1 Department of Radiology, Fudan University Shanghai Cancer Center, 200032 Shanghai, China
2 Department of Oncology, Shanghai Medical College, Fudan University, 200032 Shanghai, China
3 Department of Anatomy and Physiology, Shanghai Jiao Tong University School of Medicine, 200025 Shanghai, China
4 Department of Urology, Dushu Lake Hospital Affiliated to Soochow University, 215100 Suzhou, Jiangsu, China
5 Dushu Lake Branch of The First Affiliated Hospital of Soochow University, 215100 Suzhou, Jiangsu, China
6 Department of Radiology, Minhang Branch of Fudan University Shanghai Cancer Center, 200032 Shanghai, China
†These authors contributed equally.
Academic Editor: Francois Roman
Abstract
Background: Peripheral nerve regeneration is a coordinated process of Schwann cell (SC) reprogramming and intrinsic neuronal growth program activation. Panaxydol (PND) is a strong biologically active traditional Chinese medicine monomer extracted from Panax notoginseng rhizomes. In vitro, PND protects neurons and SCs from injury and stimulates the expression and secretion of neurotrophic factors (NTFs) by SCs. We hypothesized that PND may also promote peripheral nerve regeneration in adult animals. Methods: PND (10 mg/kg body weight) was injected intraperitoneally into the Sprague–Dawley (SD) rats for two consecutive weeks after sciatic nerve transection. The morphology of the repaired sciatic nerve was evaluated after 16 weeks, and sensory and motor function recovery was evaluated using functional and behavioral techniques. Results: PND was biologically safe at an injection dose of 10 mg/kg/day. After 14 days, it significantly increased the myelination of regenerated nerve fibers, and promoted sensory and motor function recovery. In the early stage of injury, PND significantly upregulated the mRNA expression of brain-derived neurotrophic factor (BDNF) and its receptors in distal injured nerves, which may represent a possible mechanism by which PND promotes nerve regeneration in vivo. Conclusions: Our study demonstrated that PND leads to sensory and motor recovery in a sciatic nerve transection model rat. Furthermore, we showed that BDNF mRNA level was significantly increased in the injured distal nerve, potentially contributing to the functional recovery. Further research is warrantied to examine whether direct injection is a more efficient method to increase BDNF expression compared to an exogenous BDNF administration.
Keywords
- biochemical pharmacology
- panaxydol (PND)
- traditional Chinese medicine
- peripheral nerve regeneration
- Schwann cells
- nerve growth factor
- BDNF
Peripheral nerve injury (PNI) is one of the most common neurosurgical diseases encountered in the clinical practice [1]. PNI is caused by the effects of direct or indirect externaly applied mechanic injuries, such as crushing, pulling, disconnecting, and tearing, on the peripheral nerve trunks or their branches [2]. The annual occurrence of PNI has been showing a constant rising trend, especially due to increasing harmful lifestyle implications and a better understanding of the diagnosis. In adults, a poorer peripheral nerve regeneration is observed, which easily leads to motor and sensory dysfunction and even a loss of function [3], with a high disability rate that seriously affects patients’ quality of life. Deployment microsurgical procedures have improved nerve regeneration efficiency and PNI morphological recovery since their conception and deployment. In contrast, the achievement of long-term functional recovery is still vastly underdeveloped [4]. Drug therapy is an important aspect of the treatment after surgical nerve continuity reconstruction. Therefore, finding novel compounds and therapeutic strategies is of a major importance to tackle the problem of long-term side effects and full disability, in the face of currently highly inefficacious current treatment that solely target inflammation but do not promote the actual nerve repair. In addition, the currently available administration route is invasive.
The neurotrophic factor (NTF) family, which induces neuron regeneration after nerve injury [4], is the most extensively applied; however, exogenous NTFs have limited practical utility [5]. Due to the short biological half-life of NTFs, penetration of the blood-nerve barrier is difficult [6]. Due to their complex components and various targets, Chinese herbal medicines may establish a regenerative microenvironment that is more supportive of neurophysiological needs. A range of Chinese herbal drugs and ingredients have been shown to support and accelerate the peripheral nerve regeneration [7, 8, 9, 10].
Panax notoginseng is a traditional Chinese herbal medicine (TCM) that has been used for centuries and is particularly essential in the therapeutic applications of TCM [11, 12, 13]. Panaxydol (PND), which is one of the lipophilic components extracted from Panax notoginseng, is a cytotoxic compound with high biological activity [14]. PND has been frequently studied for its potential antitumor activity [15, 16], and researchers are increasingly interested in its favorable antioxidant and neuroprotective properties [17, 18, 19, 20]. PND can protect primary cultured cortical neurons from NO-induced damage by regulating apoptotic and antiapoptotic proteins [21]. PND can also stimulate neurite outgrowth in PC12 cells by mimicking the effects of NGF through the cAMP-Epac1-Rap1-MEK-ERK-CREB pathway [22].
Recently, our research team reported that PND boosted the biological activity of RSC96 cells and primary SCs and promoted the expression of NTFs, such as NGF and BDNF, in SCs in vitro [23]. PND also shielded primary cultured neurons and SCs from hypoxic effects [24]. We confirmed that the peripheral nerve regeneration is a coordinated process that involves SC reprogramming and activation of the intrinsic neuronal growth program [6, 25]. Based on our previous research, this study investigated the hypothesis that PND promotes peripheral nerve regeneration by the increase of the BDNF expression. We established a sciatic nerve transection model in adult SD rats. PND was intraperitoneally injected for two consecutive weeks. Morphology and sensory and motor function restoration of the sciatic nerve were observed and analysed.
Nature Standard Company, Shanghai, China, isolated and purified authentic
standards of PND ((3R)-9,10-epoxy-1-ene-4,6-diyn-3-ol). Nuclear magnetic
resonance (NMR) spectroscopy (
Twenty adult SD rats (female, 7 weeks of age) were provided by the Animal Care
Facility of Shanghai Jiao Tong University, School of Medicine (Shanghai, China).
The rats were housed at a constant temperature (25
Anesthesia was administered by intraperitoneal injection of sodium pentobarbital
(40 mg/kg body weight, i.p.), and all animal studies were performed under aseptic
conditions. Sciatic nerve transection injury was established as previously
described [29]. The right sciatic nerve was exposed 1.0 cm distal to the sciatic
notch and transected in the middle with sharp scissors. The lesion site was then
repaired with four stitches of 9–0 microsutures (Jin Huan Co., China) through
the epineurium under 20–40
After surgery, the rats were randomly assigned to two groups: the PND group or the solvent control group. Simultaneously, all groups were given an intraperitoneal injection of the corresponding medication for two consecutive weeks at the same fixed time. Rats in the PND group received PND (10 mg/kg) daily, whereas rats in the control group received the same volume of solvent daily. Then, the rats were randomly divided into two separate experiments, as shown in Fig. 1; in experiment 1, n = 3 per group per timepoint, while in experiment 2, n = 4 per group.
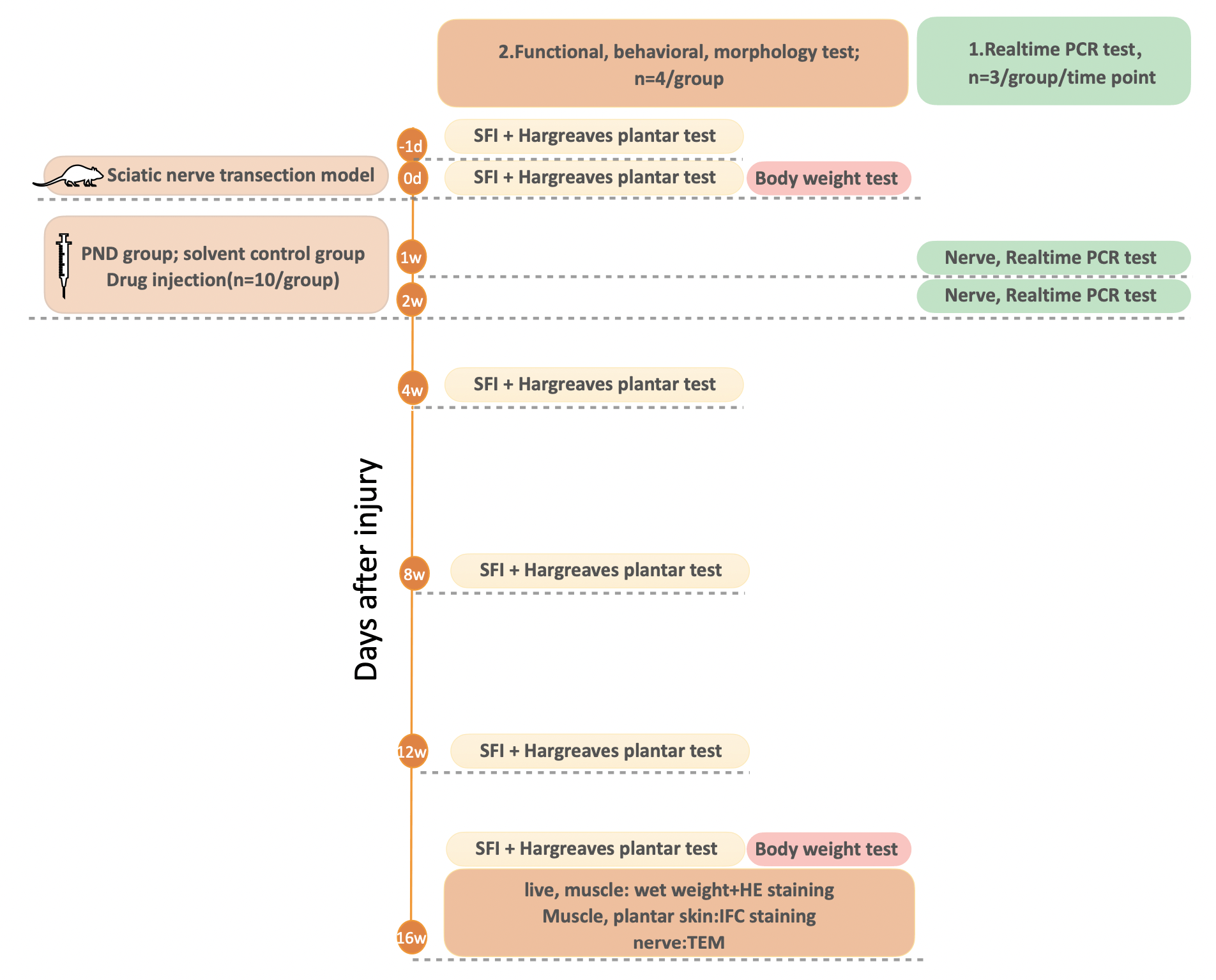 Fig. 1.
Fig. 1.Schematic diagram of the experimental procedure. On day 0, sciatic nerve transection was performed in all rats (n = 20). Following injury, the rats were randomly assigned to two groups: the PND group or the solvent control group (n = 10/group). All groups received an intraperitoneal injection of the corresponding medication for two consecutive weeks. Rats in the control group received 0.5 mL solvent daily, whereas rats in the PND group received 0.5 mL PND (10 mg/kg) daily. Then, in experiment 1, the rats were sacrificed at the indicated time points for the sampling of nerve tissues for real-time PCR (n = 3/group) analyses. In experiment 2, the walking track analysis (for evaluating motor function) along with the Hargreaves plantar test (for evaluating sensory function) were performed at the indicated time points (n = 4/group). After functional and behavioral tests were completed, the rats were weighed and sacrificed for the sampling of liver, nerve, muscle, and plantar skin tissues at 16 weeks for H&E staining, IF staining, and transmission electron microscopy (n = 4/group).
The mRNA levels of BDNF, NGF, tropomyosin-related kinase B (TrkB), and p75 neurotrophin receptor (p75NTR) in the injured distal nerve were examined by real-time PCR one and two weeks after surgery. A 1.0-cm-long section of the sciatic nerve on the distal end of the lesion was isolated and removed from anesthetized animals of both groups.
Total RNA was extracted using TRIzol (15596-018, Invitrogen, Carlsbad, CA, USA),
and 2
| Primer | Sequence |
| GAPDH-F | AGGGTGGTGGACCTCATGG |
| GAPDH-R | AGCAACTGAGGGCCTCTCTCTT |
| NGF-F | GTCTGGGCCCAATAAAGGCT |
| NGF-R | CTGTGTACGGTTCTGCCTGT |
| BDNF-F | GTCACAGCGGCAGATAAAAAG |
| BDNF-R | ATGGGATTACACTTGGTCTCGT |
| TrkB-F | CGACACTCAGGATTTGTATTGC |
| TrkB-R | ATGGTCACAGACTTCCCTTCC |
| p75NTR-F | AGCAGACCCATACGCAGACT |
| p75NTR-R | GCAGTTTCTCTACCTCCTCACG |
All behavioral experiments described below were conducted in a blinded manner in
a quiet room (temperature 25
The Hargreaves plantar test (Yuyan, Shanghai, China) was used to assess the injured hindlimb’s heat sensitivities and sensory functional recovery of rats (n = 4/group). The tests were performed at six time points: 1 day before surgery and 1 day, 4 weeks, 8 weeks, 12 weeks, and 16 weeks after surgery. The light intensity of the selected heat source beam was IR = 75%, and the upper limit of the pain threshold latency was set to 20.1 s to avoid scalding the rat’s foot. Briefly, each rat was placed on the glass plate of the pain tester, separated by a special transparent plastic box, and allowed to adapt for more than 30 min. The light source of the tester was switched on while ensuring that the heat source beam emitted by the transmitter aim was located outside of the middle part of the sole of the foot. The time interval between initial activation and paw withdrawal in response was recorded automatically. Heat stimulation was stopped to prevent thermal injury if the animals did not withdraw their paws after 20 s. The test was repeated three times for each rat (15-min intervals were included between tests), and the average value of each measurement was recorded to represent the thermal pain threshold.
The recovery of motor function in rats (n = 4/group) was examined by walking track analysis at six time points: 1 day before surgery and 1 day, 4 weeks, 8 weeks, 12 weeks, and 16 weeks after surgery. The rats were habituated to the experimental setting for three consecutive days before the start of the experiment, and the daily acclimatization time was calculated to be the duration of the experiment.
Then, the sciatic functional index (SFI) was calculated as previously described
[30, 31]. Briefly, the rats were trained to walk across a custom-made walking box
(100 cm long, 12 cm wide, and 10 cm high) leading to a darkened box. White paper
(12 cm
SFI = (–38.3
PL: the distance from the heel to the top of the third toe;
IT (intermediary toe spread): the distance from the second to the fourth toe;
TS (toe spread): the distance between the first and the fifth toe.
The normal print length (NPL), normal toe spread (NTS), and normal intermediary toe spread (NIT) represent the PL, IT, and TS recorded from the uninjured, normal foot; experimental print length (EPL), experimental toe spread (ETS), and experimental intermediary toe spread (EIT) represent the PL, IT, and TS recorded from the paired, injured, experimental foot, respectively. An SFI value of approximately 0 indicates full recovery, whereas an SFI value of approximately 100 represents complete dysfunction.
After functional and behavioral tests were completed, the rats (n = 4/group) were weighed and sacrificed carefully 16 weeks after surgery. Organs and tissues including the liver, injured nerve, plantar skin, and gastrocnemius muscles were collected from the injured and uninjured sides. The livers and gastrocnemius muscles from the injured and uninjured sides were then weighed. Blood vessels and the deep fascia covering the surfaces of the liver and muscle were removed and discarded before weighing. The following equation was used to determine the rate of body weight gain, liver hepatosomatic, and recovery rate of the gastrocnemius muscles:
Rate of body weight gain = (final body weight-initial body weight)/initial body weight
Hepatosomatic index = wet weight of the liver/final body weight
The recovery rate of the gastrocnemius muscle = wet weight of the injured muscle/wet weight of the uninjured muscle
After weighing, the liver and mid belly of the gastrocnemius muscle specimens
were cut into small pieces, fixed in 4% paraformaldehyde/PBS, and dehydrated in
a graded series of ethanol. Then, the samples were paraffin-embedded, cut into
5-
After functional and behavioral tests were completed, a 0.5-cm-long piece of the
sciatic nerve at the 1 cm distal end of the lesion was separated and removed from
the rats (n = 4/group). The nerves were sliced into 5-
Myelin staining with toluidine blue: The segments were stained with 1%
toluidine blue at room temperature for 30 min, washed gently in water, and
consecutively soaked in 95% ethanol and 100% ethanol for 2–3 s. Images were
acquired at 400
Transmission electron microscopy: Sections of regenerated nerves were fixed in precooled 2.5% glutaraldehyde for 3 h followed by postfixation in a 1% osmium tetroxide solution for 1 h, washed, dehydrated, embedded in Epon 812 epoxy resin, cut into ultrathin sections at 60 nm, and stained with lead citrate and uranyl acetate. The stained sections were observed under a transmission electron microscope (JEM-1200, JEOL Ltd., Tokyo, Japan).
Nerve, skin, and muscle were postfixed in 4% paraformaldehyde/PBS overnight
after which tissues were incubated in 30% sucrose/PBS overnight for dehydration.
Then, the tissues were frozen in Tissue-Tek O.C.T. (4583, Sakura Finetek, CA,
USA). Then, 10-
Statistical analyses were performed using GraphPad Software (GraphPad 9, La
Jolla, CA, USA). The data are presented as the means
The toxicity and safety of traditional Chinese medicine have garnered increased
attention in recent decades. The liver is the primary site of drug transformation
and metabolism and is also the main target organ for drug toxicity [32, 33, 34]. To
evaluate the side effects and toxicity of PND, we investigated body weight gain,
hepatosomatic index, and liver histopathologic characteristics of both groups of
rats. Rats in both groups survived the experiment without obvious ascites,
emaciation, or self-mutilation. Each rat gained approximately 40–52 g of body
weight in both groups, and no significant difference was observed in the rate of
body weight gain or in the hepatosomatic index between the two groups (Fig. 2C; n
= 4, p
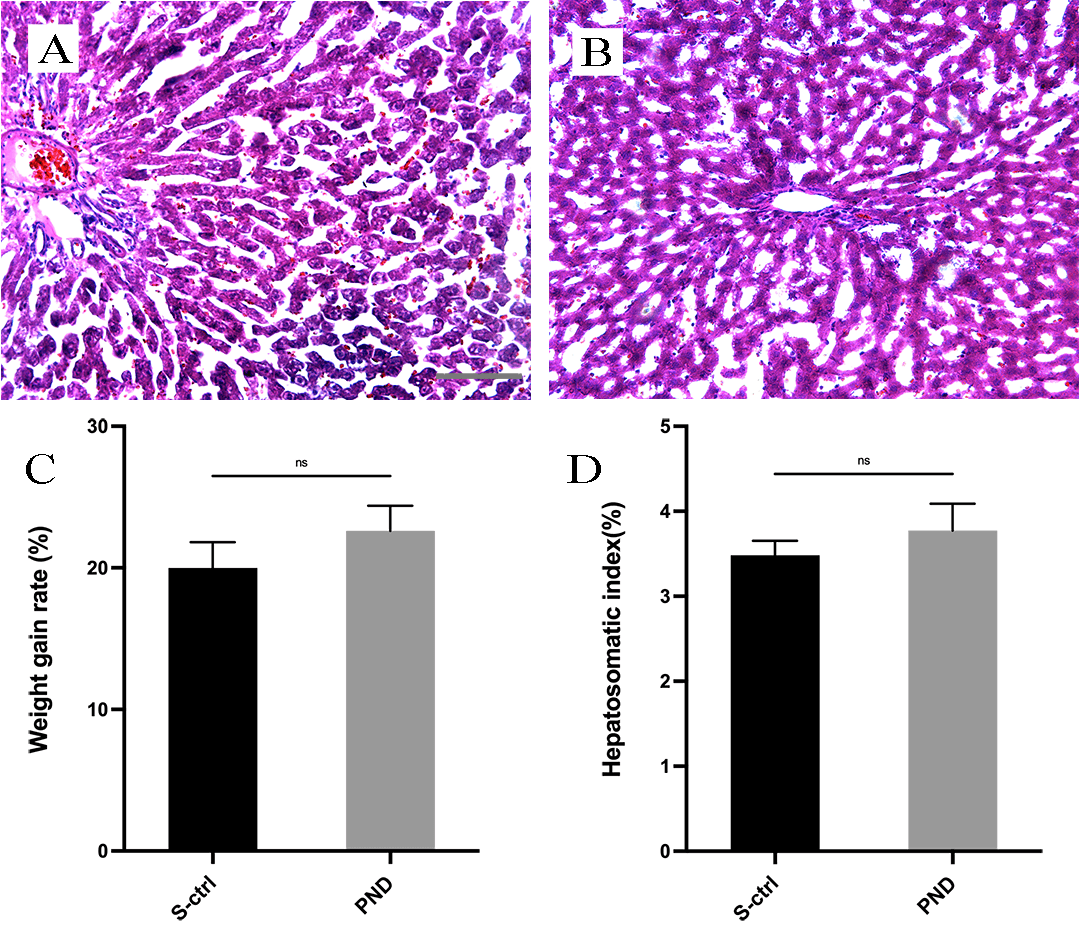 Fig. 2.
Fig. 2.H&E staining and histological analysis of the liver in each
group. Representative light micrographs of H&E-stained liver sections from the
solvent control group (A) and the PND group (B) 16 weeks after surgery. The rate
of weight gain in each group is shown in panel (C). The hepatosomatic index in
each group is shown in panel (D). All data are presented as the means
After 16 weeks, cross-sections of tissue 1 cm distal to the injury were obtained
for the morphologic analysis of the ingrowth of regenerated nerve fibers. SCs
surround regenerated nerve processes to reform myelin sheaths or unmyelinated
fibers, which provides a structural basis for the subsequent recovery of nerve
conduction function [35]. Along with the density of regenerated nerve fibers, the
thickness of the myelin sheath, the diameter of nerve fibers, and the g-ratio all
serve as important indicators of regenerated nerve fiber maturation. Toluidine
blue staining was used to assess distal nerve fiber formation and myelination.
The results of toluidine blue staining at the distal end of the injury are shown
in Fig. 3A,B. Sixteen weeks after sciatic nerve injury, both groups demonstrated
significant regeneration of nerve fibers at the distal end of the injury, with
some fibers forming myelin sheaths. Additionally, the average fiber diameter and
myelin sheath thickness were significantly greater in the PND group than in the
solvent control group (Fig. 3C; n = 4, p
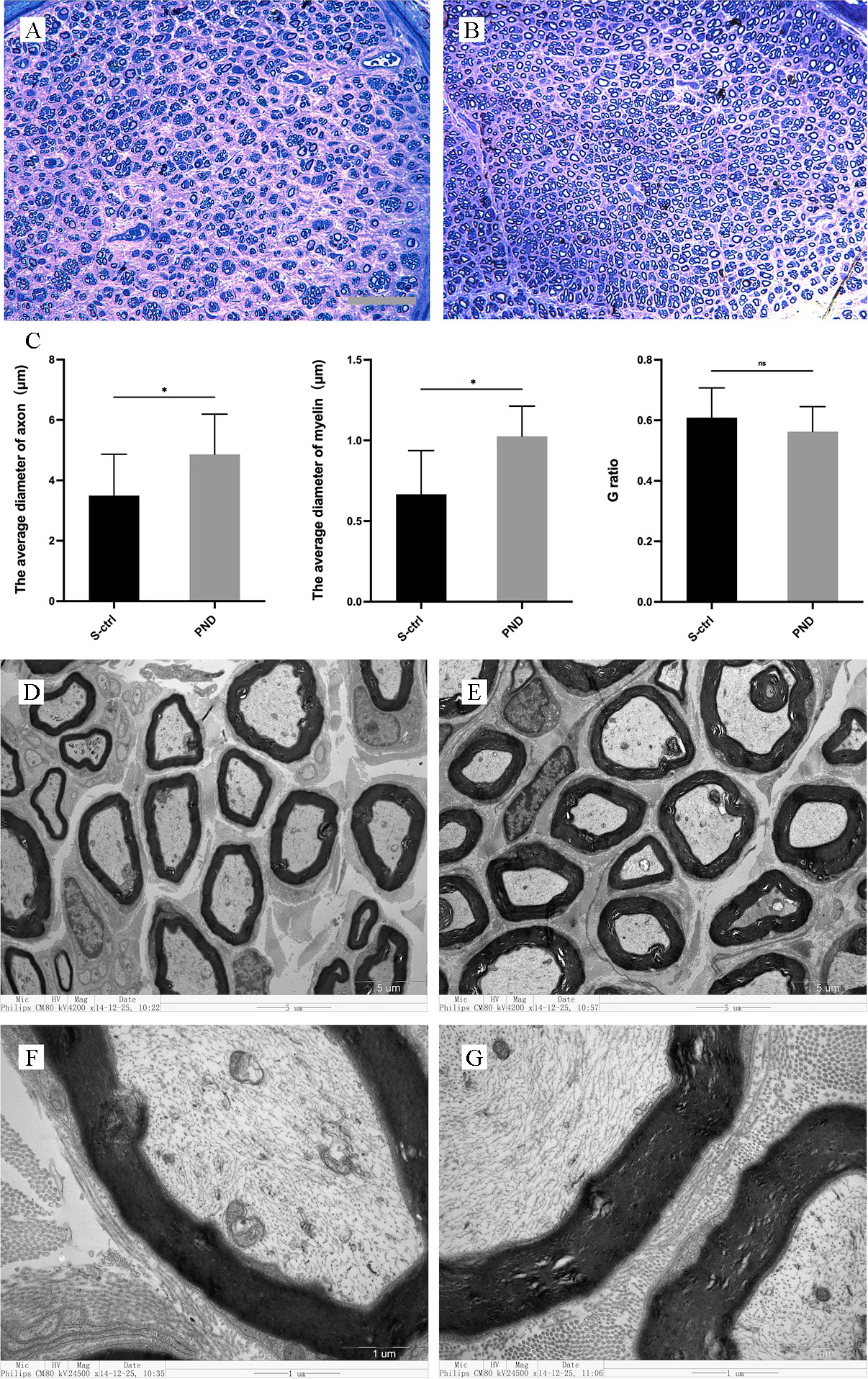 Fig. 3.
Fig. 3.Morphological appearance and morphometric assessments of
regenerated and myelinated nerves in each group. Representative images show
toluidine blue staining of regenerated axons (A,B) 1 cm distal to the injury site
in the solvent control group (A) and PND group (B) 16 weeks after injury. A, B:
scale bar: 50
By collecting, weighing, and staining the gastrocnemius muscle with H&E 16 weeks after nerve injury, we assessed the healing of target organs following treatment.
As illustrated in Fig. 4A,B, gastrocnemius muscle atrophy was detected in both groups of rats following denervation.
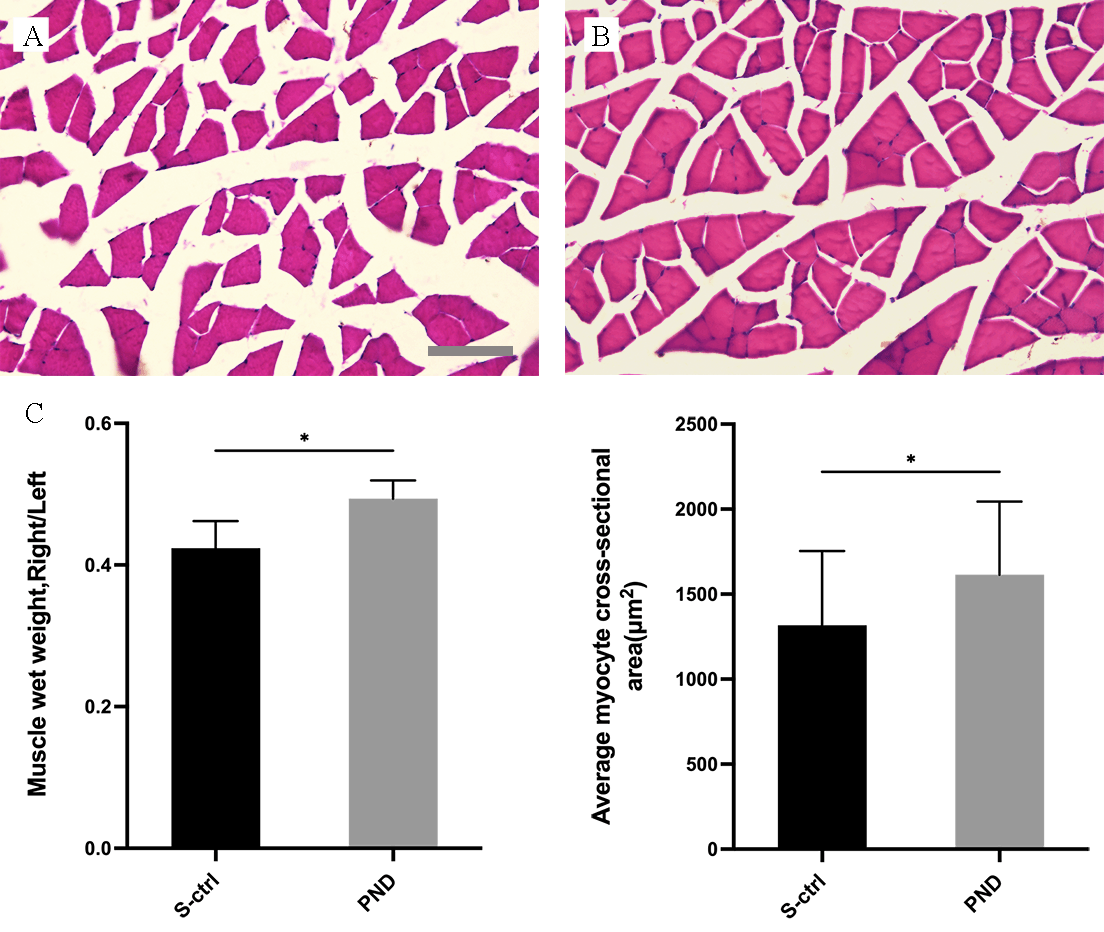 Fig. 4.
Fig. 4.Morphological appearance and morphometric assessments of
gastrocnemius muscle fiber recovery in each group. Representative light
micrographs of H&E-stained transverse sections of the gastrocnemius muscle (A,B)
from rats in the solvent control group (A) and the PND group (B) 16 weeks after
injury. A, B: scale bar: 100
Fig. 4 illustrates the morphometric evaluations (C). The gastrocnemius recovery
rate (the ratio of muscle wet weight on the injured side to that on the
contralateral side) and the area of a single myocyte in the control group were
both lower than those in the PND group (Fig. 4C; n = 4, p
To evaluate the target organ condition, immunofluorescence (IF) staining was
used to detect the reinnervation of muscle and skin by sciatic nerve terminals in
the two groups 16 weeks after injury. Neuromuscular junction staining is shown in
Fig. 5A,B. Double staining and colocalization of markers of the end plate and
nerve terminals was seen in both groups, which indicates effective reinnervation.
The number of reinnervated end plates in each visual field in the PND group was
not significantly different from that in the control group (Fig. 5C; n = 4,
p
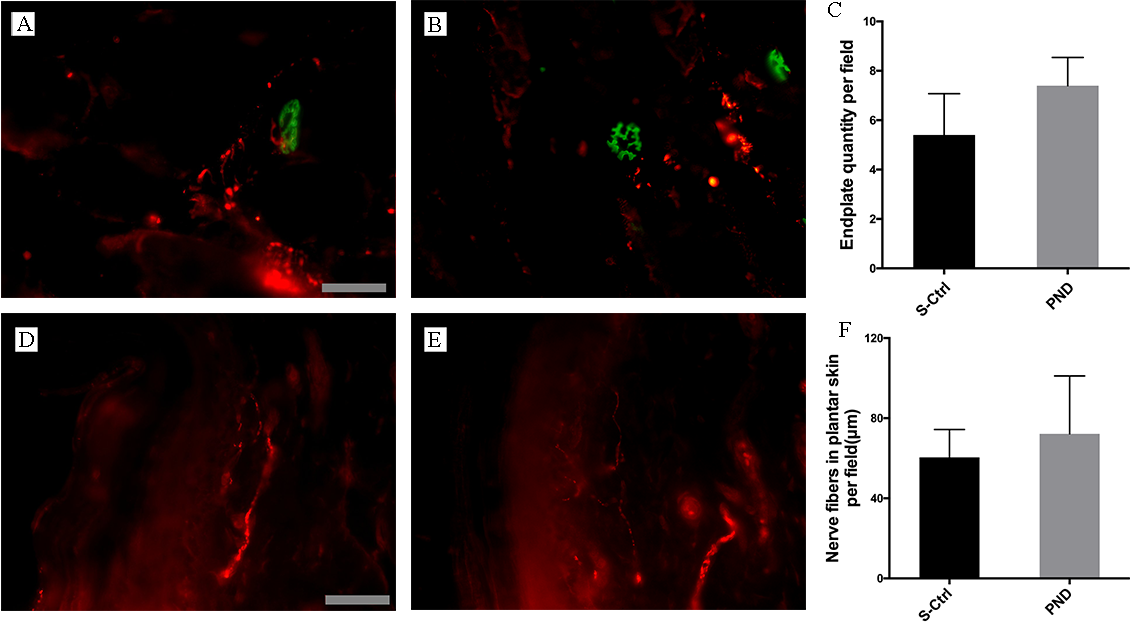 Fig. 5.
Fig. 5.Morphological appearance and morphometric assessments of
reinnervated muscle and skin in each group. Representative images of IF staining
for PGP 9.5 (red) and FITC-conjugated
Both groups displayed regenerated nerve fiber ingrowth in the plantar skin, as
illustrated in Fig. 5D,E. Imaging analysis revealed no statistically significant
difference in nerve fiber length per unit area between the PND and control groups
(Fig. 5F; n = 4, p
The Hargreaves plantar test data were collected every four weeks after injury
and revealed that sensory functional recovery was complete in both groups. As
illustrated in Fig. 6, the plantar skin of rats gradually recovered sensitivity
to heat and pain. After approximately four weeks for the PND group and after
approximately eight weeks for the control group, the rats recovered to
near-normal levels of sensory function (Fig. 6; n = 4, * p
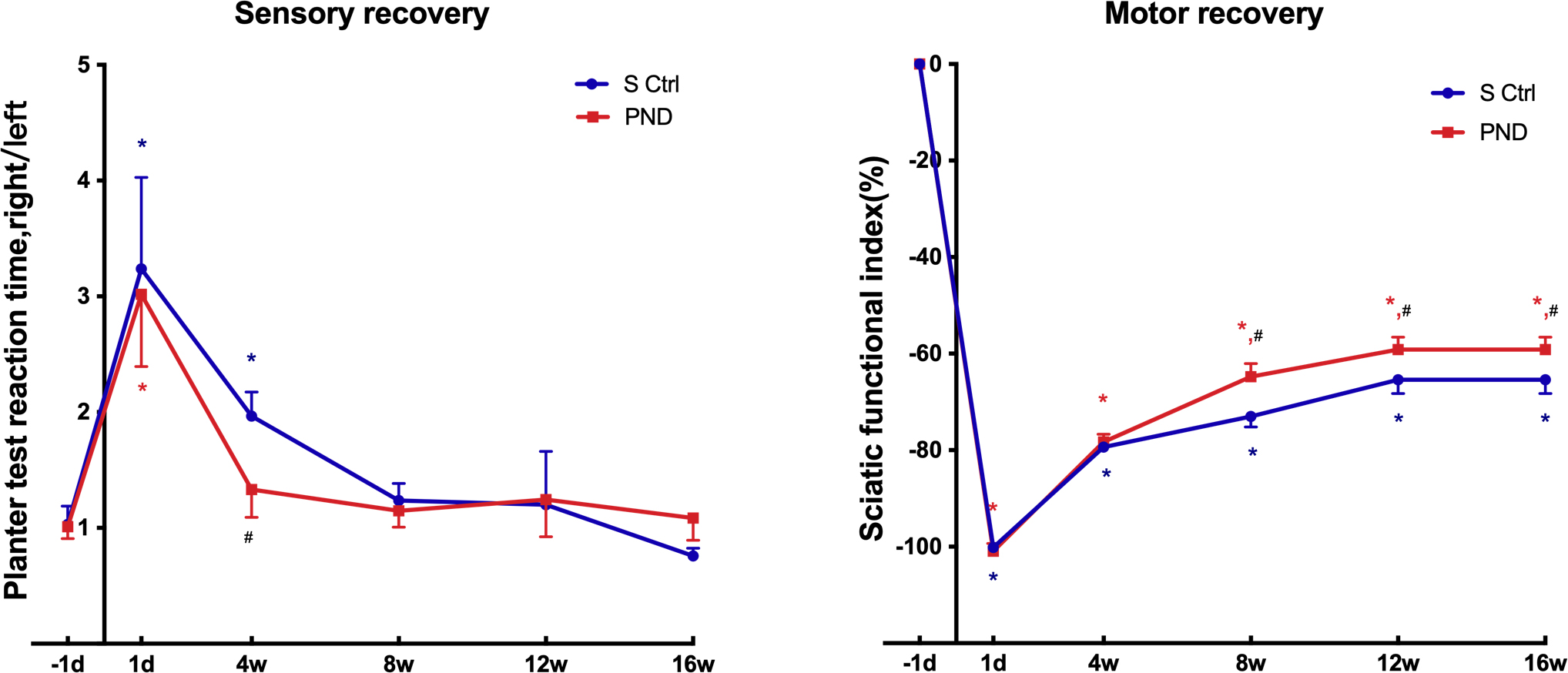 Fig. 6.
Fig. 6.Motor and sensory functional recovery in each group. The SFI (a
score of –100 and below corresponds to total paralysis, whereas uninjured
animals typically have a score of –8.8 or higher) and plantar test reaction time
rate tested 1 day before injury and 1 day, 4 weeks, 8 weeks, 12 weeks, and 16
weeks after injury. *p
A greater SFI suggests stronger sciatic nerve function. The return to preinjury
SFI levels indicates complete recovery of hindlimb motor coordination and
function, including that of the most distal paw muscles. Every four weeks,
animals in both groups were given an SFI test to measure the recovery of motor
function of the sciatic nerve. Statistical analysis of SFI data indicated that
motor functional recovery was only partially achieved in both groups. SFI values
remained low compared with preinjury levels at all time intervals evaluated up to
16 weeks (Fig. 6; n = 4, * p
NGF and BDNF mRNA levels in the injured distal nerve were examined using
real-time PCR one and two weeks after injury. As shown in Fig. 7, NGF mRNA levels
were similar in the PND group and control group at one and two weeks after
surgery (n = 3, p
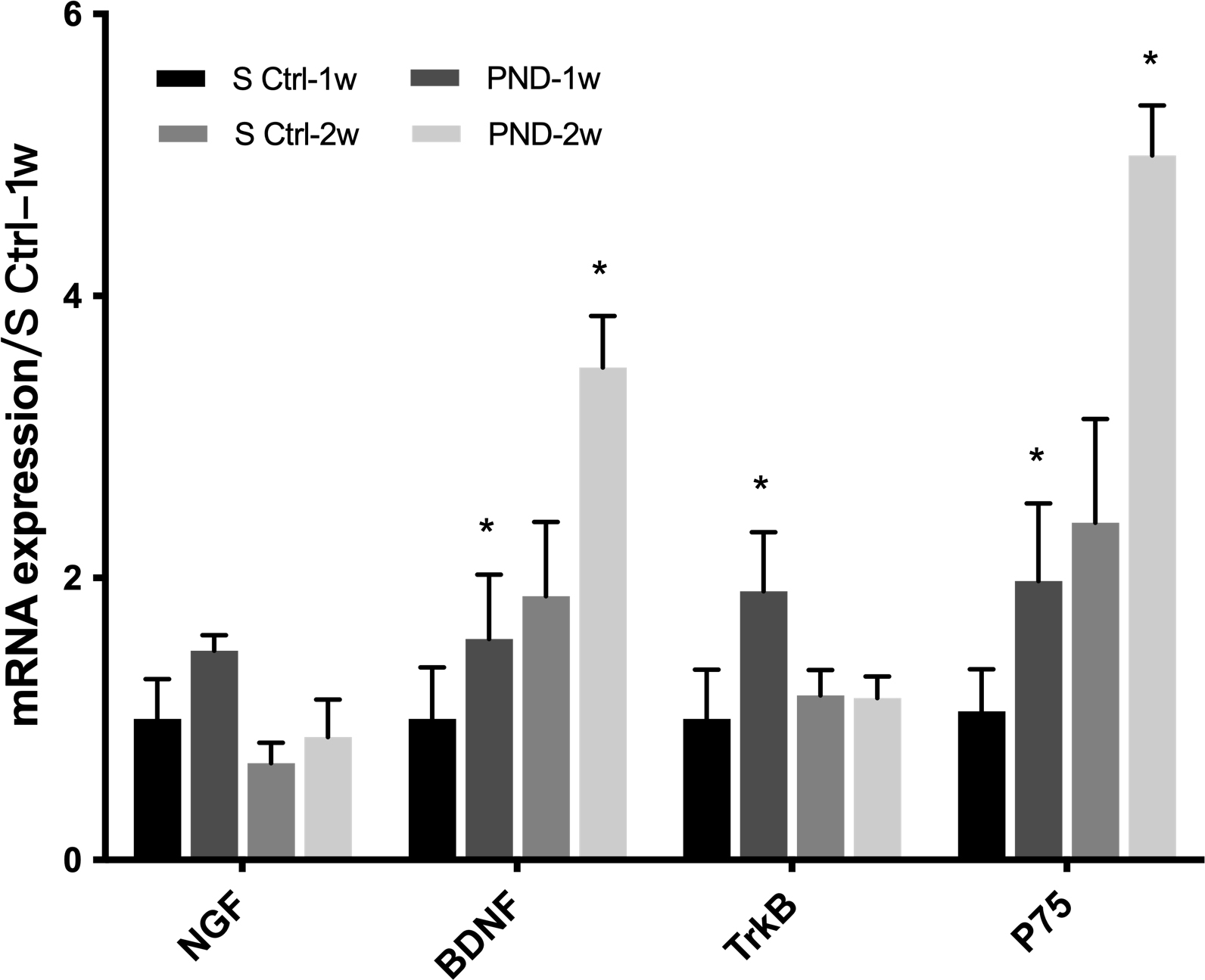 Fig. 7.
Fig. 7.Expression of neurotrophic factors and their receptors in each
group one and two weeks after injury. The mRNA levels of NGF, BDNF, and their
receptors TrkB and p75NTR at the injured sites were determined in the solvent
control and PND groups at one and two weeks after injury. Each test was repeated
three times. All data are presented as the means
The PNS is mainly composed of nerve processes and glial cells (SCs) [36]. SCs are myelinated glial cells unique to the PNS that play a key role in regeneration after nerve injury [37]. After PNS injury, both myelinating and nonmyelinating SCs undergo substantial reprogramming to facilitate and direct axonal growth [6]. SCs divide and proliferate, provide appropriate numbers of cells and a basement membrane skeleton, form a Büngner band, and provide mechanical growth channels for regenerating axons [38]. They also secrete a variety of NTFs and nerve cell adhesion factors and provide an active surface to promote the adherent growth of axons. In addition, SCs surround regenerated nerve processes to reform myelin sheaths or unmyelinated fibers, which provides a structural basis for the subsequent recovery of nerve conduction function [35].
After PNI, the use of microsurgical techniques sufficiently restores the continuity of injured nerve fibers and is an important component of subsequent clinical treatment. Drug treatment provides a good microenvironment for nerve regeneration. The most common treatments related to the NTF family have been shown to promote regeneration after nerve injury [39]. However, due to their short biological half-life, the clinical application of NTFs is still limited due to enzymolysis, inactivation, antigenic immune reactions, and the limited effect of single drugs [40]. However, a variety of Chinese herbal medicines and compounds have been shown to promote nerve repair and regeneration after injury [7, 10]. Chinese herbal medicines are characterized by complex components, a wide range of actions, and many targets. Notably, the complexity of Chinese herbal formulas and single herbal medicines results in low specificity, low efficacy, and the occurrence of side effects, particularly serious liver damage. Furthermore, due to the complexity of the components, the molecular biological basis and pharmacological mechanisms of action have not been clarified. In recent years, the screening and analysis of single active ingredients in herbal medicines have led to novel methods for pharmacological research on Chinese herbal medicine and are important for identifying small-molecule compounds with specific targets.
As a traditional medicine used in China, Panax notoginseng has been shown to possess various biological properties related to immune defense, antioxidant capability [41, 42, 43], improvements in blood pressure [11, 12, 13], and neurotrophic functions [44, 45, 46, 47], and its medicinal value has been recognized worldwide. PND is a fat-soluble component isolated from the rhizome of Panax notoginseng [48]. PND exhibits strong biological activity due to a reactive oxygen atom in its structure [49]. Our in vitro studies have shown the protective and nutritional effects of PND on cultured neurons and SCs [23, 24]. This study further confirmed the effect of PND on peripheral nerve repair in vivo and further explored the underlying mechanisms.
After PNI, the diameter and thickness of the myelin sheath of myelinated nerve fibers were significantly greater in the PND group than in the control group, which implies that PND promotes nerve fiber regeneration and SC myelination. The Hargreaves plantar test indicated that sensory function recovered more rapidly in the PND group, although no significant difference was observed in the length of the reinnervated nerve growing into the plantar skin between the PND and control groups at 16 weeks. For motor nerve target muscles, neither the area of nerve fiber growth into the gastrocnemius nor the number of NMJs in the muscle showed significant differences between the PND treatment group and the control group. However, gastrocnemius atrophy was less severe in PND rats than in control rats. The SFI is the most frequently used indicator of motor function recovery following sciatic nerve injury and is determined by a walking trajectory analysis. The SFI has a high degree of sensitivity and accuracy and clearly demonstrates the direct relationship between hind limb muscle function and footprints [50, 51]. In this study, the SFI indicated that the PND group recovered motor function slightly more quickly than the control group at week 12; however, recovery of motor function in both the PND and control groups remained suboptimal at 16 weeks.
After PNI, the recovery of sensory and motor functions is usually unequal [52]. Sakuma et al. [31] reported that sensory function was fully recovered in model rats by day 55 after transection injury; in marked contrast, the SFI remained at the lowest score even at day 90. This finding is also consistent with our results. In clinical practice, for example, the protective sensation in the fingers is restored in 90% of patients with a brachial plexus injury even though recovery of the thenar muscle is very limited, and the sensory axons can reinnervate even the most distal skin areas [53]. However, many studies have shown that even if regenerated nerve axons successfully reach the target muscle after injury and form complete anatomical NMJs with no abnormal ultrastructure, these NMJs do not normally activate the muscles, and recovery of motor function is still unsatisfactory. Researchers have proposed that this observation is due to the loss of motor function caused by the failure of chemical synapse reconstruction in the target muscle after long-term denervation. Nerve regeneration may require active nutritional signals, such as NTFs or synaptic collagen, from muscles to complete chemical synaptic reconstruction [54]. Therefore, we postulate that the condition of the motor end plate does not reflect the state of motor function recovery; the nutritional status of the target muscle may affect nerve regeneration and indirectly reflect the degree of motor function recovery. Gastrocnemius atrophy was significantly lower in rats in the PND group than in rats in the control group, which may explain the relatively better recovery of motor function in the PND rats.
By examining the NGF and BDNF mRNA levels in distal nerves in the early stage of injury (one and two weeks), we investigated a possible mechanism by which PND promotes peripheral nerve regeneration. PND upregulated BDNF mRNA expression in distal nerves, and this trend is also consistent with the previous in vitro experimental results reported by our team. To date, many studies have elucidated the important role of NTFs, including NGF and BDNF, in the process of peripheral nerve repair [55, 56, 57]. NGF is one of the most important bioactive molecules in the PNS, as it affects neuronal survival and differentiation. After PNI, NGF protects injured neurons and promotes neurite regeneration. The level of NGF produced by distal SCs peaks within 24 h of nerve transection and remains at 5–10 times the normal level for at least two weeks following axon transection. BDNF protects damaged neurons and promotes the growth of regenerated nerves [57]. Gordon and colleagues also found that BDNF levels are closely related to the selectivity of motor nerve regeneration [58]. In summary, after PNI, the increased expression of NGF and BDNF, which protect injured neurons, promotes the growth of regenerated nerve processes and nourishes denervated target organs, thereby improving the efficiency of nerve regeneration through various functions. NTF can be used as drug therapy, but usually cannot penetrate the blood-brain barrier. Whether or not this is true for PND is still unclear. Further research is needed to investigate whether and if yes, to which extent the permeability can be warranted.
In addition, the functions of BDNF are achieved through a family of high-affinity receptors (Trk family) and a low-affinity receptor (p75NTR) [59]. The expression levels of p75NTR and TrkB were upregulated in the sciatic nerve of animals in the PND group. TrkB mediates the positive neurotrophic effect of BDNF, protects damaged neurons, promotes the growth of regenerated nerves, and participates in the selective regulation of nerve regeneration. In contrast, p75NTR does not have any kinase activity [60] but rather recruits different intracellular binding proteins to activate different signaling pathways. These signaling pathways have multiple functions in different cellular environments. On the one hand, p75NTR interacts with sortilin family members to induce neuronal cell apoptosis. On the other hand, p75NTR not only directly activates survival signals but also promotes TrkB translocation and signal transduction after binding to BDNF. In addition, the myelination of BDNF depends on p75NTR, and p75NTR knockout affects myelination of the sciatic nerve during injury. In summary, PND may activate the TrkB receptor and p75NTR to initiate the positive neurotrophic effects of BDNF and promote peripheral nerve regeneration.
The main objective and scope of the study was to objectify the PND-support of peripheral nerve regeneration using a sciatic nerve transection model in adult SD rats.
We performed morphological experiments to ascertain the maturity of regenerated nerves, the number of nerve fibers reinnervating target organs, and the degree of muscle atrophy in target organs. In addition, we examined sensory and motor functions in rats by conducting functional and behavioral experiments. The nerve regeneration efficiency of the PND group was superior to that of the solvent control group. After combining the mRNA data obtained in this study and the results of previous cell-based experiments, we speculate that PND promotes the secretion of BDNF by SCs after PNI and that BDNF initiates nerve and muscle nutritional functions by activating TrKB- and p75NTR-mediated signaling pathways. Our studies have identified the effect of PND on peripheral nerve repair in vivo and further explored the underlying mechanisms in nerve repair. However, the cellular and molecular mechanisms and related pathways remain unclear. Notably, a PND injection is a more easily accessible and convenient method of increasing BDNF expression than exogenous BDNF administration. Therefore, the study demonstrated that PND’ bioavailability and lipophilic features provide a theoretical basis for the future clinical applications of PND. Further research is required, first on a larger number of various model organisms, follow by clinical trials. This study, however, brings major insights for this research field by demonstrating the core efficacy and efficiency of PND injections for PNI, including mechanism of action and active compounds.
SC, Schwann cell; PND, panaxydol; SD rats, Sprague–Dawley rats; NTFs, neurotrophic factors; PNI, peripheral nerve injury; PNS, peripheral nervous system; BDNF, brain-derived neurotrophic factor; TCM, traditional Chinese herbal medicine; NGF, nerve growth factor; NMR, nuclear magnetic resonance; HPLC, high-performance liquid chromatography; TrkB, tropomyosin-related kinase B; p75NTR, p75 neurotrophin receptor; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SFI, sciatic functional index; PL, print length; IT, intermediary toe spread; TS, toe spread; NMJ, neuromuscular junction
ZZ and YW designed the research study. YMW and JL performed the experiments. YMW analyzed the data. YMW and JL wrote the manuscript. ZZ and YW edited the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All experiments followed the Chinese Council for Animal Care guidelines and were in accordance with the Animal Care Committee of FuDan University (201903001S).
The authors thank Wenlong Ding from Shanghai Jiao Tong University School of Medicine for his experimental guidance and help. Ewelina Biskup assisted in the revision of the manuscript.
This research was financially supported by National Natural Science Foundation of China (No. 81672247), Shanghai Science and Technology Development Foundation (Grant No. 21ZR1436100, Grant No. 15ZR1408000 and Grant No. 18140901200).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.







