1 Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA 02129, USA
Academic Editor: Rafael Franco
Abstract
Background: Neurological disorders are a major source of suffering for patients worldwide. Scalp stimulation methods have been widely applied in treating a number of neurological disorders. Recently, our understanding of pathological mechanisms associated with neurological disorders has been enhanced significantly. Nevertheless, these findings have yet to be well-integrated into scalp stimulation treatments for neurological disorders. Methods: In a previous study, we proposed new brain targets for scalp stimulation in the treatment of eight common mental disorders based on the results of a large-scale meta-analyses using Neurosynth. This study aims to extend our previous findings in identifying surface brain targets for seven common neurological disorders: Alzheimer’s disease, aphasia, chronic pain, dementia, dyslexia, mild cognitive impairment, and Parkinson’s disease, utilizing a similar method. Results: We hidentified seven to eight potential scalp stimulation targets for each disorder and used both 10–20 EEG system and acupuncture points to locate these targets to facilitate its clinical application. Conclusions: The proposed target protocols may facilitate and extend clinical applications of scalp stimulation methods such as transcranial electrical stimulation and scalp acupuncture in the treatment of neurological disorders.
Keywords
- meta-analysis
- scalp stimulation
- brain stimulation transcranial electrical stimulation
- scalp acupuncture
- neurological disorder
Neurological disorders are a source of suffering and burden on the quality of life of patients across the world. Clinicians and scientists, in particular, have begun to notice a rising trend in younger patients being diagnosed with neurological disorders; calling for more treatment options [1, 2, 3].
Scalp stimulation, the application of stimulation to the scalp to modulate the function of corresponding brain areas for symptom relief [4], has recently been applied in the clinical treatment of neurological disorders, particularly those associated with brain dysfunction. For instance, scalp acupuncture: a type of scalp stimulation method and a modern branch of acupuncture techniques, has been applied to treat neurological disorders, such as Alzheimer’s disease [5], Parkinson’s disease [6], mild cognitive impairment [7] and chronic pain [8].
Another form of scalp stimulation, transcranial electrical stimulation (tES) (including transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS) and transcranial random noise stimulation (tRNS)), has been applied to treat neurological disorders. Studies demonstrated that tDCS can be applied in rehabilitating motor function and contributes to motor function improvement in stroke patients [9, 10, 11]. A recent study showed that tACS could improve memory performances in patients with mild cognitive impairment/Alzheimer’s disease [12]. Both tACS and tRNS displayed potential in increasing temporal precision of the auditory system in dyslexia patients [13].
Studies have shown that the specific location of the stimuli play a crucial role in scalp stimulation [14, 15]. However, many of these scalp stimulation studies were only applied on the dorsolateral prefrontal cortex (dlPFC) and several other limited brain regions for multiple disorders. For instance, Papazova and colleagues found that applying the anode of tDCS on the left dlPFC can enhance working memory performance in schizophrenic patients [16]. Similarly, tDCS on dlPFC has been applied in patients with chronic pain [17, 18]. Nevertheless, other brain areas should also be considered as potential targets to widen novel therapeutic avenues as brain imaging studies have indicate that a complicated network has been involved in almost any neurological disorders.
Taking advantage of rich brain research data previously collected, we applied Neurosynth, a platform that can automatically incorporate text-mining, meta-analysis and machine-learning techniques [19], to develop new scalp stimulation target protocols in the treatment of eight common mental disorders from a previous study [4]. The current manuscript aims to extend our previous findings and to identify potential brain targets for seven common neurological disorders (Alzheimer’s disease, aphasia, chronic pain, dementia, dyslexia, mild cognitive impairment, and Parkinson’s disease) utilizing an identical method. We hypothesize that different neurological disorders will be associated with both distinguishable scalp stimulation targets, and some common targets across different neurological disorders.
In this study, we applied a similar method to our previous study [4]. Please see our previous publication and Supplementary Fig. 1 for details. In summary, we first applied Neurosynth [19] to identify brain regions associated with different disorders. Research strings (“Alzheimer’s disease”, “aphasia”, “chronic pain”, “dementia”, “dyslexia”, “mild cognitive impairment (MCI)”, and “Parkinson disease”) were used; a uniformity test map was generated to identify disorder-associated brain regions. A complete list of the studies included for each disorder extracted from Neurosynth can be found in Supplementary Tables 1,2,3,4,5,6,7.
To identify disorder-associated surface brain regions, we created a brain surface cortical mask using SPM Wake Forest University (WFU) PickAtlas toolbox [20, 21, 22, 23]. We then refined the brain regions by discerning the overlap on the uniformity test map with the surface cortical masks. Next, we identified the coordinates with peak z-scores within the all-surface cluster larger than 30 voxels on the uniformity test map using the xjView toolbox (version 8, https://www.alivelearn.net/xjview/). After that, we mapped the results onto a standard brain using SurfIce (www.nitrc.org/projects/surfice) and a standard head using MRIcroGL (www.mccauslandcenter.sc.edu/mricrogl) with the international 10–20 electroencephalography (EEG) system in Montreal Neurological Institute (MNI) space [24].
We then chose seven/eight clusters (based on cluster size and peak intensity) for each disorder and identified peak coordinates of these clusters as potential scalp stimulation targets. To facilitate identifying the locations, a 2-mm radius spherical masks centered on the identified peak coordinates were mapped onto a standard brain with the international 10–20 EEG system in MNI space and international standard acupoints (Supplementary Fig. 1).
After visually assessing the results, we proposed neuroimaging-based scalp targets (e.g., AD-1 to AD-7, APH-1 to APH-7, CP-1 to CP-7, etc.) for each disorder. To help readers understand the function of identified areas, we also summarized the functions of each identified brain regions based on https://neurosynth.org and http://www.fmriconsulting.com/brodmann. In addition, we explored overlap surface regions among the different disorders using MRIcroGL (version 1.2.20210317, https://www.nitrc.org/plugins/mwiki/index.php/mricrogl:MainPage).
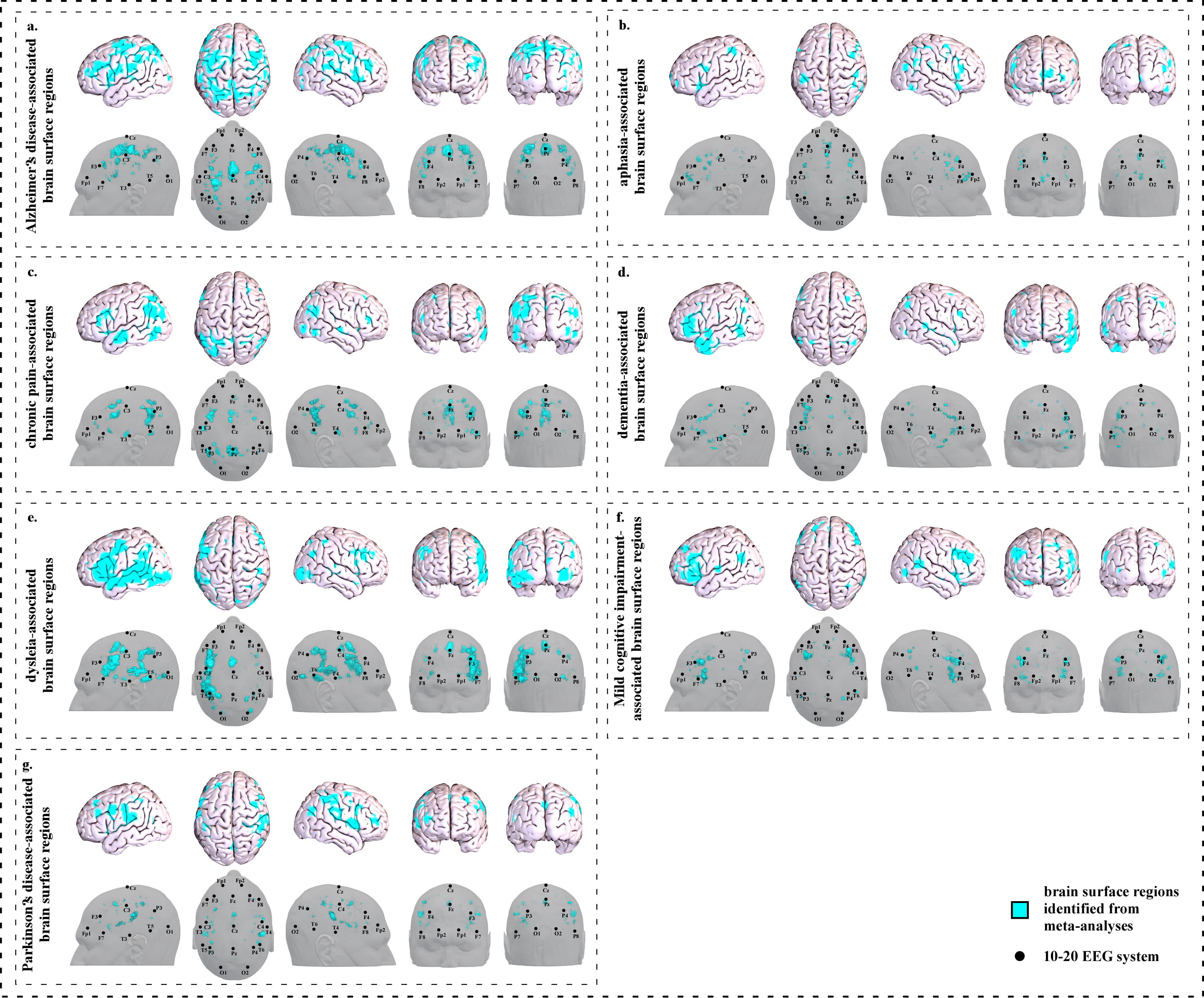
We identified seventeen clusters (Fig. 1a and Supplementary Table 1a) based on 263 studies (Supplementary Table 1b).
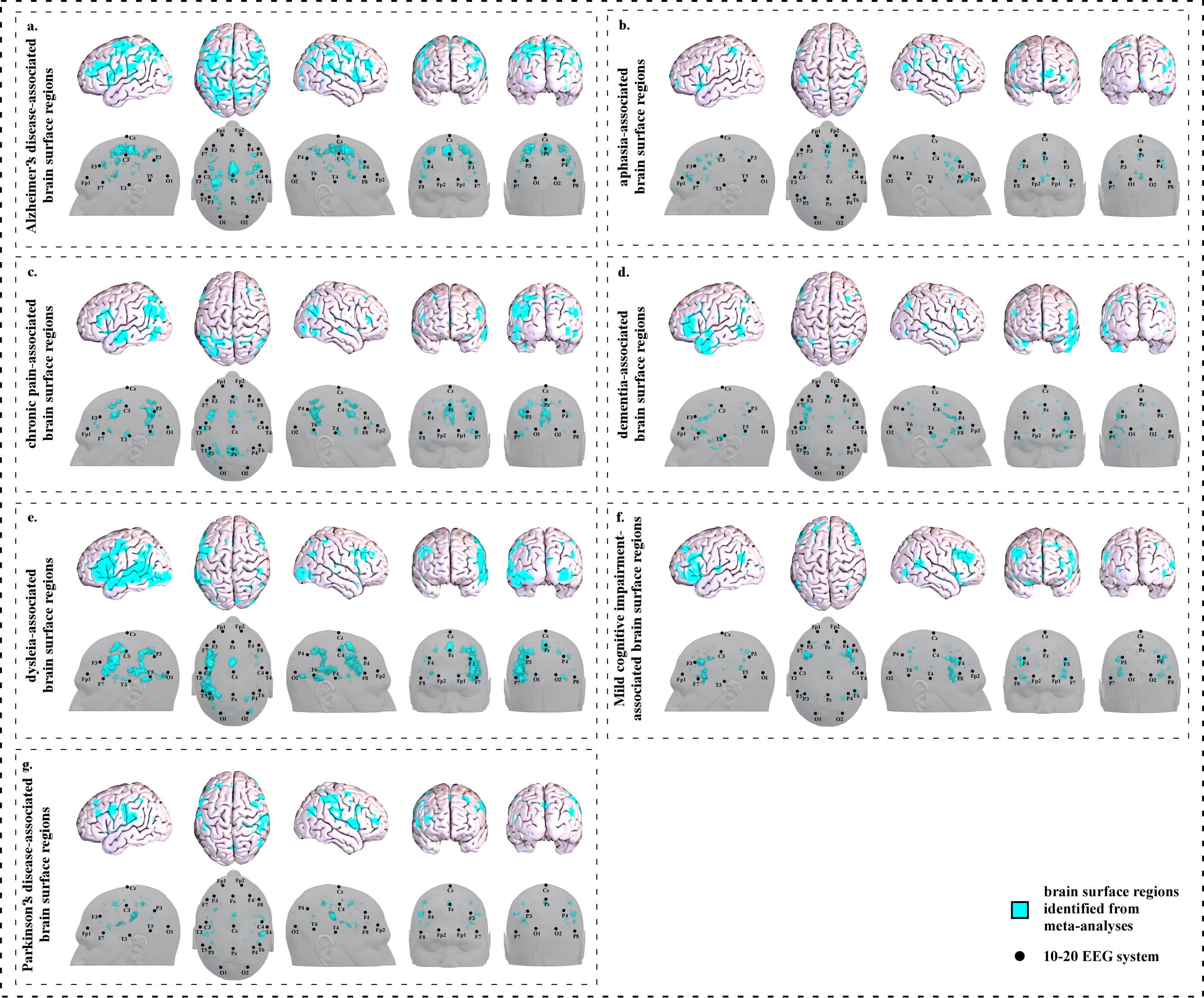 Fig. 1.
Fig. 1.Brain surface regions for scalp stimulation for neurological disorders, identified from meta-analyses of neuroimaging studies. (a) Alzheimer’s disease-associated surface regions. (b) Aphasia-associated surface regions. (c) Chronic pain-associated surface regions. (d) Dementia-associated surface regions. (e) Dyslexia-associated surface regions. (f) Mild cognitive impairment-associated surface regions. (g) Parkinson’s disease-associated surface regions.
We identified seven clusters on the brain surface (Fig. 1b and Supplementary Table 2a) based on 82 studies (Supplementary Table 2b).
We identified seventeen clusters on the brain surface (Fig. 1c and Supplementary Table 3a) based on 92 studies (Supplementary Table 3b).
We identified twelve clusters on the brain surface (Fig. 1d and Supplementary Table 4a) based on 142 studies (Supplementary Table 4b).
We identified fifteen clusters on the brain surface (Fig. 1e and Supplementary Table 5a) based on 76 studies (Supplementary Table 5b).
We identified eight clusters on the brain surface (Fig. 1f and Supplementary Table 6a) based on 81 studies (Supplementary Table 6b).
We identified ten clusters on the brain surface (Fig. 1g and Supplementary Table 7a) based on 175 studies (Supplementary Table 7b).
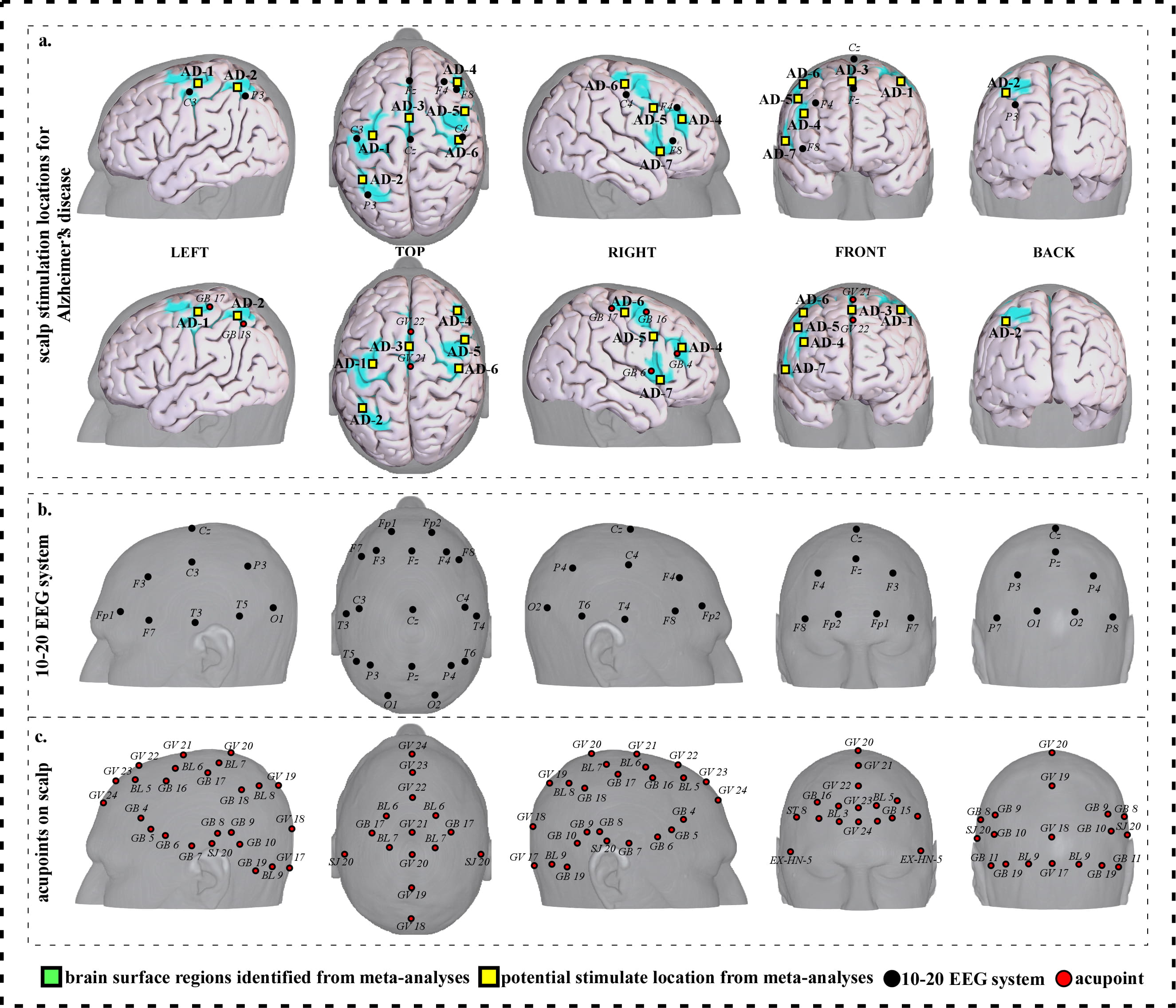
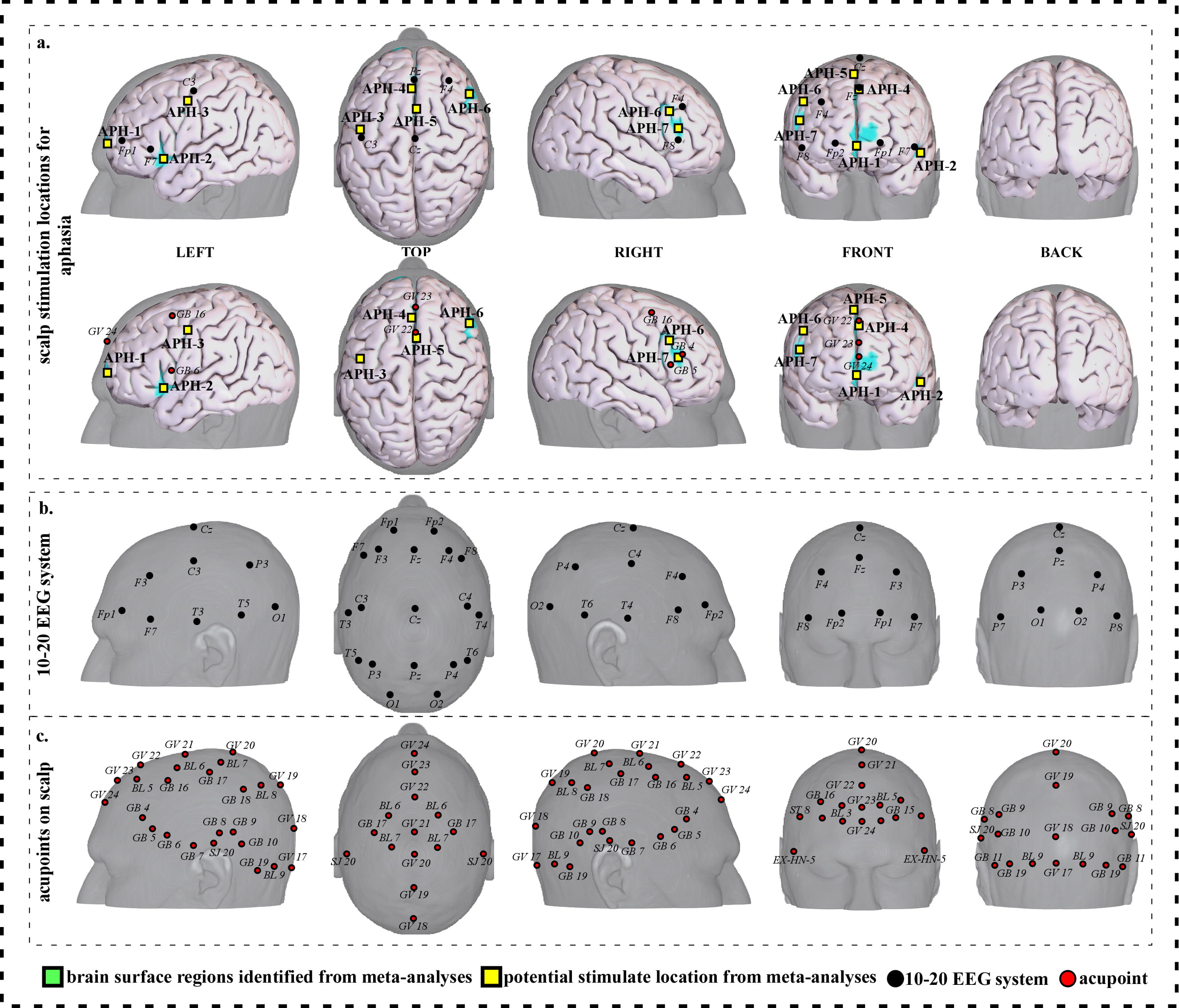
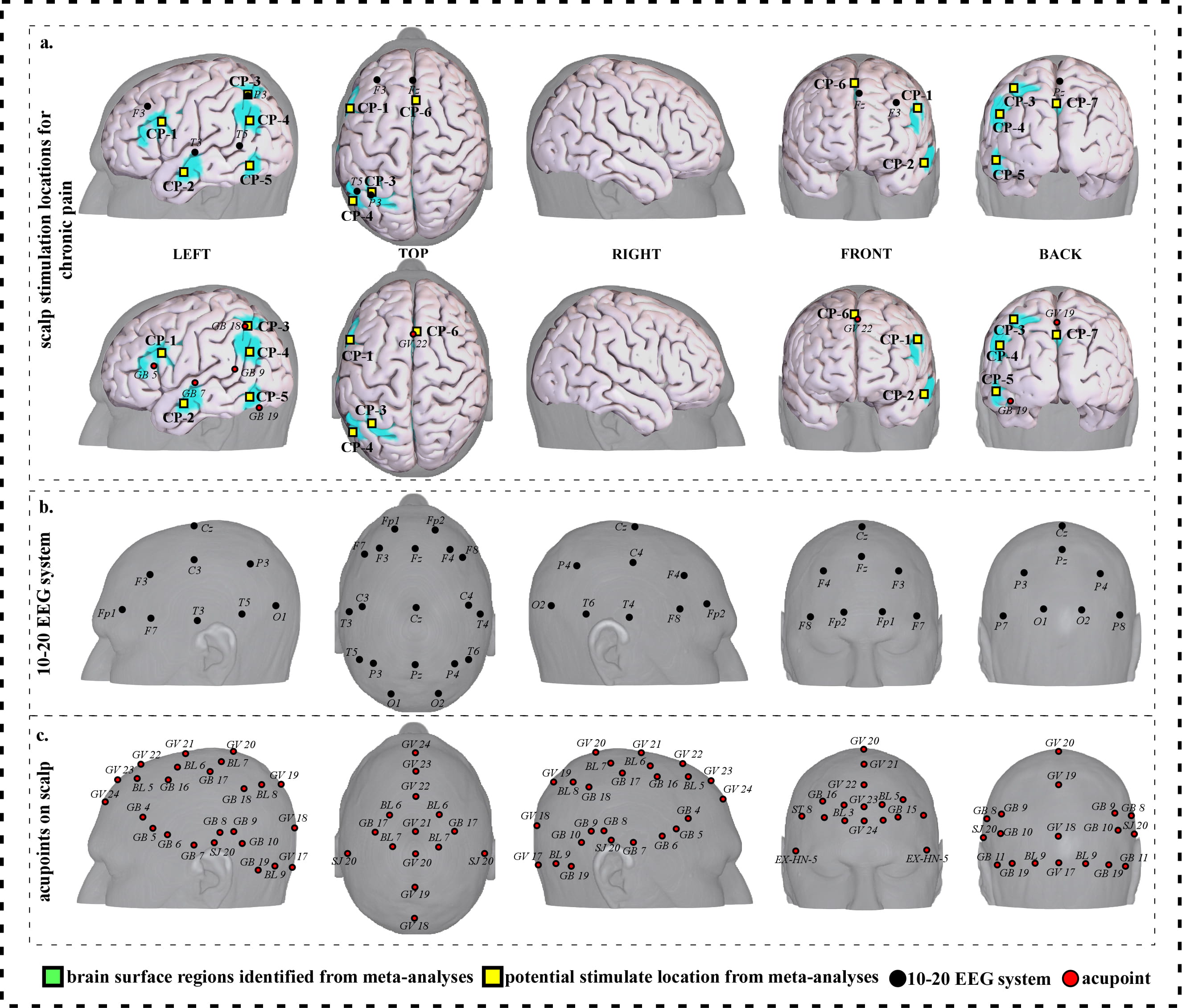
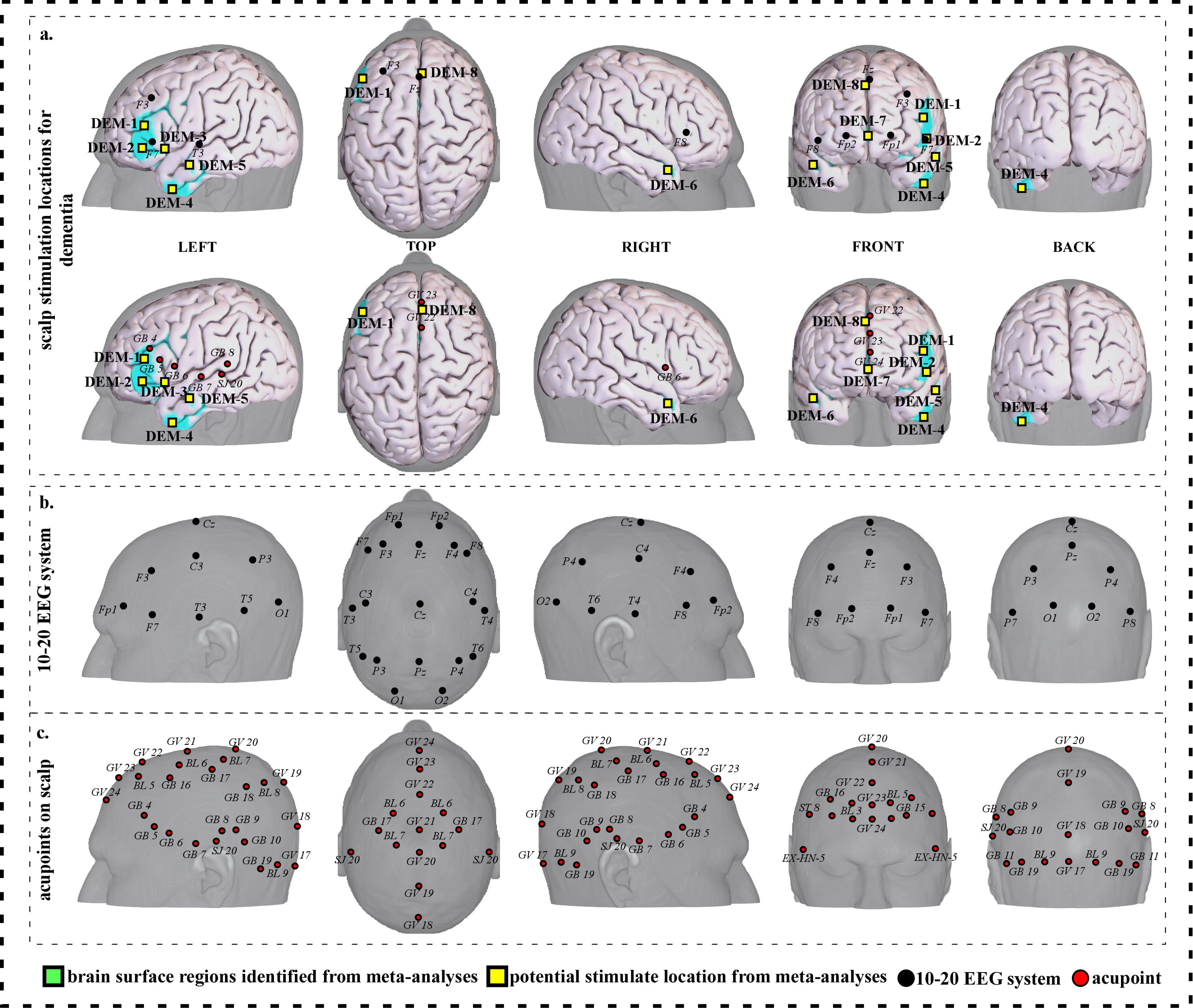
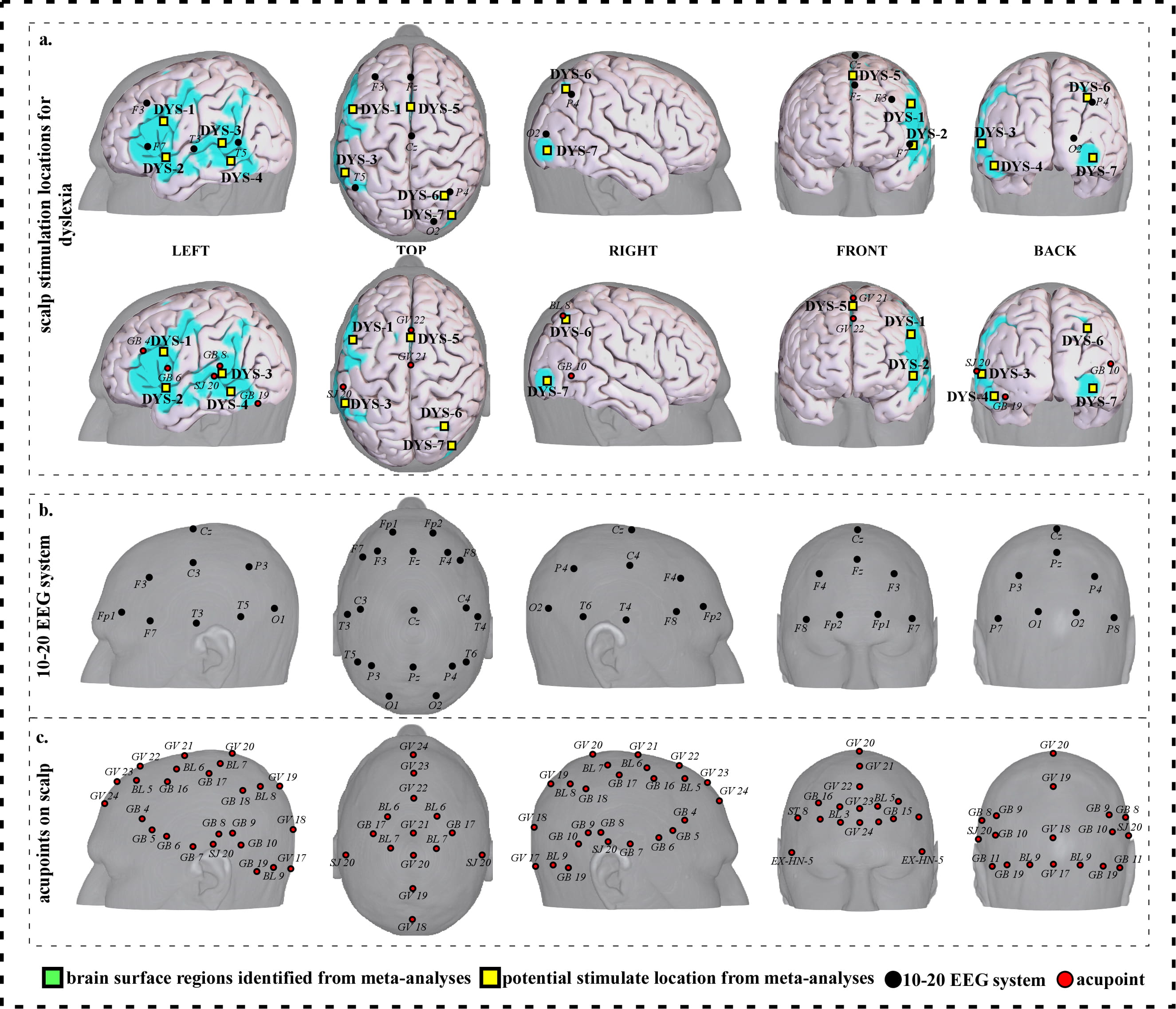
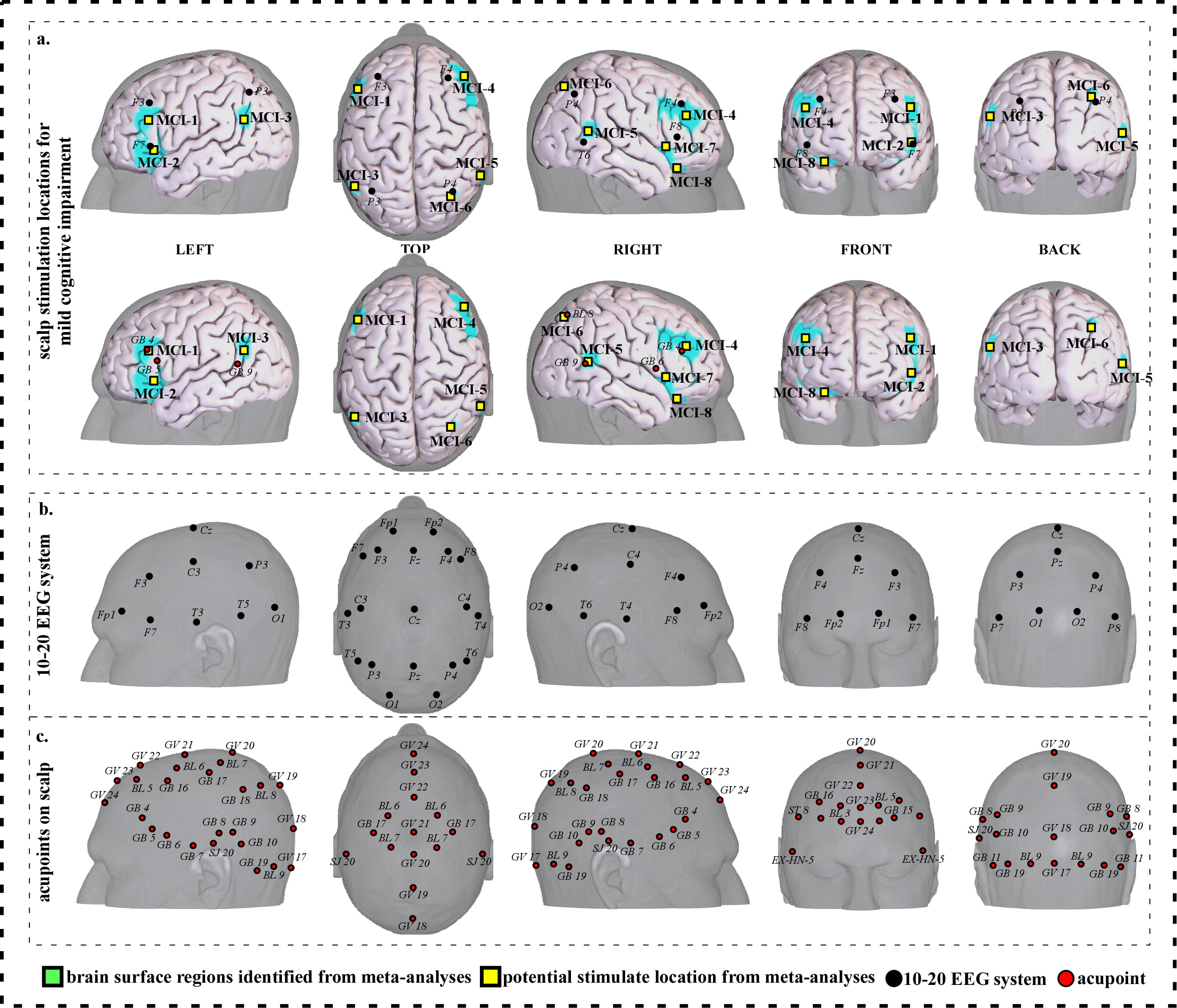
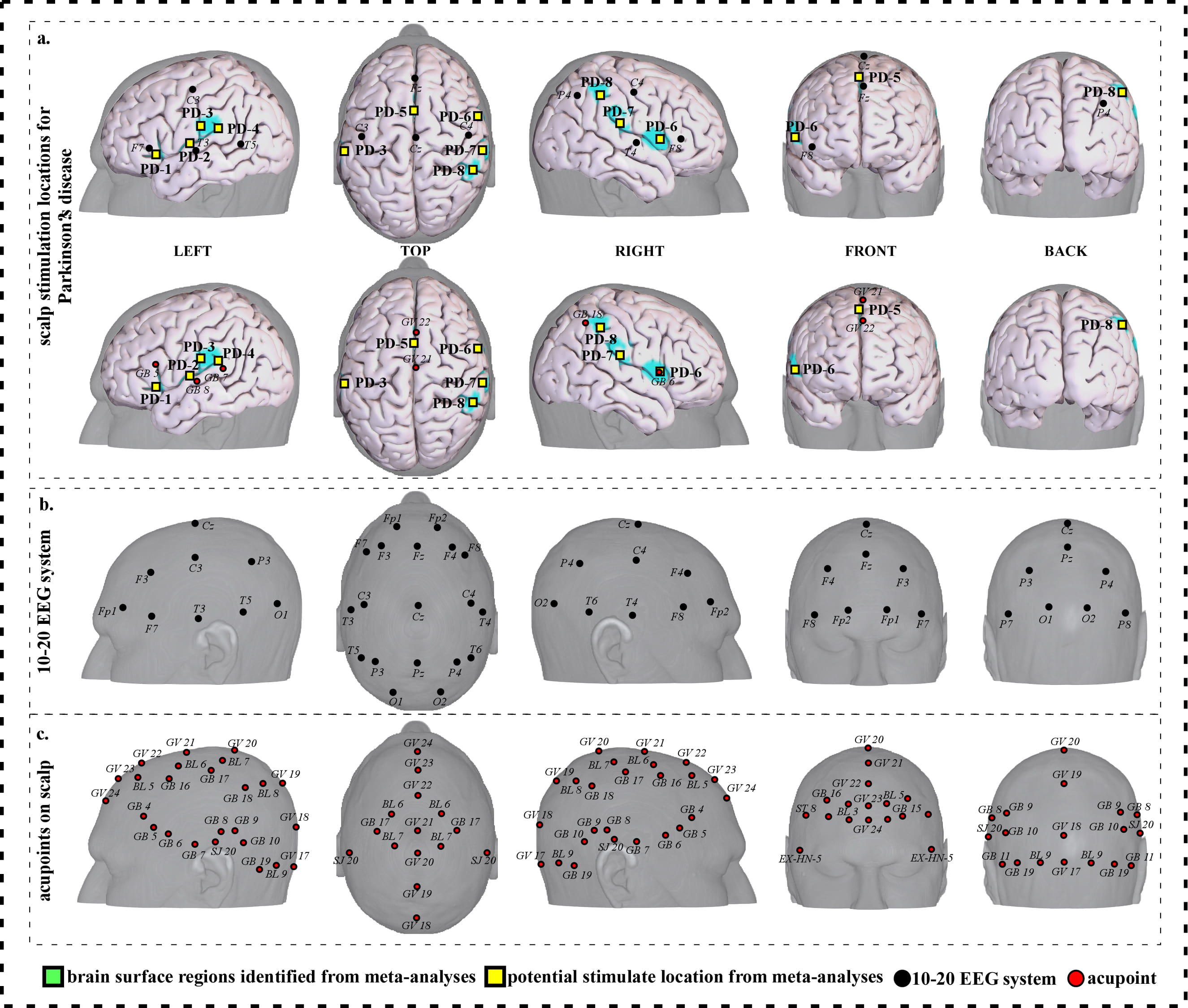
To facilitate clinical application, we further refined our results and proposed a neuroimaging-based protocol for each disorder by identifying the seven or eight surface regions based on the meta-analysis results. The 10–20 EEG system and the international standard acupoints were used to help locate the targets. We summarized the brain functions of each identified brain region associated with a corresponding neurological disorder to help the readers understand the specific brain functions of identified areas (Supplementary Tables 1c,2c,3c,4c,5c,6c,7c). Detailed descriptions of identified targets for each disorder based on 10–20 EEG system coordinates and acupuncture points can be found in Figs. 2,3,4,5,6,7,8 and Tables 1,2,3,4,5,6,7, respectively.
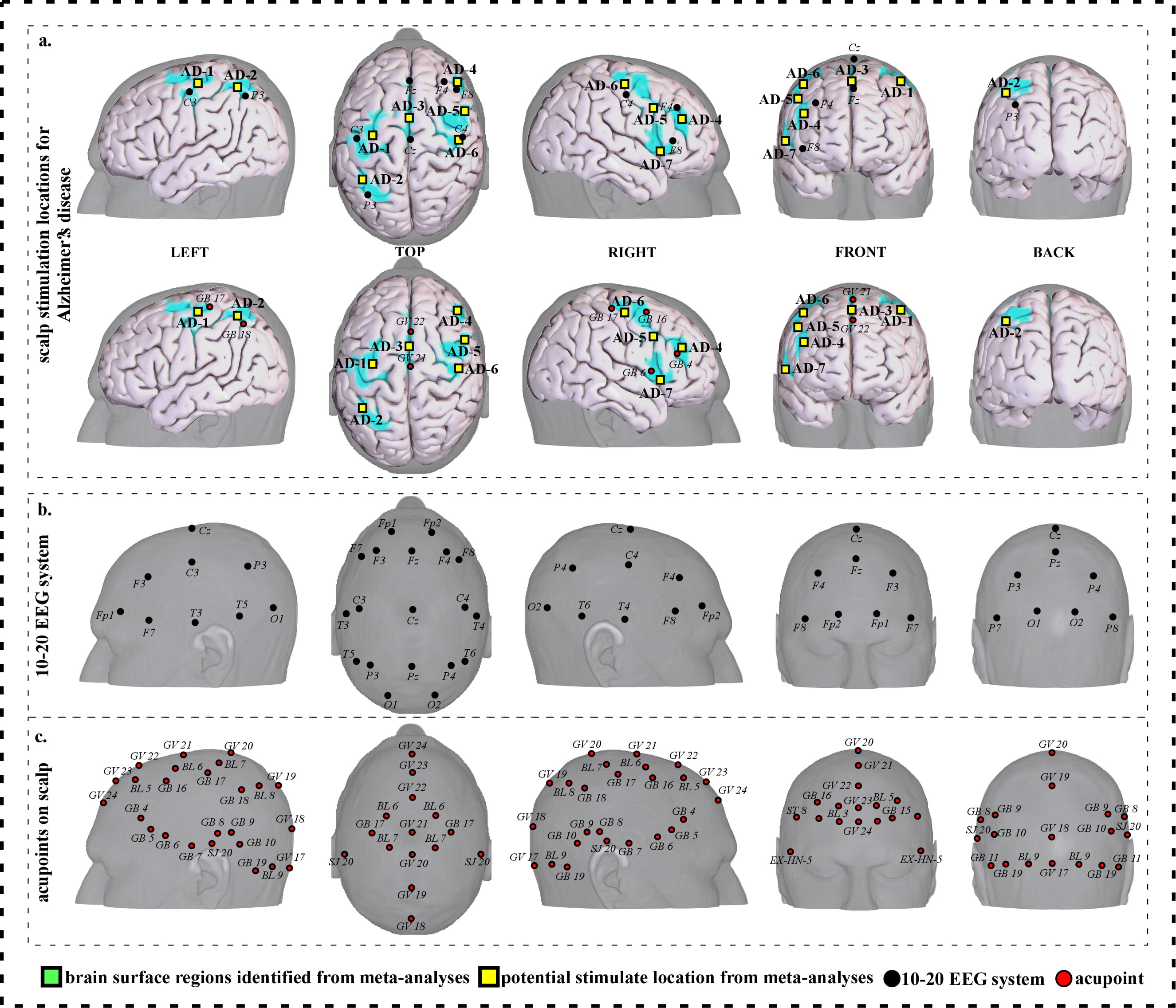 Fig. 2.
Fig. 2.Neuroimaging-based scalp stimulation protocols for Alzheimer’s disease. (a) Scalp stimulation locations for Alzheimer’s disease. Upper and lower panels of a to g. applied the 10–20 EEG system, and international acupoints, respectively, to facilitate identifying the locations. (b) 10–20 EEG system locations. (c) Acupoint locations. Abbreviations: AD, Alzheimer’s disease; GV 17, Naohu; GV 18, Qiangjian; GV 19, Houding; GV 20, Baihui; GV 21, Qianding; GV 22, Xinhui; GV 23, Shangxing; GV 24, Shenting; GB 4, Hanyan; GB 5, Xuanlu; GB 6, Xuanli; GB 7, Qubin; GB 8, Shuaigu; GB 9, Tianchong; GB 10, Fubai; GB 11, Touqiaoyin; GB 15, Toulinqi; GB 16, Muchuang; GB 17, Zhengying; GB 18, Chengling; GB 19, Naokong; BL 5, Wuchu; BL 6, Chengguang; BL 7, Tongtian; BL 8, Luoque; BL 9, Yuzhen; SJ 20, Jiaosun; ST 8, Touwei; EX-HN-5, Taiyang.
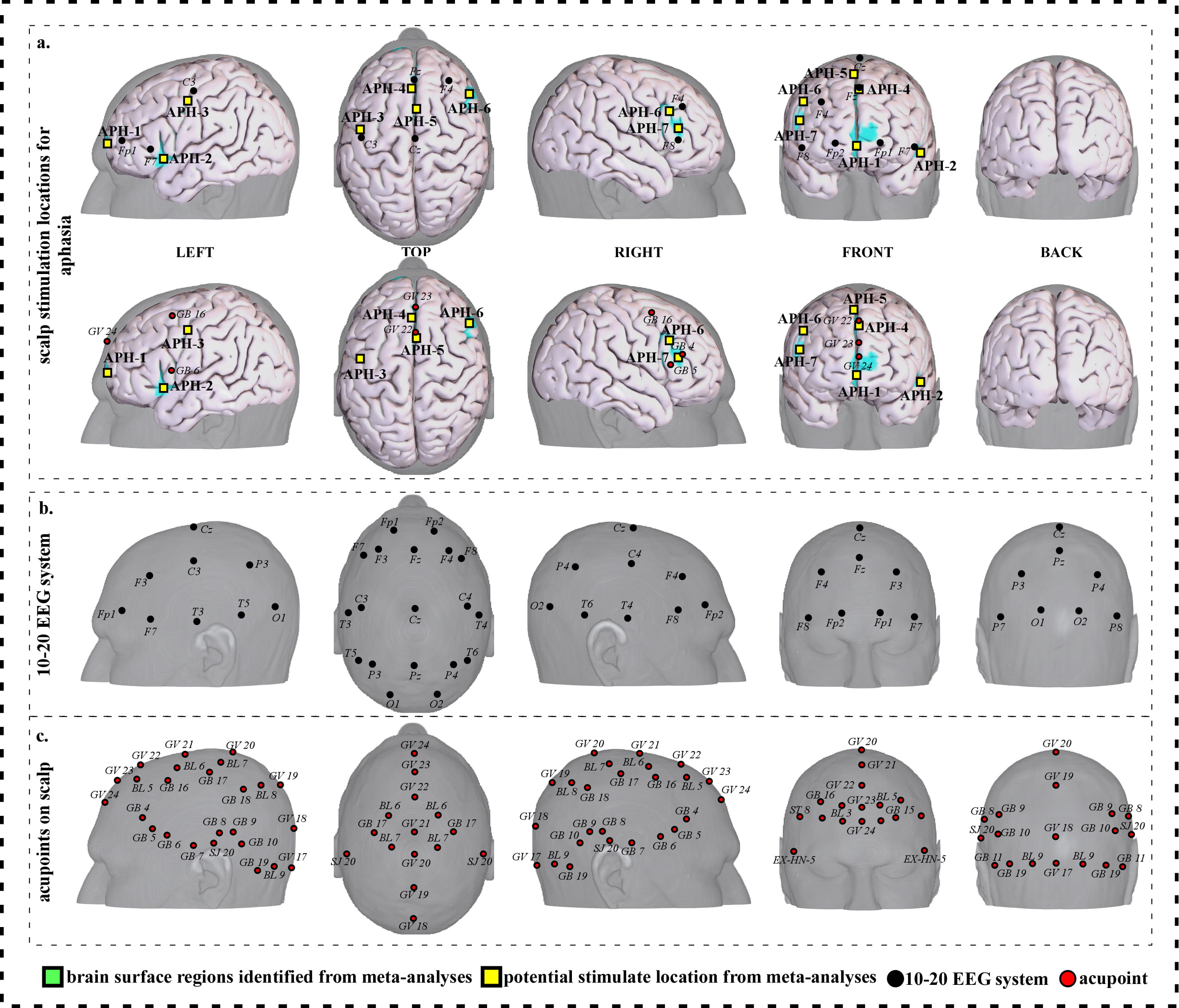 Fig. 3.
Fig. 3.Neuroimaging-based scalp stimulation protocols for aphasia. (a) Scalp stimulation locations for aphasia. Upper and lower panels of a to g. applied the 10–20 EEG system, and international acupoints, respectively, to facilitate identifying the locations. (b) 10–20 EEG system locations. (c) Acupoint locations. Abbreviations: AD, Alzheimer’s disease; GV 17, Naohu; GV 18, Qiangjian; GV 19, Houding; GV 20, Baihui; GV 21, Qianding; GV 22, Xinhui; GV 23, Shangxing; GV 24, Shenting; GB 4, Hanyan; GB 5, Xuanlu; GB 6, Xuanli; GB 7, Qubin; GB 8, Shuaigu; GB 9, Tianchong; GB 10, Fubai; GB 11, Touqiaoyin; GB 15, Toulinqi; GB 16, Muchuang; GB 17, Zhengying; GB 18, Chengling; GB 19, Naokong; BL 5, Wuchu; BL 6, Chengguang; BL 7, Tongtian; BL 8, Luoque; BL 9, Yuzhen; SJ 20, Jiaosun; ST 8, Touwei; EX-HN-5, Taiyang.
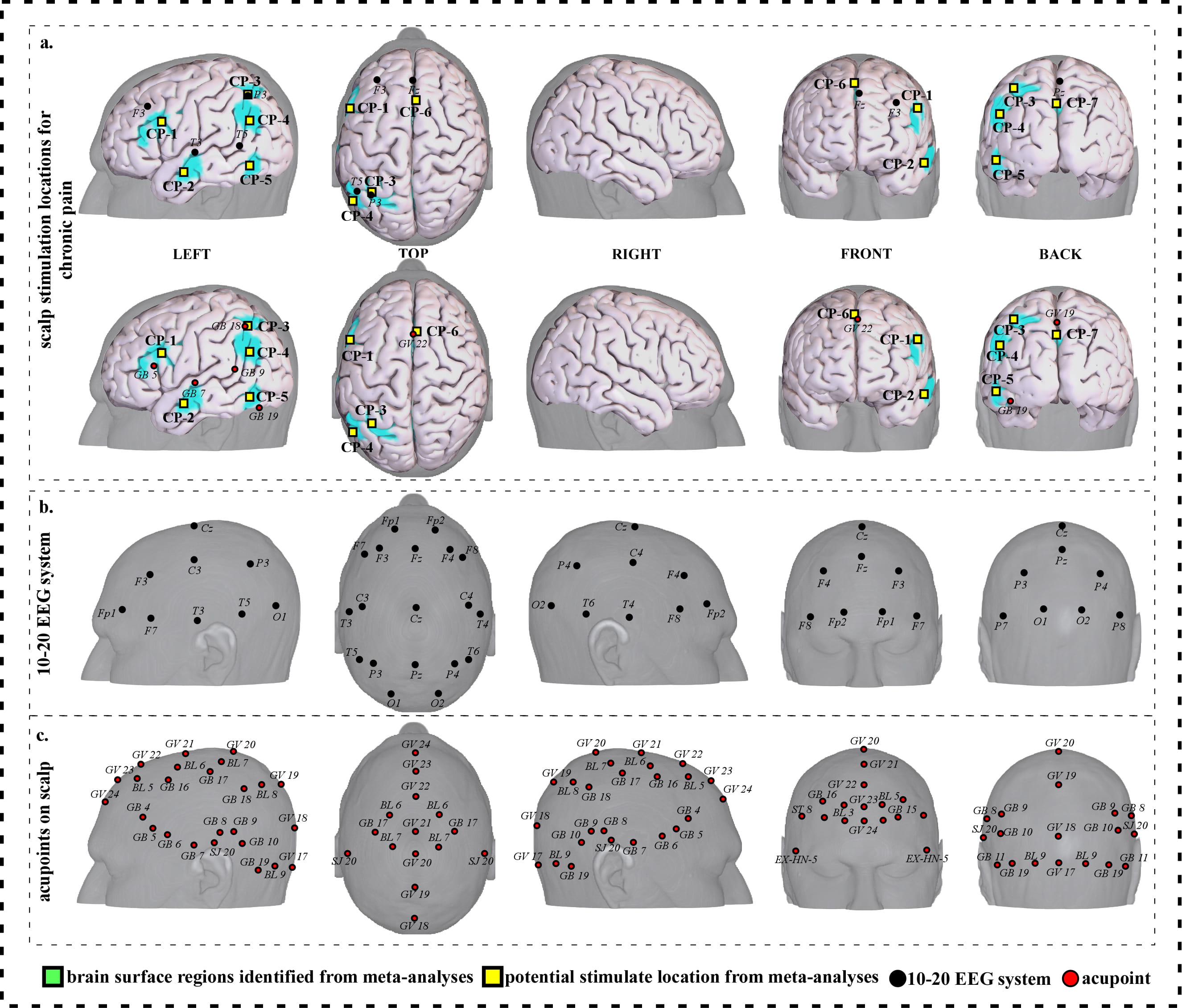 Fig. 4.
Fig. 4.Neuroimaging-based scalp stimulation protocols for chronic pain. (a) Scalp stimulation locations for chronic pain. Upper and lower panels of a to g. applied the 10–20 EEG system, and international acupoints, respectively, to facilitate identifying the locations. (b) 10–20 EEG system locations. (c) Acupoint locations. Abbreviations: AD, Alzheimer’s disease; GV 17, Naohu; GV 18, Qiangjian; GV 19, Houding; GV 20, Baihui; GV 21, Qianding; GV 22, Xinhui; GV 23, Shangxing; GV 24, Shenting; GB 4, Hanyan; GB 5, Xuanlu; GB 6, Xuanli; GB 7, Qubin; GB 8, Shuaigu; GB 9, Tianchong; GB 10, Fubai; GB 11, Touqiaoyin; GB 15, Toulinqi; GB 16, Muchuang; GB 17, Zhengying; GB 18, Chengling; GB 19, Naokong; BL 5, Wuchu; BL 6, Chengguang; BL 7, Tongtian; BL 8, Luoque; BL 9, Yuzhen; SJ 20, Jiaosun; ST 8, Touwei; EX-HN-5, Taiyang.
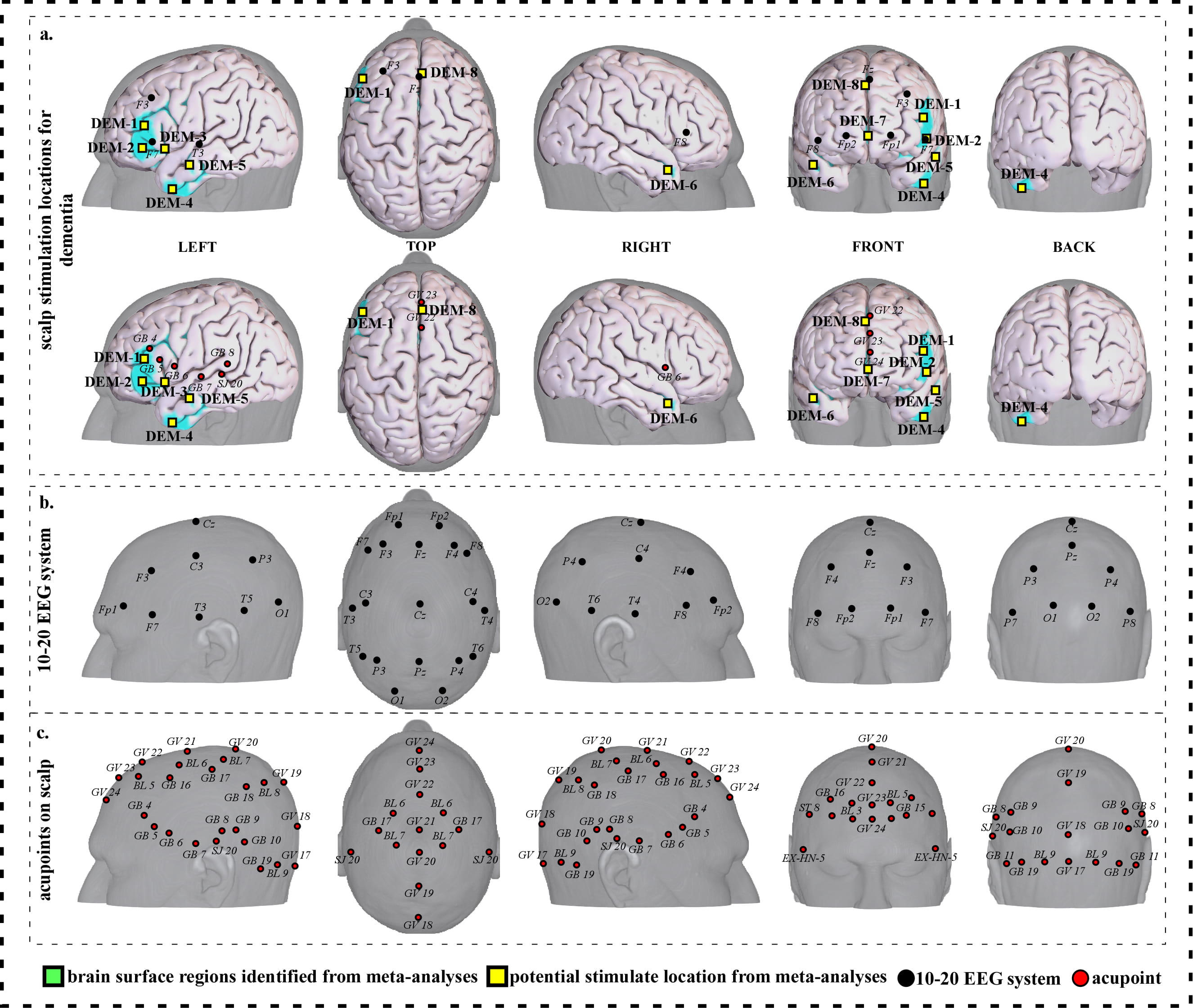 Fig. 5.
Fig. 5.Neuroimaging-based scalp stimulation protocols for dementia. (a) Scalp stimulation locations for dementia. Upper and lower panels of a to g. applied the 10–20 EEG system, and international acupoints, respectively, to facilitate identifying the locations. (b) 10–20 EEG system locations. (c) Acupoint locations. Abbreviations: AD, Alzheimer’s disease; GV 17, Naohu; GV 18, Qiangjian; GV 19, Houding; GV 20, Baihui; GV 21, Qianding; GV 22, Xinhui; GV 23, Shangxing; GV 24, Shenting; GB 4, Hanyan; GB 5, Xuanlu; GB 6, Xuanli; GB 7, Qubin; GB 8, Shuaigu; GB 9, Tianchong; GB 10, Fubai; GB 11, Touqiaoyin; GB 15, Toulinqi; GB 16, Muchuang; GB 17, Zhengying; GB 18, Chengling; GB 19, Naokong; BL 5, Wuchu; BL 6, Chengguang; BL 7, Tongtian; BL 8, Luoque; BL 9, Yuzhen; SJ 20, Jiaosun; ST 8, Touwei; EX-HN-5, Taiyang.
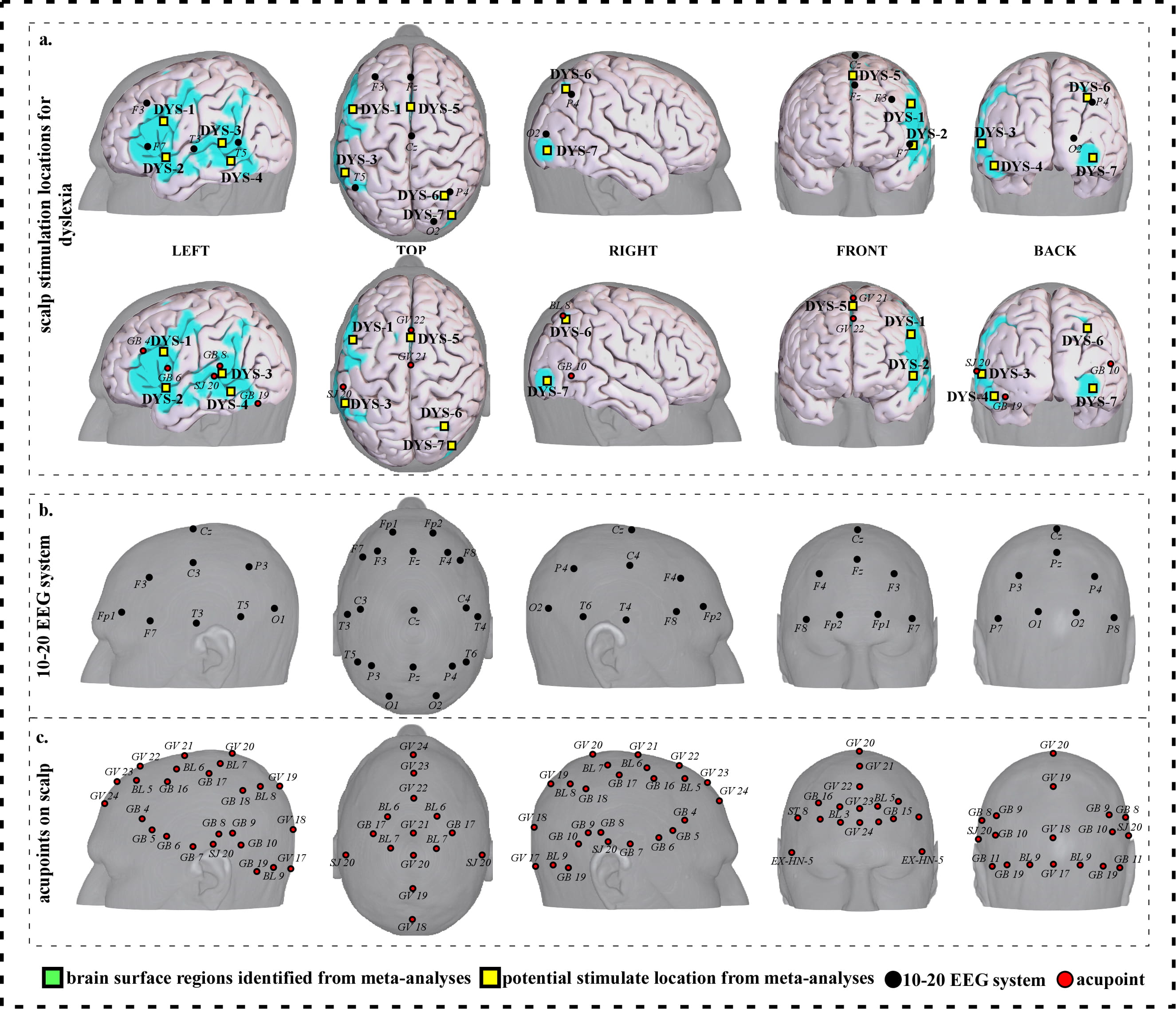 Fig. 6.
Fig. 6.Neuroimaging-based scalp stimulation protocols for dyslexia. (a) Scalp stimulation locations for dyslexia. Upper and lower panels of a to g. applied the 10–20 EEG system, and international acupoints, respectively, to facilitate identifying the locations. (b) 10–20 EEG system locations. (c) Acupoint locations. Abbreviations: AD, Alzheimer’s disease; GV 17, Naohu; GV 18, Qiangjian; GV 19, Houding; GV 20, Baihui; GV 21, Qianding; GV 22, Xinhui; GV 23, Shangxing; GV 24, Shenting; GB 4, Hanyan; GB 5, Xuanlu; GB 6, Xuanli; GB 7, Qubin; GB 8, Shuaigu; GB 9, Tianchong; GB 10, Fubai; GB 11, Touqiaoyin; GB 15, Toulinqi; GB 16, Muchuang; GB 17, Zhengying; GB 18, Chengling; GB 19, Naokong; BL 5, Wuchu; BL 6, Chengguang; BL 7, Tongtian; BL 8, Luoque; BL 9, Yuzhen; SJ 20, Jiaosun; ST 8, Touwei; EX-HN-5, Taiyang.
 Fig. 7.
Fig. 7.Neuroimaging-based scalp stimulation protocols for mild cognitive impairment. (a) Scalp stimulation locations for mild cognitive impairment. Upper and lower panels of a to g. applied the 10–20 EEG system, and international acupoints, respectively, to facilitate identifying the locations. (b) 10–20 EEG system locations. (c) Acupoint locations. Abbreviations: AD, Alzheimer’s disease; GV 17, Naohu; GV 18, Qiangjian; GV 19, Houding; GV 20, Baihui; GV 21, Qianding; GV 22, Xinhui; GV 23, Shangxing; GV 24, Shenting; GB 4, Hanyan; GB 5, Xuanlu; GB 6, Xuanli; GB 7, Qubin; GB 8, Shuaigu; GB 9, Tianchong; GB 10, Fubai; GB 11, Touqiaoyin; GB 15, Toulinqi; GB 16, Muchuang; GB 17, Zhengying; GB 18, Chengling; GB 19, Naokong; BL 5, Wuchu; BL 6, Chengguang; BL 7, Tongtian; BL 8, Luoque; BL 9, Yuzhen; SJ 20, Jiaosun; ST 8, Touwei; EX-HN-5, Taiyang.
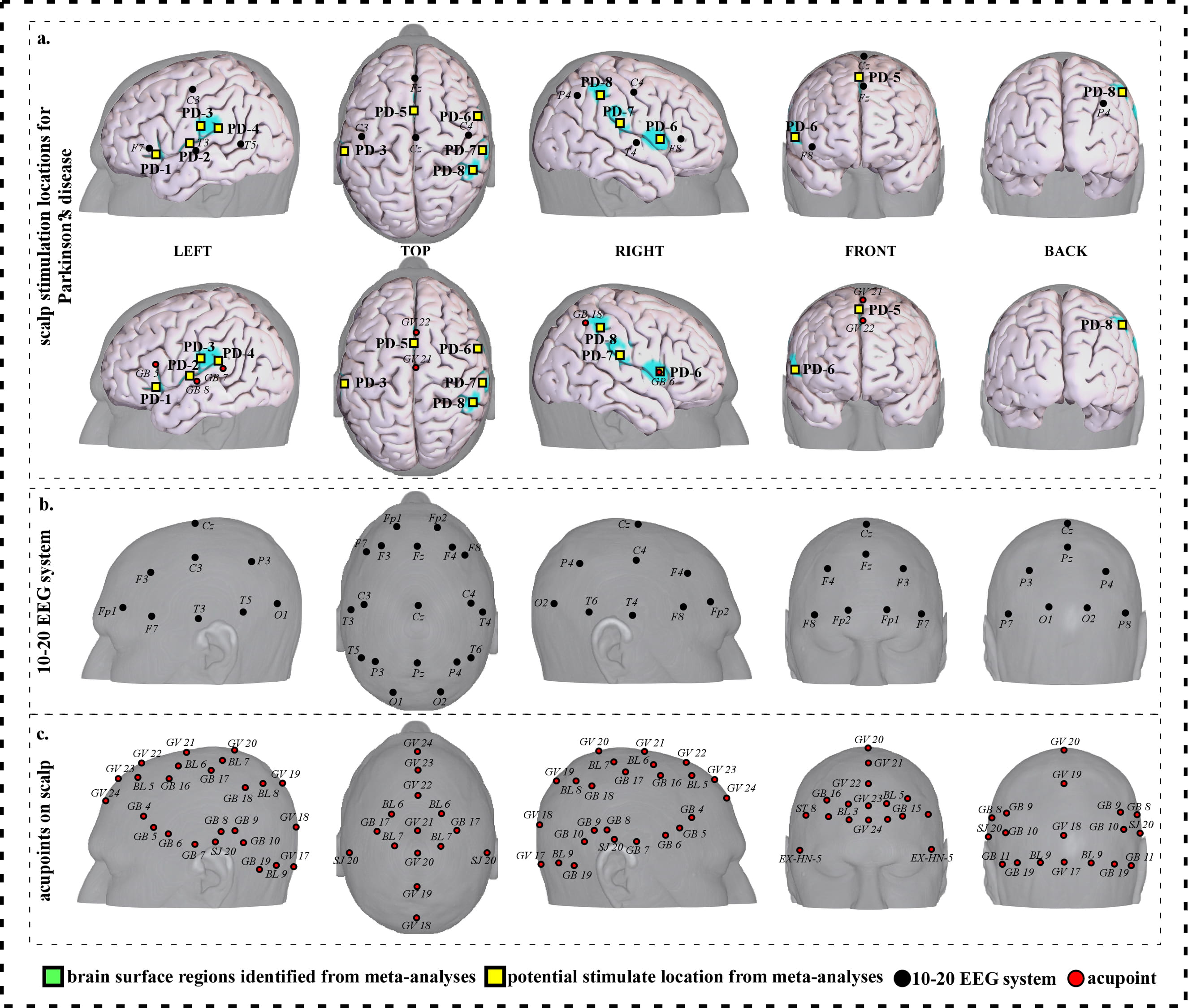 Fig. 8.
Fig. 8.Neuroimaging-based scalp stimulation protocols for Parkinson’s disease. (a) Scalp stimulation locations for Parkinson’s disease. Upper and lower panels of a to g. applied the 10–20 EEG system, and international acupoints, respectively, to facilitate identifying the locations. (b) 10–20 EEG system locations. (c) Acupoint locations. Abbreviations: AD, Alzheimer’s disease; GV 17, Naohu; GV 18, Qiangjian; GV 19, Houding; GV 20, Baihui; GV 21, Qianding; GV 22, Xinhui; GV 23, Shangxing; GV 24, Shenting; GB 4, Hanyan; GB 5, Xuanlu; GB 6, Xuanli; GB 7, Qubin; GB 8, Shuaigu; GB 9, Tianchong; GB 10, Fubai; GB 11, Touqiaoyin; GB 15, Toulinqi; GB 16, Muchuang; GB 17, Zhengying; GB 18, Chengling; GB 19, Naokong; BL 5, Wuchu; BL 6, Chengguang; BL 7, Tongtian; BL 8, Luoque; BL 9, Yuzhen; SJ 20, Jiaosun; ST 8, Touwei; EX-HN-5, Taiyang.
| Cluster ID | Cluster size | Peak T | Peak coordinates | 10–20 EEG system locations | Acupoint locations | ||
| x | y | z | |||||
| AD-1 | 878 | 8.84 | –34 | –18 | 54 | Approximately 1 cm superior and posterior to C3 | Approximately 0.3 cun inferior and anterior to GB 17 |
| AD-2 | 194 | 7.54 | –34 | –52 | 52 | Approximately 1 cm superior and anterior to P3 | Approximately 0.1 cun superior and anterior to GB 18 |
| AD-3 | 1211 | 11.44 | 4 | 2 | 58 | Approximate midpoint of Fz and Cz | Approximate midpoint of GV 21 and GV 22 |
| AD-4 | 120 | 6.89 | 42 | 36 | 22 | Approximately 1 cm inferior and anterior to F4 | Approximately 0.1 cun superior and anterior to GB 4 |
| AD-5 | 137 | 7.54 | 50 | 8 | 36 | Approximately 2 cm posterior to F4 | Approximate midpoint of GB 4 and GB 16 |
| AD-6 | 373 | 6.24 | 42 | –18 | 54 | Approximately 0.5 cm superior to C4 | Approximate midpoint of GB 16 and GB 17 |
| AD-7 | 167 | 8.19 | 46 | 16 | 2 | Approximately 2 cm and inferior posterior to F8 | Approximately 0.3 cun inferior and anterior to GB 6 |
| Abbreviations: L, left; R, right; AD, Alzheimer disease; cm, centimeter; cun, Chinese inches, 1 cun is about 3.33 centimeters; GV 21, Qianding; GV 22, Xinhui; GB 4, Hanyan; GB 6, Xuanli; GB 16, Muchuang; GB 17, Zhengying; GB 18, Chengling. | |||||||
| Cluster ID | Cluster size | Peak T | Peak coordinates | 10–20 EEG system locations | Acupoint locations | ||
| x | y | z | |||||
| APH-1 | 216 | 7.10 | 0 | 50 | –6 | Approximate midpoint of Fp1 and Fp2 | Approximately 0.6 cun anterior to GV 24 |
| APH-2 | 77 | 7.10 | –46 | 16 | –4 | Approximately 1 cm inferior and posterior to F7 | Approximately 0.6 cun inferior and anterior to GB 6 |
| APH-3 | 42 | 5.08 | –40 | –6 | 44 | Approximately 1 cm inferior and anterior to C3 | Approximately 0.6 cun inferior and posterior to GB 16 |
| APH-4 | 105 | 8.11 | –2 | 34 | 36 | Approximately 0.5 cm posterior to Fz | Approximate midpoint of GV 22 and GV 23 |
| APH-5 | 88 | 7.10 | 2 | 20 | 42 | Approximate midpoint of Fz and Cz | Approximately 0.1 cun posterior to GV 22 |
| APH-6 | 82 | 7.10 | 44 | 18 | 30 | Approximately 1 cm inferior and posterior to F4 | Approximate midpoint of GB 4 and GB 16 |
| APH-7 | 63 | 6.09 | 48 | 28 | 18 | Approximately 1 cm superior to F8 | Approximate midpoint of GB 4 and GB 5 |
| Abbreviations: L, left; R, right; APH, aphasia; cm, centimeter; cun, Chinese inches, 1 cun is about 3.33 centimeters; GV 22, Xinhui; GV 23, Shangxing; GV 24, Shenting; GB 4, Hanyan; GB 5, Xuanlu; GB 6, Xuanli; GB 16, Muchuang. | |||||||
| Cluster ID | Cluster size | Peak T | Peak coordinates | 10–20 EEG system locations | Acupoint locations | ||
| x | y | z | |||||
| CP-1 | 371 | 8.84 | –50 | 12 | 26 | Approximately 2 cm inferior and posterior to F3 | Approximately 0.3 cun superior and posterior to GB 5 |
| CP-2 | 146 | 7.77 | –56 | –10 | –16 | Approximately 2 cm inferior and anterior to T3 | Approximately 0.6 cun inferior and anterior to GB 7 |
| CP-3 | 164 | 7.77 | –32 | –58 | 54 | Approximately P3 | Approximately GB 18 |
| CP-4 | 211 | 7.23 | –48 | –62 | 28 | Approximately 2 cm inferior to P3 | Approximately 0.6 cun superior and posterior to GB 7 |
| CP-5 | 120 | 7.77 | –46 | –62 | –10 | Approximately 2 cm inferior and posterior to T5 | Approximately 0.3 cun superior and anterior to GB 19 |
| CP-6 | 319 | 8.31 | 8 | 20 | 44 | Approximately 1 cm posterior to Fz | Approximately GV 22 |
| CP-7 | 789 | 9.38 | –6 | –52 | 16 | Approximately 2 cm posterior to Pz | Approximately 0.3 cun posterior to GV 19 |
| Abbreviations: L, left; R, right; CP, chronic pain; cm, centimeter; cun, Chinese inches, 1 cun is about 3.33 centimeters; GV 19, Houding; GV 22, Xinhui; GB 5, Xuanlu; GB 7, Qubin; GB 18, Chengling; GB 19, Naokong. | |||||||
| Cluster ID | Cluster size | Peak T | Peak coordinates | 10–20 EEG system locations | Acupoint locations | ||
| x | y | z | |||||
| DEM-1 | 292 | 7.09 | –46 | 32 | 16 | Approximately 2 cm inferior and anterior to F3 | Approximately 0.3 cun inferior and anterior to GB 4 |
| DEM-2 | 44 | 5.56 | –50 | 34 | –4 | Approximately 1 cm inferior and anterior to F7 | Approximately 0.6 cun inferior and anterior to GB 5 |
| DEM-3 | 106 | 6.32 | –38 | 22 | –4 | Approximately 1 cm inferior and posterior to F7 | Approximately 0.3 cun inferior and anterior to GB 6 |
| DEM-4 | 71 | 6.32 | –46 | 4 | –42 | Approximately 3 cm inferior and anterior to T3 | Approximately 1 cun inferior to GB 6 |
| DEM-5 | 107 | 7.85 | –56 | –10 | –18 | Approximately 1.5 cm inferior and anterior to T3 | Approximately 0.5 cun inferior and anterior to GB 7 |
| DEM-6 | 47 | 7.09 | 46 | 10 | –24 | Approximately 3 cm inferior and posterior to F8 | Approximately 0.6 cun inferior to GB 6 |
| DEM-7 | 49 | 6.32 | 4 | 50 | 0 | Approximate midpoint of Fp1 and Fp2 | Approximately 0.3 cun anterior to GV 24 |
| DEM-8 | 57 | 6.32 | 10 | 12 | 46 | Approximately Fz | Approximate midpoint of GV 22 and GV 23 |
| Abbreviations: L, left; R, right; DEM, dementia; cm, centimeter; cun, Chinese inches, 1 cun is about 3.33 centimeters; GV 22, Xinhui; GV 23, Shangxing; GV 24, Shenting; GB 4, Hanyan; GB 5, Xuanlu; GB 6, Xuanli; GB 7, Qubin. | |||||||
| Cluster ID | Cluster size | Peak T | Peak coordinates | 10–20 EEG system locations | Acupoint locations | ||
| x | y | z | |||||
| DYS-1 | 1830 | 15.14 | –44 | 6 | 28 | Approximately 2 cm inferior and posterior to F3 | Approximately 0.6 cun posterior to GB 4 |
| DYS-2 | 207 | 7.07 | –50 | 6 | –6 | Approximately 2 cm inferior and posterior to F7 | Approximately 0.6 cun inferior to GB 6 |
| DYS-3 | 1809 | 12.11 | –52 | –46 | 4 | Approximately 1 cm anterior to T5 | Approximately 0.1 cun posterior to SJ 20 |
| DYS-4 | 361 | 12.11 | –42 | –50 | –16 | Approximately 1.5 cm inferior and anterior to T5 | Approximate midpoint of SJ 20 and GB 19 |
| DYS-5 | 504 | 12.11 | –2 | 8 | 54 | Approximate midpoint of Fz and Cz | Approximate midpoint of GV 21 and GV 22 |
| DYS-6 | 120 | 8.08 | 28 | –62 | 44 | Approximately 0.5 cm superior and posterior to P4 | Approximately BL 8 |
| DYS-7 | 110 | 9.09 | 38 | –86 | –6 | Approximately 1 cm inferior to O2 | Approximately 0.6 cun inferior and posterior to GB 10 |
| Abbreviations: L, left; R, right; DYS, dyslexia; cm, centimeter; cun, Chinese inches, 1 cun is about 3.33 centimeters; GV 21, Qianding; GV 22, Xinhui; GB 4, Hanyan; GB 6, Xuanli; GB 10, Fubai; GB 19, Naokong; BL 8, Luoque; SJ 20, Jiaosun. | |||||||
| Cluster ID | Cluster size | Peak T | Peak coordinates | 10–20 EEG system locations | Acupoint locations | ||
| x | y | z | |||||
| MCI-1 | 157 | 11.61 | –46 | 28 | 26 | Approximately 1 cm inferior to F3 | Approximately GB 4 |
| MCI-2 | 331 | 8.88 | –34 | 26 | –4 | Approximately F7 | Approximately 0.5 cun inferior to GB 5 |
| MCI-3 | 35 | 6.16 | –52 | –60 | 28 | Approximately 2 cm inferior and anterior to P3 | Approximately 0.3 cun superior and posterior to GB 9 |
| MCI-4 | 368 | 8.88 | 44 | 38 | 28 | Approximately 0.5 cm inferior and anterior to F4 | Approximately GB 4 |
| MCI-5 | 73 | 6.16 | 58 | –46 | 8 | Approximately 0.5 cm superior to T6 | Approximately GB 9 |
| MCI-6 | 75 | 7.06 | 32 | –62 | 44 | Approximately 0.5 cm superior and posterior to P4 | Approximately BL 8 |
| MCI-7 | 212 | 10.70 | 32 | 24 | –6 | Approximately 0.5 cm inferior and posterior to F8 | Approximately 0.2 cun inferior and anterior to GB 6 |
| MCI-8 | 49 | 6.16 | 30 | 28 | –18 | Approximately 1.5 cm inferior to F8 | Approximately 0.6 cun inferior and anterior to GB 6 |
| Abbreviations: L, left; R, right; MCI, mild cognitive impairment; cm, centimeter; cun, Chinese inches, 1 cun is about 3.33 centimeters; GB 4, Hanyan; GB 5, Xuanlu; GB 6, Xuanli; GB 9, Tianchong; BL 8, Luoque. | |||||||
| Cluster ID | Cluster size | Peak T | Peak coordinates | 10–20 EEG system locations | Acupoint locations | ||
| x | y | z | |||||
| PD-1 | 64 | 8.72 | –38 | 24 | 0 | Approximately 0.5 cm inferior and posterior to F7 | Approximately 0.5 cun inferior to GB 5 |
| PD-2 | 63 | 6.03 | –44 | –6 | 6 | Approximately 0.5 cm superior and anterior to T3 | Approximately 0.1 cun superior and anterior to GB 8 |
| PD-3 | 63 | 6.93 | –40 | –18 | 12 | Approximately 2 cm inferior and posterior to C3 | Approximately 0.5 cun superior and anterior to GB 7 |
| PD-4 | 110 | 6.93 | –56 | –26 | 24 | Approximately 2cm superior and anterior to T5 | Approximately 0.1 cun superior and anterior to GB 7 |
| PD-5 | 102 | 7.82 | –2 | 8 | 44 | Approximate midpoint of Fz and Cz | Approximate midpoint of GV 21 and GV 22 |
| PD-6 | 63 | 6.03 | 60 | 8 | 8 | Approximate midpoint of F8 and T4 | Approximately GB 6 |
| PD-7 | 200 | 8.72 | 58 | –22 | 22 | Approximate midpoint of F8 and P4 | Approximate midpoint of GB 6 and GB 18 |
| PD-8 | 68 | 6.03 | 52 | –40 | 48 | Approximate midpoint of C4 and P4 | Approximately 0.3 cun inferior and anterior to GB 18 |
| Abbreviations: L, left; R, right; PD, Parkinson disease; cm, centimeter; cun, Chinese inches, 1 cun is about 3.33 centimeters; Baihui; GV 21, Qianding; GV 22, GB 5, Xuanlu; GB 6, Xuanli; GB 7, Qubin; GB 8, GB 18, Chengling. | |||||||
We proposed seven potential targets for treating Alzheimer’s disease (named AD-1 to AD-7). These targets are located in the bilateral frontal gyrus, precentral and postcentral gyrus, the left parietal lobe and precuneus, and the right supplementary motor area (Table 1, Fig. 2).
We proposed seven potential targets for treating aphasia (named APH-1 to APH-7). These targets are located in the bilateral frontal gyrus, the left precentral gyrus, and the right supplementary motor area (Table 2, Fig. 3).
We proposed seven potential targets for treating chronic pain (named CP-1 to CP-7). These targets are located in the bilateral frontal gyrus, the left parietal lobe supramarginal gyrus, angular gyrus, precuneus/cuneus, temporal gyrus and occipital gyrus, and the right supplementary motor area (Table 3, Fig. 4).
We proposed eight potential targets for treating dementia (named DEM-1 to DEM-8). These targets are located in the bilateral frontal gyrus and temporal gyrus, and the right supplementary motor area (Table 4, Fig. 5).
We proposed seven potential targets for treating dyslexia (named DYS-1 to DYS-7). These targets are located in the bilateral frontal gyrus, parietal lobe, precuneus, angular gyrus, the left supplementary motor area, supramarginal gyrus, temporal gyrus, precentral gyrus and Rolandic operculum, and the right occipital gyrus (Table 5, Fig. 6).
We proposed eight potential targets for treating mild cognitive impairment (named MCI-1 to MCI-8). These targets are located in the bilateral frontal gyrus, parietal lobe, temporal gyrus, angular gyrus, the left supramarginal gyrus, and the right precentral gyrus (Table 6, Fig. 7).
We proposed eight potential targets for treating Parkinson disease (named PD-1 to PD-8). These targets are located in the bilateral frontal gyrus, precentral gyrus and Rolandic operculum, the left parietal lobe, supplementary motor area, postcentral gyrus and supramarginal gyrus (Table 7, Fig. 8).
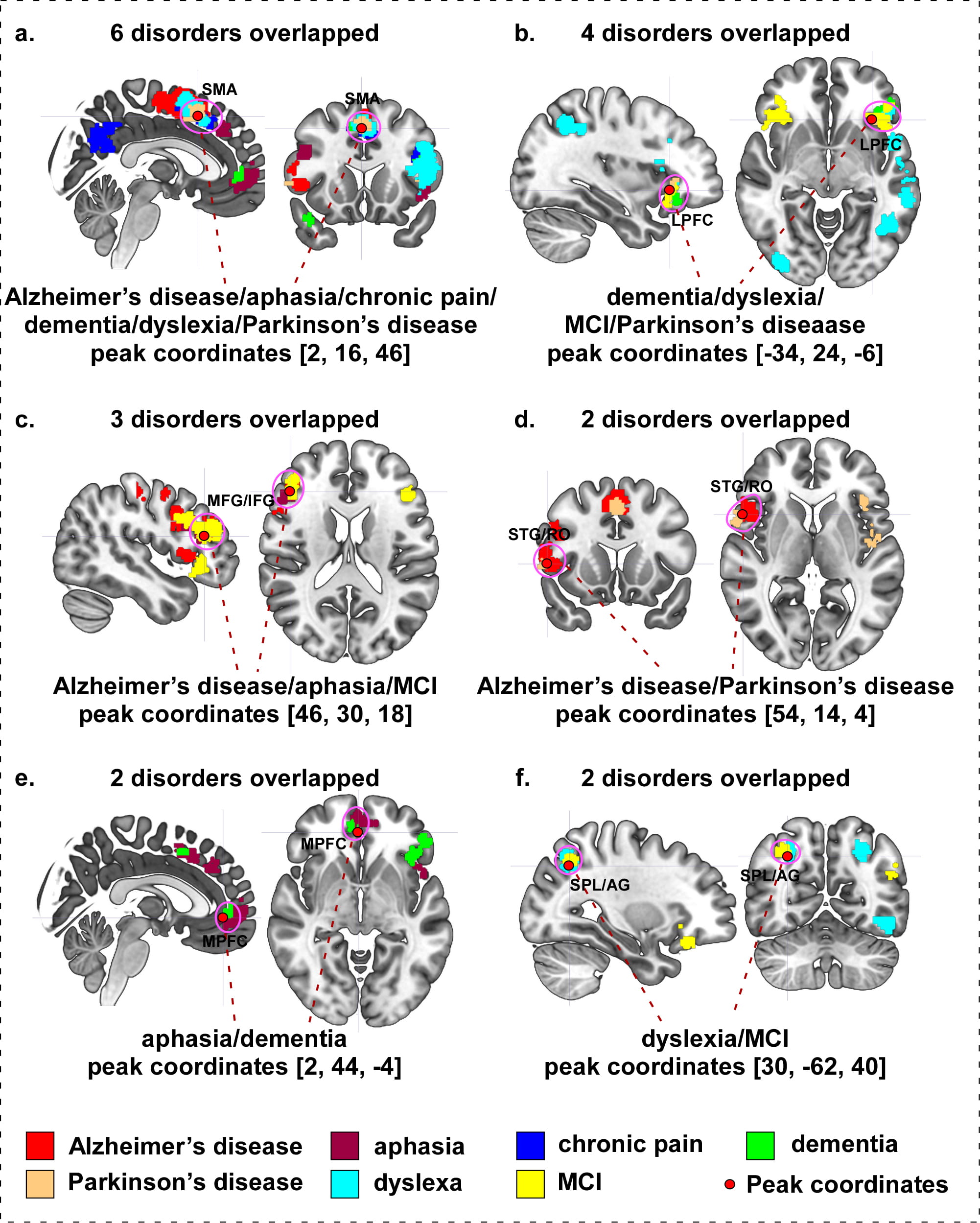
We investigated overlapping brain areas across different neurological disorders (see Table 8 and Fig. 9 for details). We found that: (a) Alzheimer’s disease, aphasia, chronic pain, dementia, dyslexia, and Parkinson’s disease show an overlap on the right supplementary motor area (SMA)/medial frontal gyrus (MedFG) (Fig. 9a); (b) the left lateral prefrontal cortex (LPFC) and inferior frontal gyrus (IFG)/orbital inferior frontal gyrus (OrbIFG) are involved in dementia, dyslexia, MCI and Parkinson’s disease (Fig. 9b); (c) the right middle frontal gyrus (MFG)/inferior frontal gyrus (IFG) are involved in Alzheimer’s disease, aphasia, and MCI (Fig. 9c); (d) Alzheimer’s disease and Parkinson’s disease display an overlap on the right superior temporal gyrus (STG)/Rolandic operculum (RO) (Fig. 9d); (e) the right medial prefrontal cortex (MPFC) are associated with aphasia and dementia (Fig. 9e); and (f) the right superior parietal lobule (SPL)/angular gyrus (AG) are involved in dyslexia and MCI (Fig. 9f).
| Cluster ID | Peak coordinates | Brain regions | Overlap disorders | ||
| x | y | z | |||
| A | 2 | 16 | 46 | R SMA/MedFG/SupMFG/SFG | 6 disorders: Alzheimer’s disease, aphasia, chronic pain, dementia, dyslexia, and Parkinson’s disease |
| B | –34 | 24 | –6 | L LPFC/IFG/OrbIFG/TriIFG/OperIFG/STG/MTG/ITG/SPL/IPL/PCu/PreCG/PoCG/SMG/AG/RO/MOG | 4 disorders: dementia, dyslexia, mild cognitive impairment, and Parkinson’s disease |
| C | 46 | 30 | 18 | R MFG/IFG/TriIFG/OperIFG/SFG/PreCG | 3 disorders: Alzheimer’s disease, aphasia, and mild cognitive impairment |
| D | 54 | 14 | 4 | R STG/RO/PreCG/IFG/OrbIFG/OperIFG/TriIFG/MFG | 2 disorders: Alzheimer’s disease and Parkinson’s disease |
| E | 2 | 44 | –4 | R MPFC/SupMFG/OrbMFG/SFG | 2 disorders: aphasia and dementia |
| F | 30 | –62 | 40 | R SPL/IPL/AG/SOG/MOG | 2 disorders: dyslexia and mild cognitive impairment |
| Abbreviations: L, left; R, right; IFG, inferior frontal gyrus; OrbIFG, orbital inferior frontal gyrus; OperIFG, opercular inferior frontal gyrus; TriIFG, triangular inferior frontal gyrus; OrbMFG, orbital medial frontal gyrus; SupMFG, superior medial frontal gyrus; LPFC, lateral prefrontal cortex; MPFC, medial prefrontal cortex; SFG, superior frontal gyrus; MFG, middle frontal gyrus; MTG, middle temporal gyrus; ITG, inferior temporal gyrus; STG, superior temporal gyrus; IPL, inferior parietal lobe; SPL, superior parietal lobe; SMA, supplementary motor area; SMG, supramarginal gyrus; PCu, precuneus; PreCG, precentral gyrus; PoCG, postcentral gyrus; RO, Rolandic operculum; MOG, middle occipital gyrus; SOG, superior occipital gyrus. | |||||
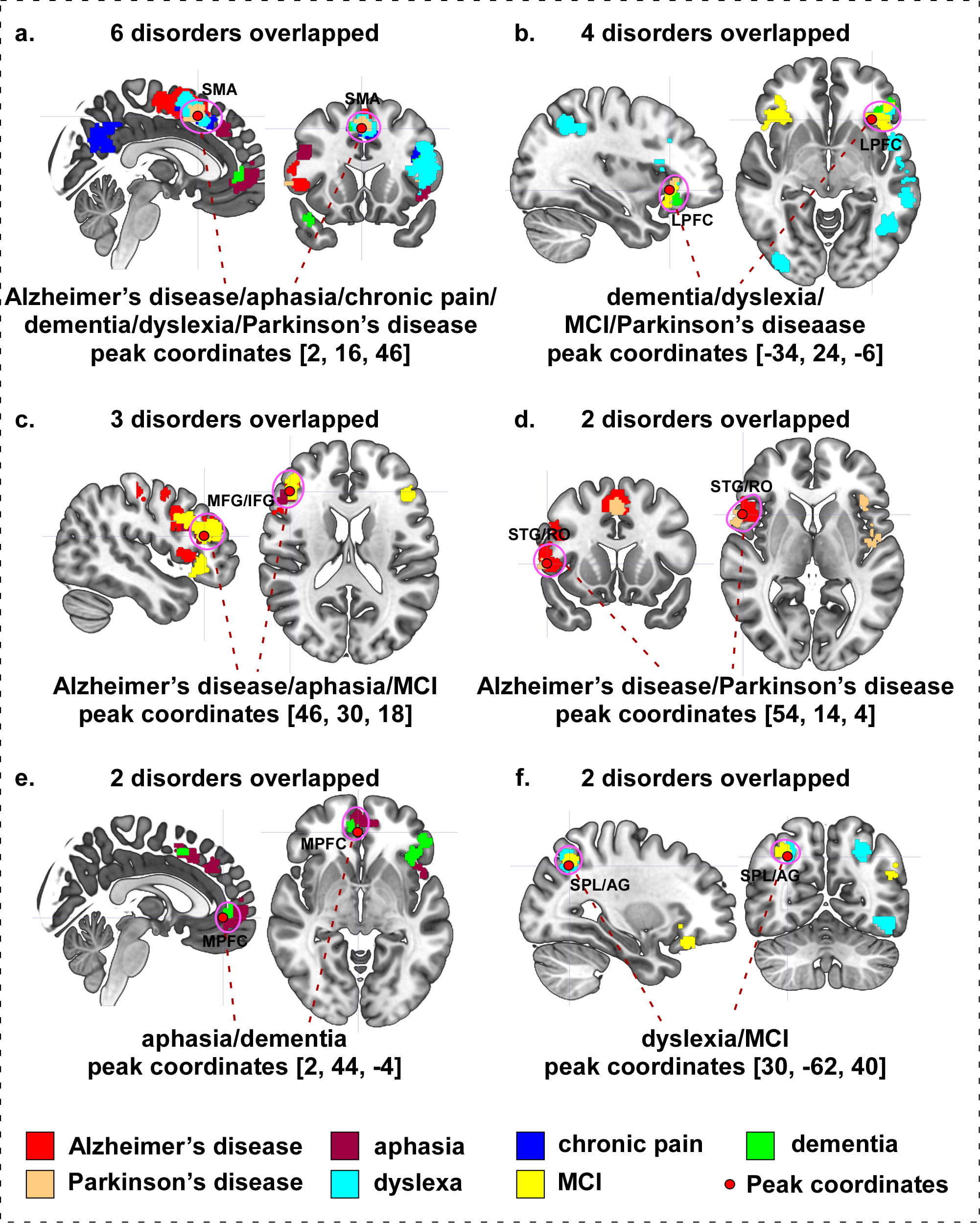 Fig. 9.
Fig. 9.Overlap surface regions among different neurological disorders. (a) Alzheimer’s disease, aphasia, chronic pain, dementia, dyslexia, and Parkinson’s disease overlapping on right SMA. (b) Dementia, dyslexia, mild cognitive impairment, and Parkinson’s disease overlapping on left dlPFC. (c) Alzheimer’s disease, aphasia, and mild cognitive impairment overlapping on right MFG/IFG. (d) Alzheimer’s disease and Parkinson’s disease overlapping on right STG/RO. (e) Aphasia and dementia overlapping on right MedFG. (f) Dyslexia and mild cognitive impairment overlapping on right SPL/AG. Abbreviation: MCI, mild cognitive impairment; SMA, supplementary motor area; LPFC, lateral prefrontal cortex; MPFC, medial prefrontal cortex; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; STG, superior temporal gyrus; RO, Rolandic operculum; SPL, superior parietal lobule; AG, angular gyrus.
This study aims to identify potential scalp stimulation targets in the treatment of seven common neurological disorders using a large-scale meta-analysis method. We selected seven to eight targets for each disorder and used both 10–20 EEG system and acupuncture points to locate these targets to facilitate its clinical application. We believe that these target protocols will provide more stimulation options in the treatment of neurological disorders using a scalp stimulation method.
As an automated tool, Neurosynth uses all applicable measurements such as activations, deactivations, connectivity, etc. appearing in a paper indiscriminately. This method may make the results difficult to interpretate. Although, it provides a way to use all data/results from different techniques and methods involved in each neurological disorder. Further, this did not impede Neurosynth from supplying robust quantitative reverse inference data consistent with other databases and methods of analysis [19, 25].
We have included chronic pain in this study, as chronic pain is a frequent component of many neurological disorders and affects about 20–40% of patients of many primary neurological diseases [26]. In addition, accumulating evidence has shown that the brain (central nerve system) plays an important role in chronic pain, and chronic pain is associated with profound brain function and structure alternations [27].
As expected, neurological disorders are associated with complicated brain circuits/networks. Our findings are consistent with previous brain imaging studies on Alzheimer’s disease [28, 29], aphasia [30, 31], chronic pain [32, 33], dementia [34, 35], dyslexia [36, 37], mild cognitive impairment [38, 39, 40], and Parkinson’s disease [41].
Although each neurological disorder is associated with specific characteristic symptoms and underlying mechanisms, the boundaries between certain neurological disorders may be complex and contentious. For example, mild cognitive impairment, Alzheimer’s disease, dementia, and Parkinson’s disease present overlapping/progressing clinical symptoms, particularly in the aspects of memory and cognitive decline [42, 43]. As different brain regions or circuits are involved in different functions, symptoms may be regarded as the effects of dysfunction in certain areas or circuits. Evidence has shown that brain regions and circuit implicated in different neurological disorders overlap and clinical symptoms may be largely shared among disorders [44, 45]. Thus, identifying overlapping regions associated with different neurological disorders may not only be useful in illuminating the common etiology of different neurological disorders, but also help to identify common treatment targets across these disorders.
We found that the SMA is conspicuously involved in six disorders, including Alzheimer’s disease, aphasia, chronic pain, dementia, dyslexia, and Parkinson’s disease. Although the primary function of SMA is to control physical movement, a number of studies have found that the SMA is also a preferential site of several different neurological disorders. For instance, a recent study found that SMA damage affects working memory, thus, working memory impairment may be part of SMA syndrome [46], which commonly presents as a transient of speech and motor function disturbance [47]. Vergani and colleagues have suggested that the SMA plays a critical role in the control of motor aspects of speech production based on its connectivity with Broca’s area [48], which is consistent with the speech impairment of Alzheimer’s disease, dementia, aphasia, dyslexia, and Parkinson’s disease.
Although the SMA is believed to primarily be a motor area, it is also involved in pain processing [49]. In a previous study, Misra and Coombes executed an fMRI study while subjects performed a motor control task, experienced a pain-eliciting stimulus on their hand, and performed the motor control task while also experiencing the pain-eliciting stimulus [50]. They found that when separate trials of motor control and pain processing were performed, overlapping functional activity was detected in the SMA. Also, as motor control and pain processing occurred simultaneously, SMA activity increased.
The prefrontal cortex is a multi-functional brain area involved in working memory and social and emotional function regulation (including behavioral control, decision making, etc.). We found that the left lateral prefrontal cortex (LPFC) is a notable region that contributes to four disorders: dementia, dyslexia, mild cognitive impairment, and Parkinson’s disease. Literature suggests that the LPFC has long been implicated in higher cognitive functions, such as attention switching and working memory formation [51, 52]. To explore the treatment effects of tDCS on patients with Parkinson’s disease, researchers applied anodal stimulation on the left LPFC and found a significant improvement of patients’ working memory [53], whereas anodal stimulation on both the left and right LPFC positively impacted the executive function [54]. Moreover, anodal tDCS targeted on Broca’s area (a brain region of the dominant hemisphere that adjacent to the LPFC with functions related to speech production) can produce positive effects on verbal fluency in patients with mild cognitive impairment [55, 56], this effect may also benefit patients with other disorders presenting speech impairments.
We also found an aphasia and dementia overlap in the medial prefrontal cortex (MPFC). A large body of evidence indicates that the MPFC is essential for theory of mind [57], response inhibition [58], reversal-learning [59], and emotional processing [60]. MPFC dysfunction is an early marker of behavioral variant frontotemporal dementia (bvFTD), the most commonly occurring subtype of frontotemporal dementia as characterized by progressive changes in personality and impaired social interaction [61]. In a previous study, Bertoux et al. [62] investigated the sensitivity and specificity of common MPFC specific tests in patient cohorts (bvFTD and Alzheimer’s disease) and age-matched healthy individuals. They found that the Mini-SEA test, which was employed to evaluate theory of mind and emotional processing, emerged as the most sensitive and specific of the MPFC tests employed, and may be used to discriminate bvFTD and Alzheimer’s disease.
In addition, our results showed that Alzheimer’s disease, aphasia, and mild cognitive disorder overlap in the middle/inferior frontal gyrus (MFG/IFG). These disorders present varying levels of language defects. Evidence for the MFG/IFG as a structure essential to speech and language function has been verified repeatedly through accumulating studies across a variety of neuroimaging approaches including functional magnetic resonance imaging [63], magnetoencephalography [64], positron emission tomography [65], and single-photon emission computed tomography [66]. These studies indicate that the MFG/IFG is involved in multiple language-specific tasks including phonologic, semantic, and sentence/discourse-level processing, as well as detection of the emotional content of speech [67]. Thus, the MFG/IFG possess the potential to be a scalp stimulation target in modulating disorders which exhibit language/speech-related disabilities.
We found the superior temporal gyrus (STG) and the Rolandic Operculum (RO) may be considered potential brain region targets in treating Alzheimer’s disease and Parkinson’s disease (two of the most common neurodegenerative diseases). A previous neuroimaging study found that Parkinson’s disease involves greater grey matter loss in frontal areas and the temporal lobe in Alzheimer’s disease relative to Parkinson’s disease dementia (PDD) [68]. These findings are consistent with another MRI study, in which researchers applied voxel-based morphometry (VBM) analysis in a group of PD patients with and without depression and reported gray matter volume reduction in the orbitofrontal cortex and the STG of PD patients with depression symptoms [69]. To investigate the clarification of regional morphologic changes in the brain associated with normal aging and AD, Ohnishi and colleagues perfromed an MRI study on 26 AD patients and 92 healthy individuals [70]. Negative correlations between age and regional gray matter volume of STG have been detected in healthy individuals (i.e., significant reduction of STG volume with age). Swallowing impairment is a growing concern in both AD [71] and PD patients [72], particularly in the disease’ later stages [73]. Numerous studies have suggested that the operculum, a critical cortical region in involved in normal swallowing, is affected by these diseases [74, 75]. For instance, Humbert et al. [73] revealed that AD patients had a significantly lower Blood-Oxygen-Level-Dependent (BOLD) response in many cortical areas that are traditionally involved in normal swallowing, especially in the Rolandic and frontal operculum.
Lastly, we found that dyslexia and mild cognitive impairment (MCI) display an overlap on the superior parietal lobe (SPL) and angular gyrus (AG). Converging evidence, from a number of lines of investigation, indicates that dyslexia may represent a disorder within the language system; such reading difficulties are also presented as prominent symptom in patients with MCI [76]. The French neurologist Dejerine highlighted the importance of the angular gyrus and the parietal lobe in reading performance as early as 1891 [77]. In a previous study, researchers found that dyslexic subjects showed a significant brain volume reduction in the right SPL compared to healthy controls [78]. Pugh et al. [79] also found that the AG is involved in reading dysfunction in dyslexic readers. Additional in vivo imaging studies have also linked dyslexia to abnormalities in the structures associated with the parietal and angular gyrus [80].
The stimulation targets we selected from the current study are partly consistent with the published prescriptions from scalp acupuncture (or traditional acupuncture), and neuromodulation studies [23, 81, 82, 83, 84, 85].
Take Parkinson’s disease for example, literature suggests that the MS 4 (Epangxian III, line 3 lateral to forehead,), MS 6 (Dingnie Qianxiexian, anterior oblique line of vertex-temporal), MD8 (Dingpangxian I, line 1 lateral to vertex, n), MS 9 (Dingpangxian II, line 2 lateral to vertex,), and MS 14 (Zhenxia Pangxian, lower-lateral line of occiput) are widely recognized for alleviating symptoms of Parkinson’s disease when using scalp acupuncture [6]. Overlapping regions exist in comparison of the neuroimaging-based targets and literature-documented locations, involving the SMA, PreCG, PoCG, SMG, and IPL. We also included the frontal gyrus (SFG/MFG/MedFG/IFG) into our proposed protocol. In parallel, as suggested by the literature, the occipital gyrus may be considered as a potential target in treating Parkinson’s disease [6], but our protocol did not include the occipital area.
In terms of neuromodulation interventions, most of the previous studies applied different neuromodulation techniques at primary motor cortex (M1) to improve the motor disability of PD. A recent literature review summarized the therapeutic application of repetitive transcranial magnetic stimulation (rTMS) in patients with Parkinson’s disease and found that stimulation targets of the twenty included research studies mainly targeted on the M1, SMA, dorsolateral prefrontal cortex (dlPFC), and MPFC [86]. In addition, tDCS on the M1 and dlPFC have been shown in several studies to ameliorate gait function in PD patients [87, 88]. Furthermore, patients with PD showed positive improvement of cortico-muscular coupling and motor performance after receiving 20 Hz tACS on the M1 [89].
In comparison with above studies, our targets also include additional brain regions, such as PoCG, Rolandic operculum, SMG, and IPL, which may expand the selection of potential targets in neuromodulation techniques for the treatment of Parkinson’s disease.
In the development of brain stimulation, how and where to stimulate remains a challenge for clinicians and researchers. In this study, we provide a set of stimulation location protocols for seven common neurological disorders based on the development of brain research using the Neurosynth. We believe that the brain regions included in the neuroimaging-based protocol reflect an enhanced understanding of the neural network involved in neurological disorders and thus, stimulating these brain regions using different techniques may hold the potential to regulate the pathophysiology associated these disorders and further relieve associated symptoms. Nevertheless, this assumption/hypothesis needs to be evaluated by additional clinical studies.
We provided multiple targets for each neurological disorder. Some of these targets may not have been used in previous studies, which provides more options for brain stimulation in neurological disorders. Readers may choose the targets based on symptoms associated with each individual patient and the function of different targets. To facilitate the reader to choose targets, we tried to summarize the functions of each identified brain regions; due to the complexity of the brain, we are almost certain these listed functions are incomplete and under certain circumstance, even may be misleading. The readers should make their judgement with cautions.
There are several limitations to our study. Since the protocol is based on the brain imaging meta-analysis, further clinical studies are needed to validate our findings. Additional functional (resting-state functional connectivity) [20, 21], and anatomical analyses (diffusion tensor imaging) [23, 90] may further enhance the proposed protocols, particularly in the target for patients at the individual level. The aim of this study was to explore potential targets for neurological disorders; the application and optimization of different scalp stimulation techniques to modulate these brain regions is beyond the scope of this manuscript. In order to facilitate clinical application, we simplified the protocol by including only seven or eight peak targets. Other brain regions may also play a critical role and should be considered in clinical practice. Finally, the exact location may alter if more studies are included in the analysis. Since scalp acupuncture may influence a relatively large area, the change should not influence the clinical effect significantly. Nevertheless, the protocol should be updated with the enhancement of our understanding of the pathology of these disorders and the brain function.
We identified neuroimaging-based scalp stimulation target protocols for the treatment of seven common neurological disorders. Our findings may facilitate and extend the clinical applications of scalp acupuncture, tES, and other neuromodulation techniques for the treatment of these neurological disorders.
Data supporting the findings of this study are from Neurosynth, a public platform for large-scale, automated synthesis of functional magnetic resonance imaging (fMRI) data.
Concept of the idea—JK, JC; data analysis—JC, JK; manuscript preparation—JC, JK, TCCZ, CMM.
Not applicable.
Not applicable.
Jian Kong is supported by R01 AT008563, R33 AT009310, R33 AT009341, R34 DA046635 (through the NIH HEAL Initiative), and R01 AG063975 from National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative.
The authors declare no conflict of interest. Jian Kong has a disclosure to report (holding equity in a startup company, MNT, and a patent to develop new peripheral neuromodulation devices).
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.jin2103083.