1 Clinic for Anesthesiology and Critical Care, Military Medical Academy, 11000 Belgrade, Serbia
2 Department of Physiology, Faculty of Medical Sciences, University of Kragujevac, 34000 Kragujevac, Serbia
3 Department of Science, Institute for Information Technologies Kragujevac, University of Kragujevac, 34000 Kragujevac, Serbia
4 Department of Histology and Embryology, Faculty of Medical Sciences, University of Kragujevac, 34000 Kragujevac, Serbia
† These authors contributed equally.
Abstract
Cisplatin therapy is often accompanied by neurotoxicity manifestation, and since the prefrontal cortex is strongly involved in emotion regulation, the aim of this study was to analyze the alterations in the oxidative and apoptotic status of this brain region, with its behavioral impact in rats, following cisplatin administration, with or without N-acetylcysteine supplementation. Thirty-two male Wistar albino rats were randomly divided into four equal experimental groups: control, cisplatin group (single dose of 7.5 mg/kg, intraperitoneally (i.p.), on the fifth day), N-acetylcysteine group (500 mg/kg i.p., on the first and the fifth day), cisplatin + N-acetylcysteine group. Behavioral testing was performed in the tail suspension test. Oxidative stress and apoptotic markers were determined in the prefrontal cortex tissue samples. Cisplatin administration increased lipid peroxidation and decreased the activity of antioxidant enzymes in the prefrontal cortex. Also, cisplatin induced increase in Bax and decrease in Bcl-2 relative gene expression. Simultaneous application of N-acetylcysteine diminished cisplatin-induced alterations in oxidative stress and apoptotic markers. The results obtained in the tail suspension test that nominally resembles antidepressant action of cisplatin (attenuated by N-acetylcysteine), should be attributed to strong motor expression of anxiogenic response to cisplatin (also reversed by N-acetylcysteine). The antioxidant supplementation with NAC diminished cisplatin-induced oxidative damage and pro-apoptotic action in the prefrontal cortex, and significantly influenced specific behavioral alterations.
Keywords
- Cisplatin
- N-acetylcysteine
- Oxidative stress
- Apoptosis
- Depression
- Tail suspension test
- Rats
The first discovery that platinum mesh electrodes inhibited Escherichia coli cell division in the early 1960s opened the possibility for new therapy options in the treatment of cancer cells [1]. Such a discovery was a good example that small chemical alteration in the structure of the platinum molecule can substantially affect their basic activity in a target cell. Cisplatin (cis-diamminedichloroplatinum (II)) structure allows platinum ion to form bonds with DNA bases, which is the basic antitumor mechanism of its action [2]. It was the first platinum compound approved for cancer treatment of different types of neoplasms including head and neck, lung, ovarian, testicular, breast, kidney and brain neoplasm [3, 4].
Cisplatin action starts in the cytoplasm, forming the hydrolyzed products that are able to react with DNA, which leads to irreversible apoptosis. Cisplatin binds to purine residue N7 reactive center of DNA and forms DNA-DNA interstrand and intrastrand crosslinks. Cisplatin has cytotoxic effects due to intrastrand adducts and the consequence of their occurrence is inhibition of DNA replication and transcription. In the further process it is created DNA damage recognition which is followed by the making of proteins which transmit DNA damage signals to downstream signaling cascades, which results in definitive apoptotic cell death [5, 6]. Furthermore, cisplatin increase expression of pro-apoptotic genes and also reduce the expression of anti-apoptotic genes, which also favors apoptosis [5, 6].
Under physiological conditions, cells control the level of ROS using scavenging systems, such as reduced glutathione-GSH, the activity of antioxidant enzymes (superoxide dismutase-SOD and catalase-CAT), balancing the excessive ROS production. However, the appearance of various pathophysiological mechanisms, such as observed in cancer cells, inflammation episodes, traumas and toxic medications may result in overcoming ROS production, which cannot be balanced by cell antioxidant capacity, that in turn can damage cellular protein, lipids, and DNA, leading to cell death. Since the cisplatin initially affects the mitochondria (disturbing the membrane structure, potentials, and calcium transfer), the oxidative damage originating from this natural source of ROS presents the most important mechanisms of cisplatin toxicity [7, 8].
Apoptosis is a complexly regulated process of cell death control during which a cell undergoes self-destruction [9]. This process can be triggered by numerous factors that include different stresses such as ROS, RNS, DNA-damaging agents and others [10]. Since the brain is extremely vulnerable to oxidative damage (due to high oxygen utilization), the excessive ROS generation can cause damage of neuronal cells inducing cell death [11]. It is worth to mention that the exposure to chemotherapeutic drugs can also trigger apoptosis, which is often mediated by ROS [12].
Unfortunately, besides the confirmed beneficial effects in the treatment of various malignancies, cisplatin application is accompanied by a broad spectrum of serious adverse effects that include neurotoxicity, hepatotoxicity, nephrotoxicity, ototoxicity, myelosuppression, gastrointestinal toxicity and cardiotoxicity [13]. Common neurological side effects of cisplatin are manifested by cognitive deficits, disorientation, visual perception and hearing disorder [14]. Recent studies performed on animal experimental models for evaluation of cisplatin neurotoxicity also confirmed serious mood disorders, such as increase anxiety levels [15, 16]. Pathophysiological mechanisms underlying mentioned cisplatin toxicities, not surprisingly, include the very same antitumor actions, including oxidative damage and cell apoptosis [17].
As expected, the antioxidant supplementation is widely accepted as a therapeutic approach in the treatment of cisplatin-induced toxicities. Certainly, it has been reported that exogenous antioxidant, N-acetylcysteine (NAC) may be beneficial in the prevention of anxiogenic response to cisplatin in rats [16]. Since the prefrontal cortex (PFC) is also strongly involved in emotion regulation [18], but not investigated following cisplatin treatment, it seems useful to analyze the alterations in the oxidative and apoptotic status of this brain region, as well as its impact on depressive behavior in rodents [19].
The aim of this study was to allow additional information considering the behavioral manifestations of cisplatin-induced neurotoxicity. Following one of the confirmed patophysiological mechanism underlying this adverse effect of cisplatin, we also intended to evaluate the potential role of antioxidant supplementation with NAC in the prevention of alterations in depressive-like behavior in rats.
A total number of three-month-old male Wistar albino rats (250–300 g) were
housed in groups of 4 per cage, under standard environmental conditions (23
Experimental procedures were conducted according to the ARRIVE guidelines, the
European Directive for welfare of laboratory animals N
On the day 10, the animals were accommodated in the testing room (1–2 h prior to the TST). The tail suspension test is considered as a standard behavioral test for the evaluation of the depressive state level in rodents [20]. The basic principle of this test is bringing the experimental animals into the uncomfortable position (suspension by the tail attached to the adhesive tape, head facing the ground). A physiological reaction to this acute stress-inducing situation is an active effort in reposition, manifested by the variety of motor patterns. Nevertheless, the active avoidance of this position will take place, which will be manifested as immobility (state of no visible voluntary movement of the body and the limbs). This “giving up behavior” is commonly considered as an equivalent to anhedonia. This six-minute lasting test was performed on the apparatus and according to previously described procedure [21]. The following parameters were obtained in TST: the latency to the first immobility-LFI (s), the number of immobility episodes-NIE, the total duration of immobility-TDI (s), and an average duration of an immobility episode-ADIE (s).
After completing the behavioral testing the animals were anesthetized (ketamine
and xylazine, 10 and 5 mg/kg, i.p., respectively) and sacrificed by decapitation.
After quick brain removal from the skull, the prefrontal cortex samples were
dissected (1 mm thick), tissue samples were homogenized (in phosphate-buffered
saline, 50 mM, pH 7.4), centrifuged (4000 rpm, 4
The total RNA was extracted from PFC tissue using PureZOL reagent (Bio-Rad, USA)
according to the manufacturer’s instructions. Reverse transcription was done
using iScript Reverse Transcription Mastermix (Bio-Rad, USA), and quantitative
RT-PCR was performed using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad,
USA). mRNA specific primers for Bax, Bcl-2, and
| Forward | NCBI reference sequence | Reverse | NCBI reference sequence | |
| AAGATCCTGACCGAGCGTGG | XM_032887061.1 | CAGCACTGTGTTGGCATAGAGG | XM_032887061.1 | |
| Bax | CGGCGAATTGGAGATGAACTGG | XM_032915032.1 | CTAGCAAAGTAGAAGAGGGCAACC | U49729 |
| Bcl-2 | TGTGGATGACTGACTACCTGAACC | XR_005492200.1 | CAGCCAGGAGAAATCAAACAGAGG | NM_016993.2 |
Statistical analysis was performed with SPSS version 20.0 statistical package
(IBM SPSS Statistics 20, IBM Corp., NY, USA). The parameters were analyzed by tests of normality
(Shapiro-Wilk) and homogeneity (Levene’s). One-way ANOVA, followed by Bonferroni
post-hoc analysis, was performed for comparisons between the groups. The results
are expressed as the means
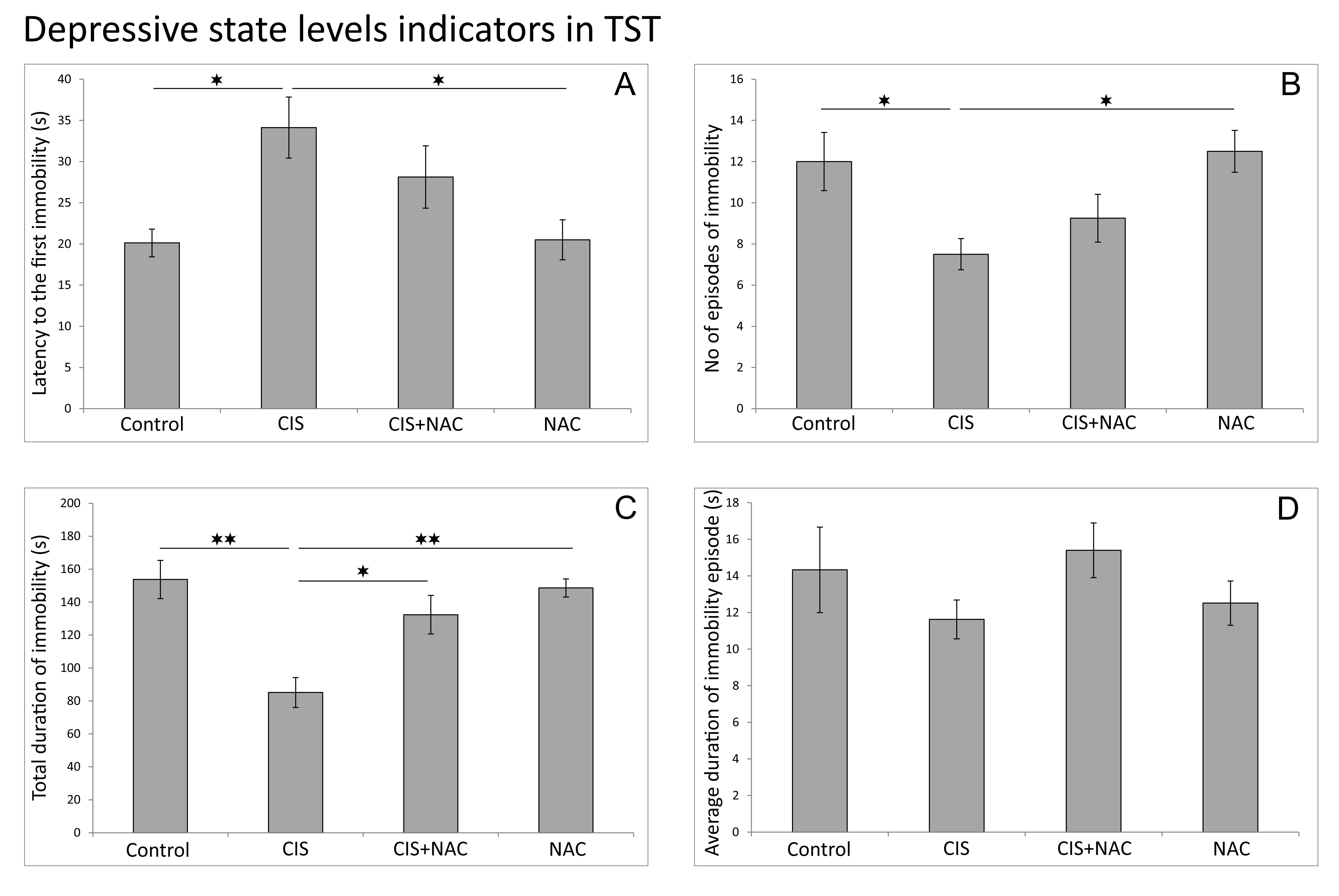
The applied protocols significantly altered the latency to the first immobility
(Fig. 1A) in TST (F = 4.905, df = 3). A single dose of cisplatin increased LFI
(p
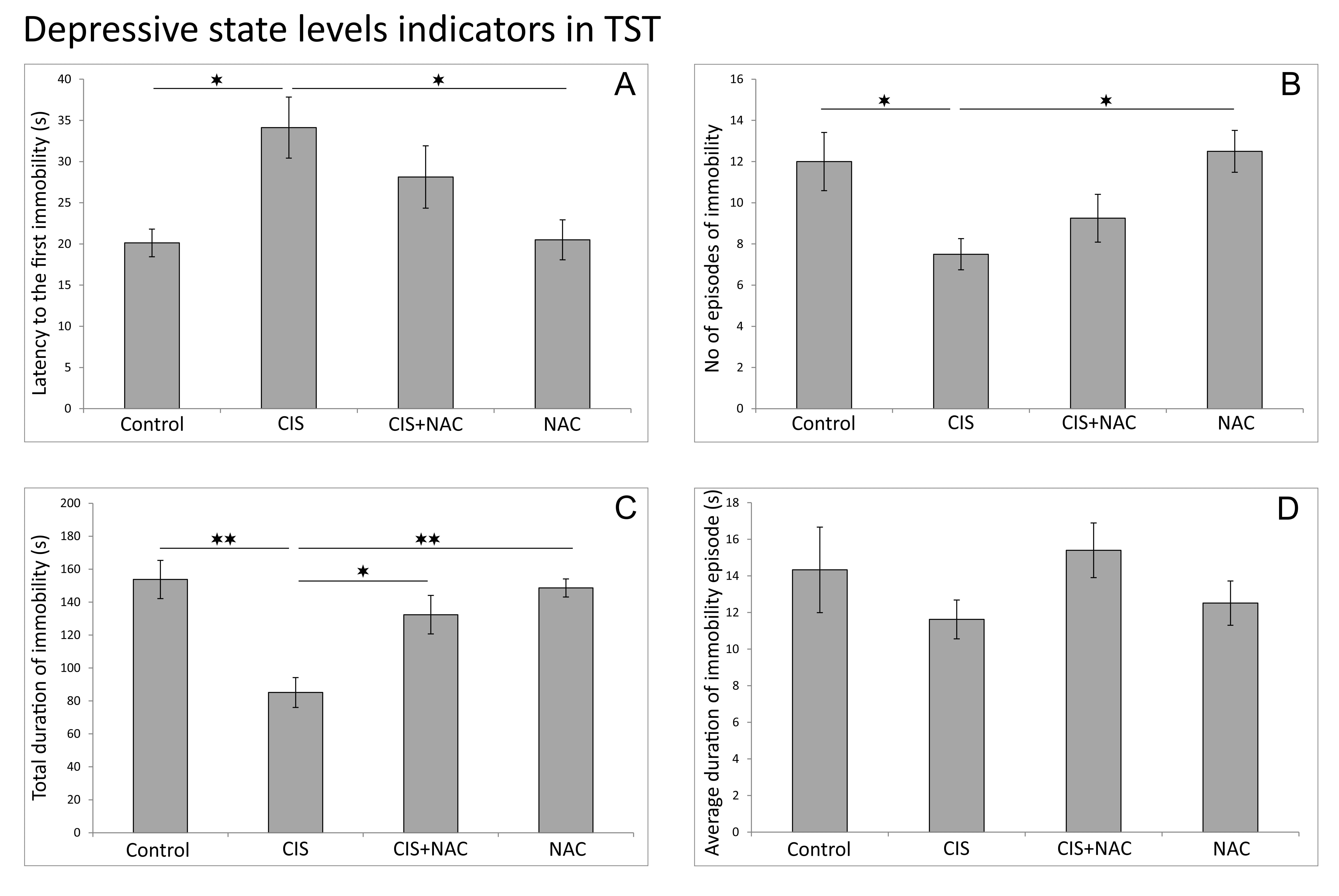 Fig. 1.
Fig. 1.The parameters obtained in the tail suspension
test. (A) The latency to the first immobility. (B) The number of
immobility episodes. (C) The total duration of immobility. (D) An average
duration of immobility episode. Bars represent means
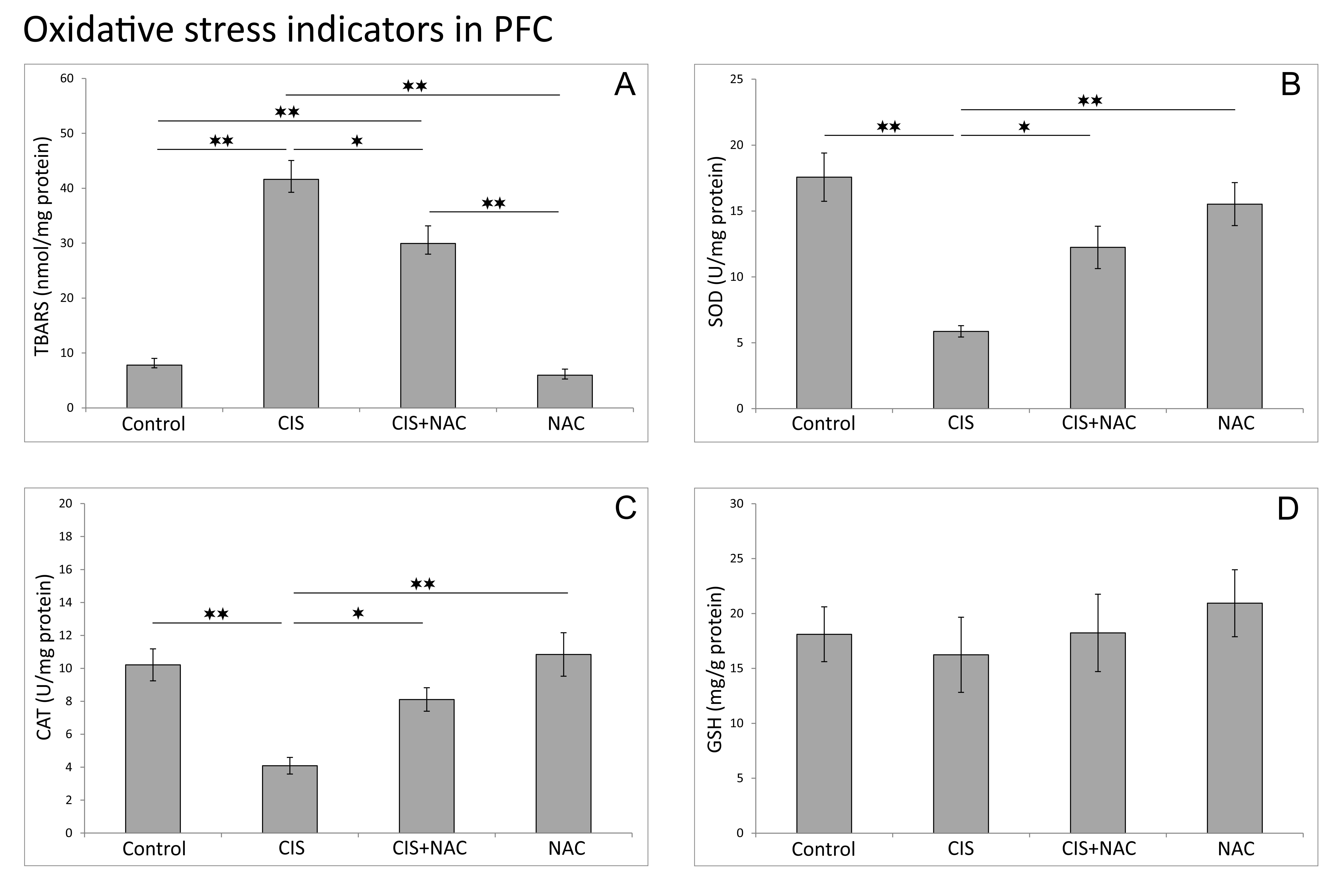
As shown in Fig. 2A, the applied protocols showed a significant impact on the
lipid peroxidation, expressed as TBARS (F = 48.633, df = 3). A single dose of
cisplatin produced significant enhancement of TBARS when compared to the control
(p
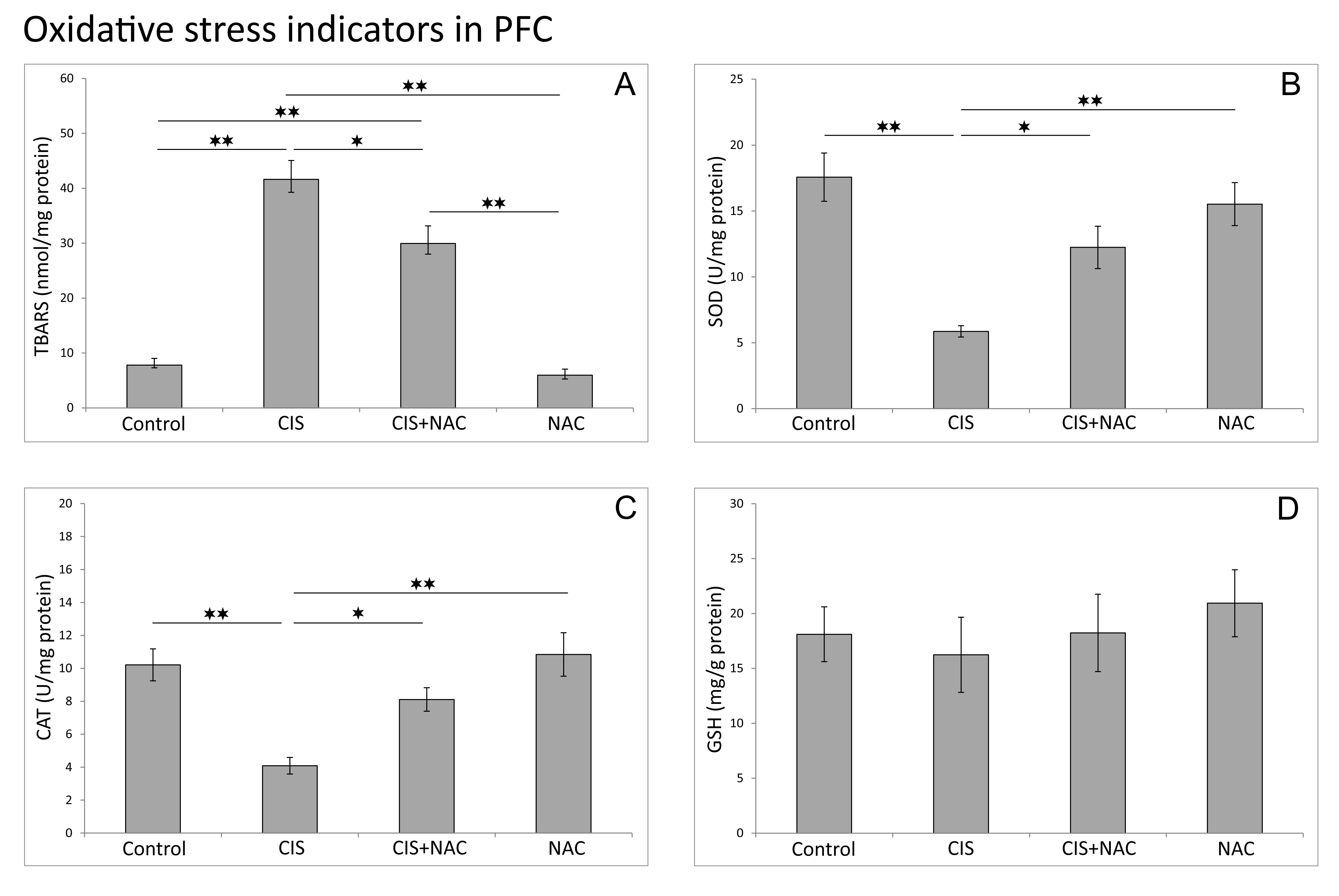 Fig. 2.
Fig. 2.The oxidative stress markers in the prefrontal cortex.
(A) The index of lipid peroxidation. (B) The activity of SOD. (C) The activity of
CAT. (D) GSH level. Bars represent means
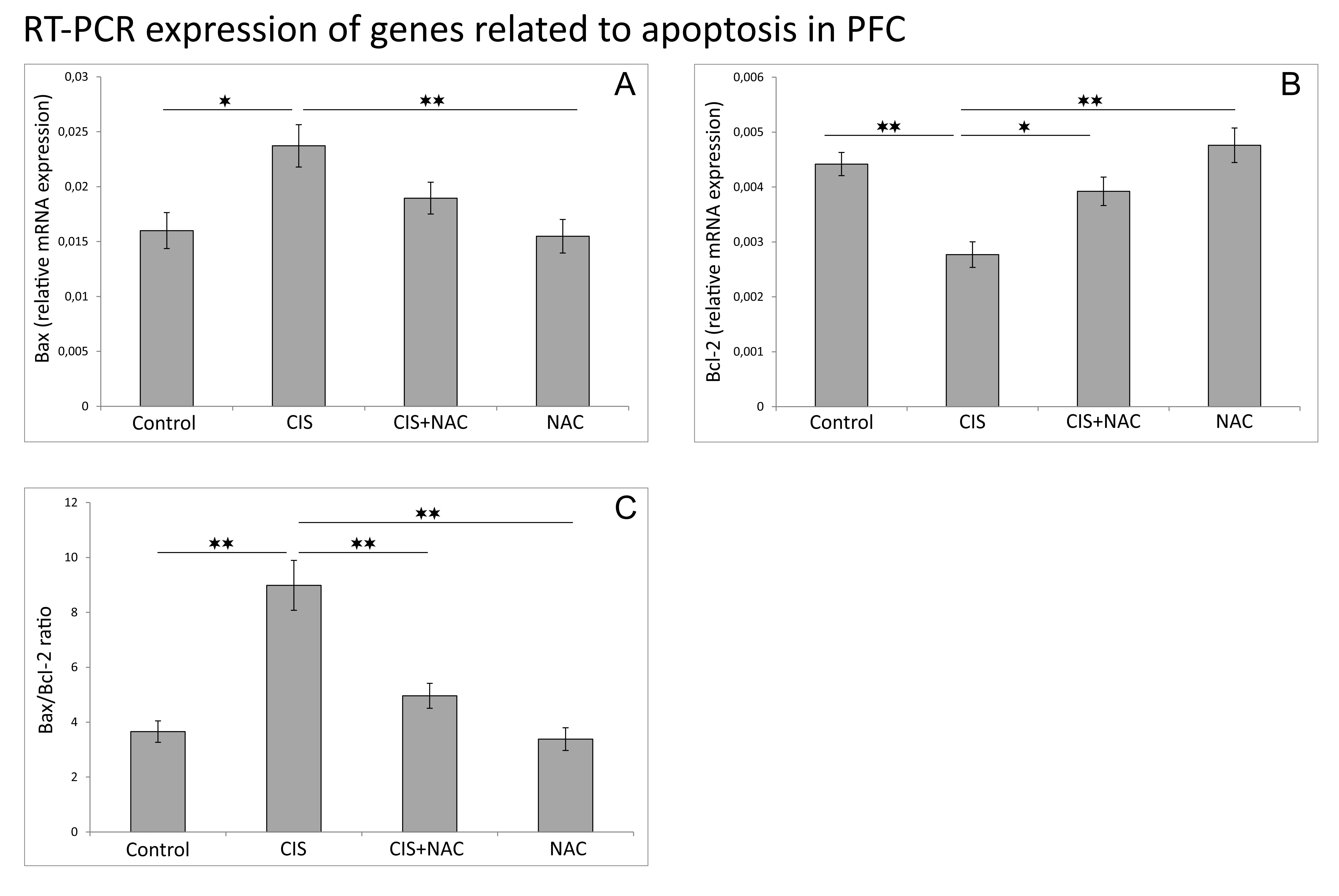
As shown in Fig. 3A, the relative gene expression of Bax in PFC was
significantly altered by the applied protocols (F = 5.254, df = 3). The
application of cisplatin resulted in increased Bax relative gene expression
(p
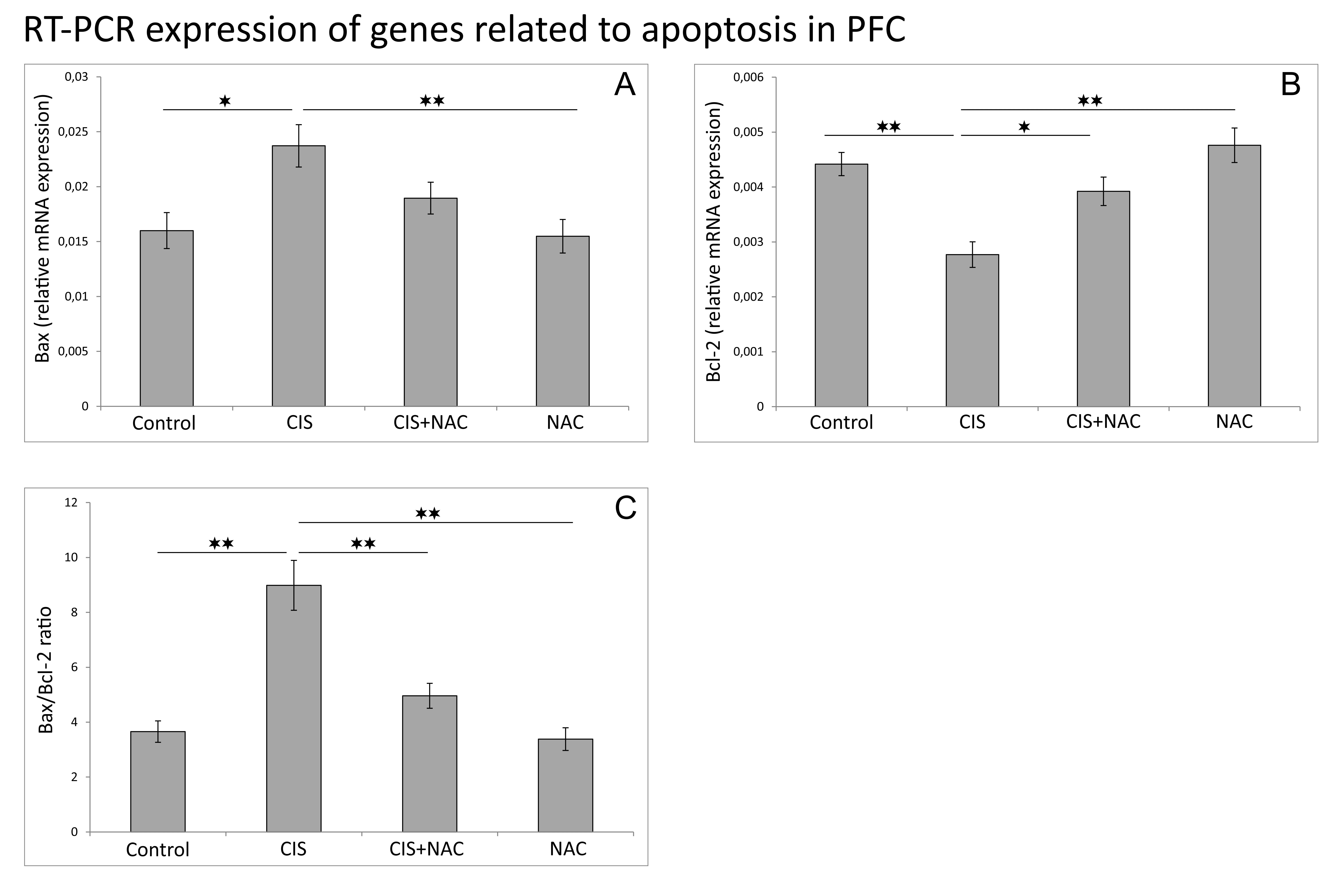 Fig. 3.
Fig. 3.The relative gene expression of pro- and anti-apoptotic
markers in the prefrontal cortex. (A) Bax. (B) Bcl-2. (C) Bax/Bcl-2 ratio. Bars
represent means
Although proclaimed as “a drug of the century” almost 50 years ago, cisplatin therapy showed numerous limitations due to severe adverse effects. The numerous toxicities accompanied by cisplatin clinical administration were addressed, among others, to an uncontrolled oxidative damage in various tissues, including the central nervous system. Therefore, the antioxidant supplementation, as a potential protective mechanism against cisplatin-induced neurotoxicity, has been one of the most targeted topics in the investigations performed on the animal experimental models.
The analysis of oxidative stress markers alterations in PFC strongly confirmed the prooxidative action of cisplatin. This was confirmed by both increased lipid peroxidation, and diminished activity of antioxidant enzymes (SOD and CAT). This is in line with the previously described prooxidative impact of a single dose of cisplatin manifested by the enhanced TBARS values and decline in the antioxidant capacity, expressed by means of decreased SOD and CAT activity in rat hippocampus [15, 16]. A similar deterioration of the antioxidant capacity following cisplatin administration in a single dose was also reported in rat frontal cortex [28]. Cisplatin-induced oxidative damage in rat brain has been accompanied with the specific morphological damage manifestations in frontal cerebral cortex [29]. On the other hand, glutathione levels in PFC remained unaltered following cisplatin administration in this study. Due to a lack of literature data for GSH levels in PFC, our results can only be compared to the previously reported decline of GSH in different brain regions, but only after higher doses of cisplatin and/or prolonged administration [30].
The antioxidant supplementation with NAC applied simultaneously with cisplatin, was sufficient to attenuate the prooxidative action of cisplatin by means of oxidative stress markers decline. Thus, NAC significantly reduced lipid peroxidation and, at the same time, enhanced the antioxidant capacity in PFC, expressed by the restoration of antioxidant enzymes capacity. The results for NAC antioxidant action in PFC obtained in this study are in accordance with the previously described neuroprotective role of antioxidant supplementation in treatment of platinum-based drugs induced adverse effects [31].
Not surprisingly, the pro-apoptotic action of cisplatin, even in a single dose, followed the described prooxidative outcome in this experimental model. The numerous data that confirmed the causal interconnection between the oxidative damage and enhanced apoptotic mechanisms [12] was also obvious in this study. Namely, cisplatin administration resulted in both enhancement of pro-apoptotic mechanisms (increase in Bax relative gene expression) and diminished anti-apoptotic activity (decrease in Bcl-2 relative gene expression). The observed pro-apoptotic action of cisplatin is in line with previously reported cell death mediated by altered expression of pro- and anti-apoptotic genes involved in cell proliferation in the specific brain regions responsible for mood regulation [5]. In general, it has been confirmed that several experimental models for depression induction may simultaneously lead to the pro-apoptotic action in neocortex [32]. It is already known that major depressive disorder is accompanied with significant augmentation of pro-apoptotic mechanisms [33]. More specific, the decline in anti-apoptotic factors, by means of decline in bcl-2 gene family, has been reported in studies obtained in both humans [33] and rodents [34] that evaluated the connection between apoptosis and depression.
The other drug employed in this study, NAC, had also been confirmed to affect the depressive level, when applied alone (10–50 mg/kg, intraperitoneally), via AMPA glutamate receptors in mice [35]. Also, NAC administration significantly altered the response to antidepressant drugs when applied simultaneously, by means of increase of the minimum effective dose [36]. In addition, there is evidence that NAC (100 mg/kg) had prevented the increased cisplatin-induced (in almost the same dose as applied in this study) oxidative damage in whole brain samples in mice [37], but with no evidence for the impact on apoptotic mechanisms. Since the oxidative stress has been attributed as trigger mechanism for neural apoptosis [38], the results of our study offer the confirmation for significant pro-apoptotic action of cisplatin in PFC, as the region responsible for depressive state level regulation.
Again, the antioxidant supplementation with NAC, applied in the presented dose, was sufficient to attenuate the pro-apoptotic action of cisplatin. The observed protective role of NAC administration is in accordance with the previously reported beneficial role of NAC in rat hippocampus [16], and may be considered as almost common characteristic of antioxidant supplements in the treatment of platinum-based compounds-induced neurotoxicity [31].
Unlike for the oxidative stress and apoptotic mechanisms in PFC, the analysis of behavioral testing in this study seems to be far more complex. To be more precise, each of the described alterations in the parameters obtained in TST individually, and especially taken together, would lead to the conclusion that cisplatin administration (in this dose) resulted in antidepressant effect. However, taking into account previously published results for the behavioral estimation of anxiety, it seems reasonable that the outcome in some of the behavioral tests for depression evaluation (including TST) may be substantially altered by anxiety level alterations. Namely, the motoric manifestations of extremely strong anxiogenic response may overcome prodepressant effect by means of hyperactivity [39], as an overreactive response to standardized stressful stimuli. This conclusion can also be supported by the confirmed prodepressant effect of cisplatin [40]. However, the controversial conclusions related to this topic may be additionally addressed to different experimental design applied in similar investigations. So, the prodepressant action of cisplatin was reported after chronic administration (10 weeks) [40], while this adverse effect was not observed following cisplatin application in a single dose in this study. The results of behavioral testing obtained in this study imply strong initial anxiogenic response to cisplatin, but it should not be excluded that potential prolongation of cisplatin treatment would finally result in prodepressant manner. This assumption may be also based on the previously commented results for cisplatin-induced oxidative damage and pro-apoptotic action in PFC.
In that sense, it should be noticed that the clinical trials confirmed the significant comorbidity for the anxiety and depression (app. 50% patients) under various pathophysiological circumstances [41]. Not surprisingly, PFC (as the morphological substrate for the behavioral alterations in this study) is crucially involved in the control of depression [42], but also in the anxiety level regulation [43]. Furthermore, it has been reported that lesion of ventral parts of PFC was manifested through the anxiogenic response [44], so it seems that the neurotoxic effect of cisplatin, as observed in this study, may be initially expressed in ventromedial PFC. On the other hand, it may be expected that prolonged administration of cisplatin would additionally involve the damage of other parts of PFC with the consequent prodepressant effect [45]. After all, it seems clear that the better insight on cisplatin action in PFC, and its behavioral manifestations, requires specific regional analysis of this complex brain region. Commenting on the results obtained in behavioral testing following cisplatin administration should also include the analysis of behavioral outcome following the antioxidant supplementation with NAC. So, the attenuation of oxidative damage and anti-apoptotic action of NAC in PFC should be rather addressed to anxiolytic effect than to the prodepressant action.
In summary, the results obtained in this study lead to the conclusion that antioxidant supplementation with NAC diminished cisplatin-induced oxidative damage and apoptosis in PFC, which is in turn manifested by specific behavioral alterations.
RV, DS, JSKS, IK, NJ, and GR conceived and designed the experiments; RV, DS, JSKS, IK, NJ, and GR performed the experiments; RV, DS, JSKS, IK, NJ, and GR analyzed the data; GR contributed reagents and materials; RV, DS, JSKS, IK, NJ, and GR wrote the paper.
Experimental procedures were conducted according to the ARRIVE guidelines, the
European Directive for welfare of laboratory animals N
We thank anonymous reviewers for excellent criticism of the article.
This study received no external funding.
The authors declare no conflict of interest.