reference to previous studies, the cortex where the activated channels reside was confirmed [49, 50, 51, 52]. Our study showed that, after tDCS stimulation, the patient’s frontopolar cortex (FPC) (channels 36 and 38) was activated. In recent years, the PFC has no longer been defined as the site of simple “central execution”. Studies have found that the execution process is divided into different cognitive functions related to motivational behavior (evaluation and decision-making) and control behavior (cognitive control) [52]. Among these, cognitive control requires working memory, response inhibition, and cognitive flexibility, which was mentioned above [53]. Cognitive control was related to the DLPFC and the lateral parietal cortex. The evaluation, motivation, reward learning, and decision-making functions in the cognitive process were mainly related to the FPC, orbitofrontal lobe, and intra-abdominal PFC [52, 54, 55]. Specifically, the FPC participates in determining when to transfer “external” information (for example, sports stimulation) to “internal” information (for example, preservation of episodic memory), and plays a role in supervision, integration, and decision-making [56]. Minati et al. [57] showed that stimulating the DLPFC can increase the confidence of subjects in risk decision-making and can lead them to execute better strategic decision-making [58].
Considering this, it seems that tDCS may improve the executive function and overall cognition of patients with PSCI by activating the FPC to improve their evaluation and decision-making abilities. Activation of the DLPFC (channel 29), FPC (channel 37), and Broca area (channel 41) on the left side of the patient was observed after iTBS stimulation. In other words, the improvement of PSCI by iTBS was accompanied by activation of the left DLPFC stimulation site and part of the distal area. The DLPFC has extensive connections with almost all cortical and subcortical brain structures, allowing it to coordinate and integrate the functions of all other brain regions [59]. Studies have shown that patients with cognitive impairment not only have decreased local activation of the DLPFC but also have decreased connectivity with other brain regions (the disconnection effect [60]). After iTBS stimulation, the DLPFC at the stimulation site was possibly activated and then returned to normal [51], and its connectivity with other brain networks was enhanced. Our research had shown not only that iTBS stimulation can activate the DLPFC at the stimulation site but also that the iTBS effect can gradually change from local punctate activation at the stimulated site to flake activation with enhanced connection between the DLPFC and the edge network [61]. As mentioned above, the DLPFC and the FPC, respectively, participate in cognitive control and evaluation, and decision-making in the execution process. Therefore, iTBS stimulation can improve executive function and overall cognitive status by improving patients’ evaluation and decision-making abilities while enhancing cognitive control via elements such as working memory and response inhibition. We also observed activation of the Broca area after iTBS stimulation. It is believed that Broca’s area plays a vital role in the VFT [62]. The improvement of patients’ cognitive function will affect the expression of speech and semantics to a certain extent, causing activation of the Broca area.
In this study, we did not observe obvious activation of the tDCS stimulation site (the left DLPFC), which may be related to the pathophysiological mechanism of tDCS. Generally, the spatial focus of iTBS is more accurate than that of tDCS: iTBS can directly affect the discharge of neurons under the stimulation coil [63], while tDCS influences the resting membrane potential and participating neural oscillations [64], resulting in less focus and impacting more scattered brain regions. Therefore, the neurophysiological effects of the tDCS stimulation may be slightly smaller than those of the iTBS [23]. However, tDCS has the advantages of small size, easy operation, and portability, making tDCS more attractive for scenarios such as home remote treatment [65].
This study has some limitations. First, the sample size was relatively small, especially that of the fNIRS measurements. It is possible that observations with larger sample sizes will identify more regional activation. In future studies, we will expand the sample size to more comprehensively observe the brain activation state and functional connectivity after iTBS or tDCS stimulation. Second, LOCTA, which we used as our evaluation tool, cannot analyze other cognitive domains of PSCI patients such as memory and language function. Third, due to the lack of follow-up evaluation, we were unable to determine the long-term efficacy and continuous changes of the two stimulations.
This study provided therapeutic potential of adding two stimulation protocols (iTBS and tDCS) to cognitive rehabilitation for PSCI. We confirmed that both iTBS and tDCS of the left DLPFC can improve executive and cognitive function in patients with PSCI, which was ultimately projected to improve the patient’s quality of life. Approaches appeared to be associated with changes in activation patterns. The clinical efficacy of the two stimulation schemes was accompanied by activation changes in different areas of the PFC.
PSCI, post-stroke cognitive impairment; NIBS, Non-invasive brain stimulation; rTMS, Repetitive Transcranial Magnetic Stimulation; TBS, Theta Burst Stimulation; iTBS, Intermittent TBS; DLPFC, Dorsolateral Prefrontal Cortex; tDCS, Transcranial Direct Current Stimulation; fNIRS, Functional Near-infrared Spectroscopy; HbO, Oxygenation; HHb, Deoxyhemoglobin; MMSE, Mini Mental State Examination; RMT, Relaxed Motor Threshold; LOTCA, Loewenstein Occupational Therapy Cognitive Assessment; MoCA, Montreal Cognitive Assessment; MBI, Modified Barthel Index; ADL, Activities of Daily Living; VFT, Verbal Fluency Task; PFC, Prefrontal Cortex; TDDR, Time Derivative Distribution Repair; MNI, Montreal Neurology Institute.
JC, MC and YueZ designed the research study. MC, YueZ, ZH, HY, FZ, XY and JL performed the research. WC, YueZ and YY provided help and advice on the experiments. JC, WC, YueZ and YY obtained financing. ZH, HY, FZ, XY and JL collected data. MC and YouZ analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
We confirm that we have read the Editorial Policy pages. This study was conducted with approval from the Ethics Committee of the Third Clinical Medical College, Zhejiang Chinese Medical University (ZSLL-ZN-2021-018-01). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
We would like to acknowledge the hard and dedicated work of Hangkai Xie, Zixi Wang, Chao Zeng, Hui Li that implemented the intervention and evaluation components of the study.
Key Research and Development Program of Zhejiang Province (No. 2021C03050); Scientific Research Program of Zhejiang Rehabilitation Medical Center (No. ZK2001).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.







