1 Department of Clinical Neurosciences, Faculty of Medicine, University Ostrava, 70800 Ostrava, Czech Republic
2 Research and Training Institute Agel, Department of Neurology, Hospital Ostrava Vitkovice, 70384 Ostrava, Czech Republic
3 Department of Neurology, University Hospital Ostrava, 70800 Ostrava, Czech Republic
4 Faculty of Medicine in Hradec Kralove, Charles University, 11000 Hradec Kralove, Czech Republic
5 Department of Medical Imaging, St. Anne's University Hospital and Faculty of Medicine, Masaryk University, 60177 Brno, Czech Republic
Academic Editors: Foteini Christidi, Emilia Salvadori and Rafael Franco
Abstract
Computed tomography perfusion (CTP) is a functional examination of brain tissue that characterises the state of cerebral perfusion and provides information about the current status of the circulation. CTP can improve diagnostic accuracy of ischemic stroke. Published studies showed that perfusion imaging improves the prognosis of patients with acute ischemic stroke in anterior circulation and allows patients to be referred for treatment outside the time window for administration of intravenous thrombolysis (IVT) or mechanical thrombectomy (MT). In this review we discuss technical aspects of CTP, clinical significance of CTP in anterior circulation stroke (ACS) and its role in diagnostics of stroke mimics.
Keywords
- Computed tomography perfusion
- Ischemic stroke
- Stroke mimics
In the context of acute stroke, computed tomography (CT) of the brain is the
most widely used imaging method. All patients with suspected acute ischaemic
stroke (AIS) must have a native CT scan of the brain (NCCT), which can assess the
age and extent of ischaemia and exclude other pathology of sudden neurological
deficit that will exclude the patient from recanalization therapy (tumour,
haemorrhage) [1]. In the first hours of stroke onset, no changes may be apparent
on the NCCT scan. Subsequently, early ischaemic changes develop (developing
cytotoxic oedema, white-grey matter de-differentiation, loss of gyrification,
flattering of the liquor spaces at the brain convexity, dense artery sign) until
a hypodense rim corresponding to cerebral infarct is formed [1]. In clinical
practice, we use the Alberta Stroke Program Early CT score (ASPECTS) to assess
the extent of early ischaemic changes in the middle cerebral artery (MCA)
territory [1]. Nowadays, automatic assessment of early ischaemic changes
(Brainomix, iSchemaView RAPID) is being developed and used. In addition, CT
perfusion (CTP) imaging is becoming clinically available. CTP is a functional
examination of brain tissue that characterises the state of cerebral perfusion
and provides information about the current status of the
circulation/microcirculation [2]. In 2017, a review of 27 studies by Shen
et al. [3] was published that evaluated and compared the diagnostic
accuracy of CTP with NCCT and CT angiography (CTA) in the detection of AIS. The
pooled sensitivity and specificity of CTP for acute ischaemic stroke were 82%
and 96%, respectively. CTP was more sensitive than NCCT and had similar accuracy
to CTA in detecting AIS. There were no statistically significant differences in
sensitivity and specificity between patients who underwent CTP within 6 hours of
symptom onset and 6 hours after the symptom onset [3]. However CTP has limited
diagnostic utility in acute ischaemic posterior circulation stroke (PCS) [4].
Other limitations of CTP are lacunar strokes with 50% false negative cases and
small cortical and subcortical strokes with a size of infarction under 3 cm
Published studies indicate that perfusion imaging may have a prognostic value in anterior circulation acute ischemic stroke and aid in the selection of patients outside the time window for administration of intravenous thrombolysis (IVT) or mechanical thrombectomy (MT) [5]. Although CTP has value in assessing tissue perfusion status in the hyperacute stroke setting and the long-term clinical prognosis of patients with AIS receiving reperfusion therapy, the prognostic use of CTP requires optimization and further validation [6].
The aim of this review was to give an overview of CTP acquisition and processing, clinical significance of CTP in anterior circulation stroke according to published meta-analysis and randomised controlled trials and its role in diagnostics of stroke mimics.
CTP represents a dynamic acquisition capturing a passing contrast bolus (its wash-in and wash-out) through the brain tissue [7, 8]. In other words, the brain is repeatedly scanned as the contrast medium flows through the region of interest and its relative increase, peak and decrease is measured, and an attenuation-time curve is generated [9].
Based on the derived attenuation-time curves that are obtained for arterial input function (representing arterial flow/wash-in) and venous output function (representing venous flout/wash-out), perfusion parameters are calculated for each voxel on a CTP map representing different hemodynamic properties [8, 9].
The essential hemodynamic parameters in CTP studies:
(1) Cerebral Blood Flow (CBF) refers to the volume of blood flowing in a unit of brain mass during a unit of time, measured in milliliters/100 g/min (mL/100 g/min). CBF is often expressed proportionately (relative CBF) as normalized measure to a presumed normal reference region (in the contralateral hemisphere).
(2) Cerebral Blood Volume (CBV) refers to the fraction of a tissue that is vascularized, expressed in the milliliters/100 g.
(3) Mean Transit Time (MTT) represents the average time that takes a contrast bolus to traverse the capillary bed; MTT is reported as an absolute in seconds.
(4) Time to maximum of the residual function (Tmax) expresses the delay from the start of scan acquisition to the maximum intensity of contrast bolus in each voxel.
CBF, MTT, and CBV are mathematically related by the equation: CBF = CBV/MTT, known as the central volume principle [10]. Therefore, measurement of any 2 of these parameters is sufficient to derive the third parameter [8], Fig. 1 (Ref. [11]).
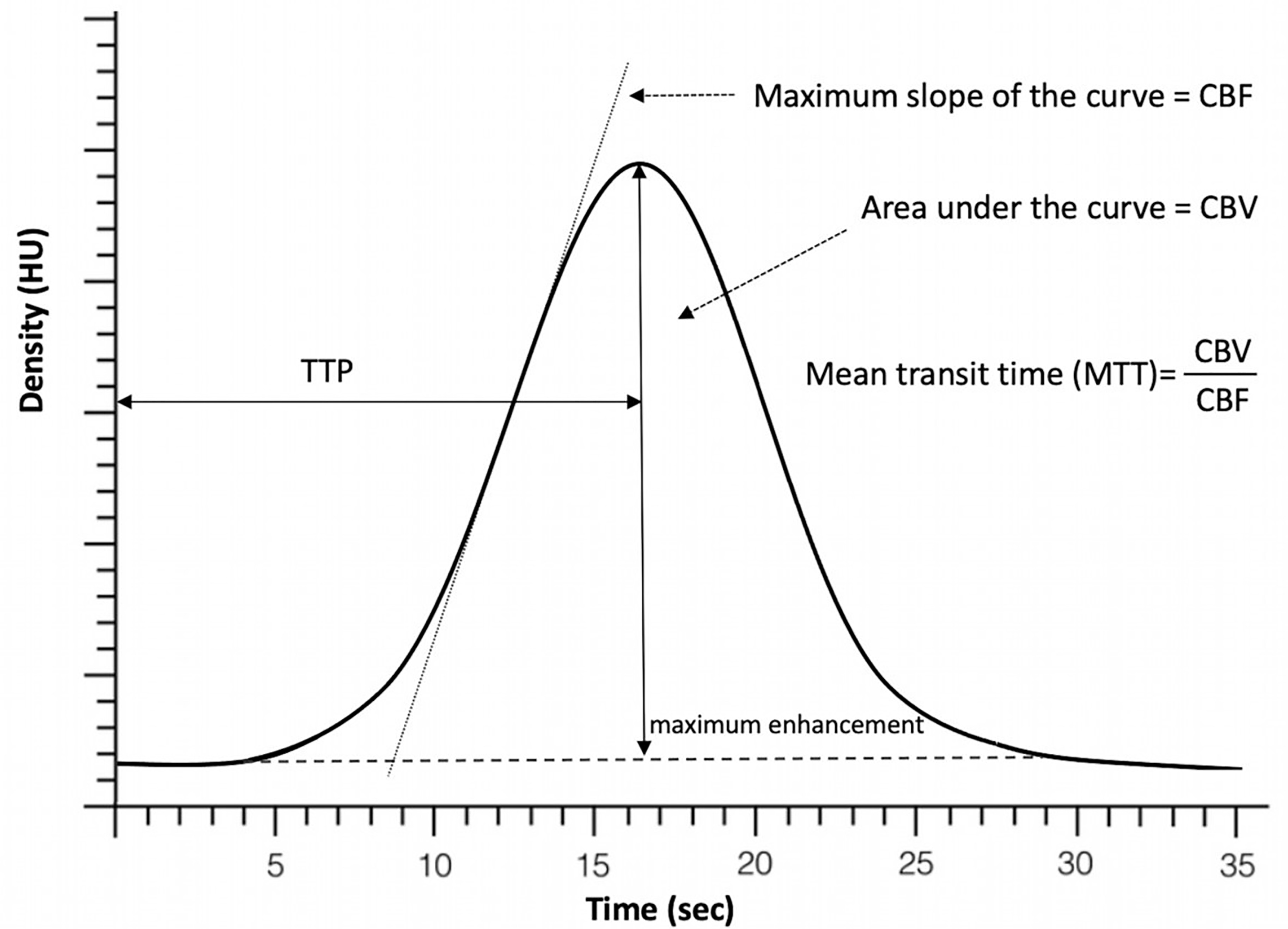 Fig. 1.
Fig. 1.Perfusion time attenuation curve. Time to peak (TTP) represents time it takes the contrast to achieve its maximum within the area of interest. Cerebral blood flow (CBF) is represented by the slope of the curve as contrast arrives into the brain and the cerebral blood volume (CBV) is expressed by the volume/area under the curve. Mean transit time (MTT) is then calculated as the ratio of CBV and CBF [11].
The contrast passage and subsequent attenuation-time curves are dependent on arterial flow (expressed as arterial input function) and tissue characteristics. Among factors influencing the arterial flow of contrast media through the tissue belong impaired cardiac output, severe carotid stenosis or factors related to the contrast bolus injection (injection rate, saline chase). These factors may result in delay or dispersion of the contrast bolus which may introduce an error in the quantification of CBF [8, 12].
Raw CTP data can be processed with 2 techniques to generate perfusion maps, non-deconvolution and deconvolution methods. Non-deconvolution methods are based on first-pass iodine extraction measurements resulting in a simplified and less computationally intensive processing algorithm. Deconvolution methods account for physiological variations in arterial flow, the collateral flow effect, and venous outflow components of cerebral perfusion [8, 13].
There exist different deconvolution methods: singular value decomposition (SVD) [14], delay- and dispersion-corrected singular value deconvolution (dd-SVD) [15], and Bayesian methodologies [16]. dd-SVD or Bayesian-estimated generate the arterial delay time in contrast Tmax derived from SVD model. Use of different deconvolution methods may result in the measurement variability of the core and penumbra based on the deconvolution algorithm [8].
The goal of perfusion analysis in the clinical use is to quantify tissue with significant hypoperfusion which is likely to infarct if reperfusion is not achieved (ischemic penumbra) and identify tissue that is likely irreversibly infarcted (ischemic core) [5]. Some form of thresholding is usually applied to derived perfusion maps to obtain more consistent and objective result.
It is important to note that the CTP threshold is not absolute. It has been shown that the ischemic core may be overestimated if the perfusion imaging is performed very early after the symptom onset [17] and the use of more strict thresholds should be considered especially if the imaging is performed within the first hour (“golden hour”) [18]. The core overestimation was also demonstrated in those patients with achieved rapid reperfusion [17]. Although it is know that the white matter is more resistant to the hypoperfusion and therefore more strict thresholds should be applied to distinguish grey and white matter at risk of infarction with a higher accuracy [19], this differentiation is not widely used in clinical practice and remains to be so far of a research interest.
Prediction of the tissue fate is also influenced by the severity of
hypoperfusion [17, 20]. Severely hypoperfused tissue defined as Tmax delay
Multiple software packages generating perfusion maps are available. However, comparison of commercially available software shows significant differences in core and penumbra volume calculation due to different methods of generating perfusion parametres [17, 21, 22]. For instance, one software may generate highly delay-sensitive MTT maps, but other packages may generate delay insensitive maps [17]. Such a differences may subsequently influence patient selection for reperfusion therapy.
However, the automated image analysis offers the potential to improve patient outcomes by reducing triage, transfer and treatment times [23, 24] by identifying the patients with perfusion deficit especially in smaller, rural hospitals where physicians with experience in stroke imaging or manual processing of perfusion scans are not always available [25].
Within a time window of up to 4.5 hours for administration of systemic
thrombolysis and up to 6 hours for mechanical thrombectomy, evaluation of native
CT is considered sufficient preferably using ASPECTS score and CT angiography to
indicate recanalisation therapy. Studies with both endovascular therapy and
systemic thrombolysis (EXTEND-IA, DEFUSE3, DAWN, EXTEND, WAKE-UP) have shown
benefit in patients treated outside the guideline recommended therapeutic window.
The significance of CTP, which allows differentiation between necrotic core and
penumbra, lies primarily in the extension of the possibility of treating patients
with systemic thrombolysis or endovascular therapy. These patient groups are:
wake up stroke, unknown time of onset and time of onset
A 2017 meta-analysis by Ruy et al. [26] examined the effect of treatment using perfusion imaging compared with CT protocols without CTP. 994 patients from 8 randomised and prospective studies published between 2011–2016 were analysed. In most studies, patients in the perfusion imaging group were treated in extended time window, up to 26 hours or with an unknown time of onset. Patients with both recanalization therapy (intravenous thrombolysis plus mechanical thrombectomy) were 1.9 more likely to achieve good clinical outcome, as expressed by modified Rankin Scale (mRS) of 0–2, when using perfusion imaging.
Another meta-analysis by Katyal in 2020 looked at randomised and retrospective studies with recanalization therapy (intravenous thrombolysis plus mechanical thrombectomy, or a combination of both) conducted from 2005 to 2020). Recanalization therapy was evaluated in patients with unknown time of onset, wake up stroke and stroke after 4.5 h for intravenous thrombolysis and after 6 h for mechanical thrombectomy, who were compared with patients treated within the standard window. From a total of 2226 studies, 15 with good quality methodology were selected. Nevertheless, the limitations of the meta-analysis mentioned that it comprised of prospective studies without a blinded placebo group, it was partly sponsored by commercial entities, and the CTP protocols were heterogeneous. A total of 5687 patients were analysed, 2040 (36%) with CTP guided recanalization therapy compared to 3647 (64%) with standard therapy. The following criteria were evaluated: good clinical outcome at 90 days (mRS 0–2), symptomatic intracranial haemorrhage (SICH), percentage of recanalization, and mortality. The odds of good clinical outcome were 2.3 times greater in the CTP-guided recanalization group (OR = 2.3; 95% CI 1.6–3.21). In the subgroup with endovascular therapy after 6 h compared to patients without recanalisation therapy, the good clinical outcome was even 5 times higher (OR = 5.01; 95% CI 3.07–8.17). There was no significant difference between groups in SICH, 5% in the CTP group versus 4.7% in control group. There was a significant reduction in mortality (OR = 0.75; 95% CI 0.59–0.74) and a higher likelihood of recanalization (OR = 4.37; 95% CI 2.52–7.58) in the CTP group [27].
CTP thus opens up the possibility of therapy for patients who would otherwise not be treated due to failure to meet the time specific criteria. Select 2 study with endovascular treatment and the use of CTP is currently underway looking for efficacy and safety in patients with large core infarcts and extended time windows [27].
Besides the role of CTP in the diagnosis of AIS, its role in prognosis of cerebral ischaemia was studied. Meta-analysis of Campbell showed that increasing ischaemic core volume (CTP time to maximum longer than 6 s) was independently associated with functional independence and functional improvement [28].
Thrombolytic therapy after 4.5 h was studied in the randomised,
placebo-controlled study – EXTEND (Extending the Time for Thrombolysis in
Emergency Neurological Deficits). Patients with known onset and duration of
symptoms between 4.5–9 hours and patients with wake up stroke were included (if
within 9 hours from the midpoint of sleep). 113 patients in the intravenous
thrombolysis (alteplase) group and 112 in the placebo group. Imaging criteria for
ischemic core and were rCBF below 30% and Tmax above 6 s as the area of
hypoperfusion. Patients with mismatch ratio
Other randomised trials studying the effect of intravenous thrombolysis in patients selected based on the moultimodal imaging were the ECASS4-EXTEND and EPITETH studies. The ECASS4-EXTEND study used the same inclusion criteria as the EXTEND study, but used visual assessment of MRI perfusion-diffusion imaging. The study was terminated prematurely due to low patient recruitment. EPITETH study randomly allocated patients to alteplase or placebo within 3–6 h of stroke onset based on evaluation of MRI perfusion-diffusion processed offline to determine the presence of perfusion mismatch [30].
Campbell et al. [28] conducted a meta-analysis of all 3 studies in
2019. From the EPITETH study, only patients treated within the 4.5–6 h of stroke
were analysed. A total of 414 patients were analysed, 213 (51%) in the alteplase
group and 201 (49%) in the placebo group. For the analysis of the primary
outcome- excellent functional outcome (mRS score 0–1) after 3 months, 211
patients from the treatment group and 199 from the placebo group were available.
In the alteplase group, 76 patients (36%) achieved it versus 58 (29%) in the
placebo group (OR 1.86, 95% CI 1.15–2.99). In the alteplase group, the number
of symptomatic intracerebral haemorrhage (SICH) was significantly higher. Ten
(5%) of 213 patients vs one (
In another meta-analysis of the EPITETH and EXTEND trials, the association of
reperfusion and 90-day clinical outcome was examined in the 4.5–6 h, 6–9 h and
wake up stroke groups. 270 patients were analysed, of whom 226 (83%) had a
positive baseline perfusion mismatch. The alteplase group had a significantly
higher percentage of reperfusion 51% vs 28% (risk ratio RR, 1.84; 95% CI
1.34–2.53; p
Thomalla’s meta-analysis, published in 2020, comprised patients treated with intravenous alteplase for stroke with unknown time of onset guided by advanced imaging. Patients with perfusion CT (EXTEND study, ECASS-4 study), perfusion-diffusion MRI, or MRI with diffusion-weighted imaging-fluid-attenuated inversion recovery (WAKE-UP study, THAWS study) mismatch were eligible. The outcomes confirmed results of the above mentioned meta-analysis:
In patients who have had a stroke with unknown time of onset with perfusion mismatch, intravenous alteplase resulted in better functional outcome after 90 days than placebo or standard care despite an increased risk of symptomatic intracranial haemorrhage [33].
The effect of mechanical thrombectomy after 6 hours demonstrated in the DEFUSE 3
study, which randomized patients within 6–16 hours after stroke based on
automated CTP analysis. Patients with middle cerebral artery (MCA) or internal
carotid artery (ICA) occlusion meeting perfusion criteria were included in the
study. An initial volume of core
and significantly more patients reached mRS 0–2 in the treated group (45% vs
17%, p
The DAWN study combined clinical/imaging mismatch inclusion criteria. Patients with MCA or ICA occlusion, 6–24 hours from the stroke onset were divided into 3 groups. Severity of neurological deficit was assessed by NIHSS, and the size of the ischaemic core was measured in mL on DWI-MRI or CTP by Rapid software. Group A included patients over 80 years of age with NIHSS over 10 and ischaemic volume less than 21 mL. Group B included patients younger than 80 years with NIHSS above 10 and size of ischaemia below 31 mL. The last group, C, also included patients below 80 but with a more severe NIHSS of 20 and above and a larger ischaemic volume of 31–51 mL. There were 107 patients in the thrombectomy group and 99 in the control group. The study was terminated prematurely after an interim analysis demonstrating superiority of mechanical thrombectomy. 90-day functional independence (mRS 0–2) was significantly better in the thrombectomy group 49% vs 13% in the control group. The rate of symptomatic intracranial haemorrhage did not differ significantly between the two groups (6% in the thrombectomy group and 3% in the control group, p = 0.50), 90-day mortality was similar (19% and 18%, respectively; p = 1.00). Despite the limited strength of statistical analysis for individual groups, no heterogeneity was found in the effect of thrombectomy depending on mismatch between the severity of the clinical deficit and infarct volume, with mismatch criteria defined according to age (under 80 years or more than 80 years) [35, 36].
The DAWN study demonstrated the beneficial effect of mechanical thrombectomy in patients selected on the basis of perfusion imaging and severity of neurological deficit treated in extended treatment window [37, 38].
The frequency of stroke mimics is variable and depends on many factors. The diagnosis of stroke mimics is made approximately in 20–50% of cases of suspected acute stroke depending on whether the patients are evaluated by the emergency personal or stroke physicians [39].
According to the aetiology, stroke mimics can be divided into two groups: medical stroke mimics and functional stroke mimics. Diseases that may not have a positive finding in NCCT and therefore their diagnosis are difficult are as follows: recrudescence, migraine, epilepsy, vertigo associated with peripheral vestibular syndrome, attacks in multiple sclerosis and functional psychogenic disease. The most common of those is recrudescence which is defined as a sudden worsening of clinical symptoms in patients with neurological deficits after previous ischaemic stroke. The most common underlying cause is infection or worsening of a known cardiac disease [40]. The suspicion of stroke mimics is mainly based on the clinical presentation and neurological symptoms. Neurological symptoms typically do not correspond clearly to vascular territories and often fluctuate in nature. In stroke mimics, the suddenness of the onset is not always evident, fluctuations in symptom severity are common, and systemic signs are also often present [40] (Table 1).
| Trial | No of Pts | Recanalization | Time window | mRS 3M treated | mRS 3M placebo | OR 95% CI | Mortality treated | Mortality placebo | OR 95% CI |
| No (%) | No (%) | No (%) | No (%) | ||||||
| Metaanalysis EXTEND, EPITHET, ECASS 4 | 414 | Intravenous thrombolysis | 4.5–9 h | 0–1 | 0–1 | 1.86 | 29 (14%) | 18 (9%) | 1.55 |
| Wake up | 76 (36%) | 58 (29%) | 1.15–2.99 | 0.81–2.96 | |||||
| DEFUSE 3 | 182 | Mechanical | 6–16 h | 0–2 | 0–2 | 2.67 | 13 (14%) | 23 (26%) | 0.55 |
| thrombecotmy | 41 (45%) | 15 (17%) | 1.6–4.5 | 0.3–1.02 | |||||
| DAWN | 206 | Mechanical | 6–24 h | 0–2 | 0–2 | Not calculated | 17 (16%) | 18 (18%) | Not calculated |
| thrombecotmy | 52 (49%) | 13 (13%) | |||||||
| EXTEND, Extending the Time for Thrombolysis in Emergency Neurological Deficits; EPITHET, Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial; ECASS 4, European Cooperative Acute Stroke Study 4; DEFUSE 3, A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke; DAWN, Diffusion Weighted Imaging (DWI) or Computerized Tomography Perfusion (CTP) Assessment With Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention; RCT, randomized controlled trial; CTP, Computerized Tomography Perfusion. | |||||||||
It is not uncommon that patients presenting with stroke mimics have a previous history of epilepsy, migraine, depression or other psychiatric symptoms. The highest accuracy for ischaemic stroke diagnosis is achieved by CTP, whose pooled sensitivity is 82% (95% CI 75–88) and specificity 96% (95% CI 89–99). Sensitivity decreases in the diagnosis of posterior circulation stroke to 76% (70–81) and specificity to 93% (87–97) [39]. Although DWI MRI has a higher sensitivity in the diagnosis of ischaemic stroke, performing a multimodal CT examination in the acute phase in patients with suspected stroke mimics has multiple benefits. First, multimodal CT examination is able to exclude stroke mimics, second, the results are valuable for the treatment strategy and the patient’s prognosis [41, 42].
Some stroke mimics have typical CTP imaging patterns such as migraine and epileptic seizures. Up to 25% of migraine patients may present focal neurologic deficit imitating acute ischaemic stroke [43].
Migraine aura is usually associated with a perfusion deficit not limited to a specific vascular territory, and only a moderate increase of TTP [44].
In epileptic seizures neurologic deficit may last up to 48 hours after the convulsion [45].
Perfusion MRI studies can show a typical CBF increased (hyperperfusion) at epileptogenic area Outside the area, in the postictal phase, the perfusion parameters are normal or reduced but there is the absence of a typical vascular lesion distribution [46]. The CT perfusion study and CT angiography demonstrated a dramatic reduction in the cerebral blood flow and blood volume involving the entire left hemisphere, but with relative symmetry of mean transit time, ruling out a large vessel occlusion [47].
Acute treatment with systemic thrombolysis should be continued even if stroke mimics are suspected based on a negative multimodal CT scan. The systemic thrombolysis in stroke mimics is safe, the rate of intracranial haemorrhage is very low (0–2%), similar to the symptomatic ICH rate in patients treated with IVT for acute myocardial infarction [48]. In the SITS international stroke thrombolysis registry with 10,436 treated subjects, only 429 (4.1%) treated patients were diagnosed with stroke mimics. The most common mimics treated with thrombolysis were functional disorder (30.8%), migraine (17.5%) and seizures (14.2%) [39]. The correct diagnosis in cases where ischaemic stroke is suspected is crucial and there is a need to also improve the diagnostics of stroke mimics.
Education of paramedics and effective prehospital care can improve the overall quality of the provided stroke care. The possibility of teleconsultation increases the correct diagnosis of ischaemic stroke in the prehospital phase. The availability of stroke physician with combination of multimodal CT examination can further improve the rates of correct diagnosis of stroke and distinguished stroke mimics [40]. In case of negative multimodal CT examination on admission, 24 hours follow up of MR is recommended for stroke mimics exclusion [49, 48]. Misdiagnosis of stroke leads to an unnecessary use of stroke prevention medication, psychosocial problems and stigmatisation [40].
Our work is a brief overview of the importance of CT and CTP in stroke, this is not an extensive systematic review or metanalysis. Our own analysis was not performed, only the data from carried out analysis were comprised in our work.
CTP-based patient selection for recanalization treatment has led to extension of crude time windows for intravenous thrombolysis and mechanical thrombectomy. The use of automated software bridges the shortcomings of standard commercial software programs and enables the introduction of CTP into clinical practice. CTP appears to be a useful tool in detecting stroke mimics during the initial CT scan in acute patients with suspected AIS.
Other standardisation of perfusion criteria and imaging processing will help to widespread CTP to clinical practise. More evidence is needed for CTP in indication of reperfusion therapy in patient with large core infarct, treatment beyond 24 hours and futile reperfusion up to standardised time window.
DV has made substantial contributions to conception and design, has given final approval of the version to be published. OV has made substantial contributions to conception and design. PC has made substantial contributions to conception and design. KŠ has made substantial contributions to acquisition of data and interpretation of data. KD has made substantial contributions to acquisition of data and interpretation of data. MB has been involved in drafting the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.

