1 Department of Pharmaceutical Sciences, University of Perugia, 06126 Perugia, Italy
Abstract
Mast cells are the major effectors in allergic reactions through degranulation and release of inflammatory, vasoactive and nociceptive mediators associated with the pathogenesis of a variety of inflammatory disorders. Mast cells are strategically positioned as gatekeepers at host/environment interfaces, like the skin, airways, gastrointestinal and urogenital tracts, and their presence also in the brain allows them to act not only as sentinels of invading microorganisms but also as targets to respond to different allergens, pathogens and other dangerous agents that can be ingested, inhaled or encountered after the breakdown of the epithelial barrier. Mast cells can respond to any change in the environment by communicating with the different cells involved in the immune response and giving rise to an amplification signal network through feedback loops. They secrete both preformed mediators within minutes of stimulation and de novo synthesized molecules acting as effectors in the relationship between nervous, vascular and immune systems. For this peculiarity, mast cells are master regulators and key players of the immune system and important sources of essential and beneficial mediators with crucial roles in regulating various physiological processes.
Keywords
- Mast cells
- Gut
- Nerve
- Brain
- Neuroinflammation
- Neuroimmune interaction
- Gut-brain axis
- Neural diseases
- Microbiota
Mast cells (MCs) develop from hematopoietically derived immune CD34 +/CD117 + multipotential stem cells originating in the bone marrow and circulating in the blood in low numbers as immature precursors. They migrate up to localize in the mucous and connective tissues in the vicinity of blood vessels, lymphatic vessels and nerves, completing their differentiation in mature MCs under the influence of the local microenvironment, which characterizes them phenotypically and, therefore, functionally [1, 2, 3, 4]. Therefore, MC precursors show a wide heterogeneity in their terminal differentiation within the tissue, giving rise to a specific subset of MCs with characteristic profiles of mediator content, secretory responsiveness, receptors at a particular site within the body species’ dependence [5]. MCs are strategically positioned to respond to different allergens, pathogens and other agents that can be ingested, inhaled or encountered after the epithelial barrier’s breakdown. Although they are numerically few, upon being activated, MCs are first responders and their position as gatekeepers at host/environment interfaces, like the skin, airways, gastrointestinal and urogenital tracts, and their presence also in the brain, allows them to act not only as sensors of invading microorganisms but also as effectors. As such, MCs can respond to any change in the environment by alarming and communicating with the different cells involved in the immune response and giving rise to an amplification signal network [6]. MCs are crucial players in innate and adaptive immune responses. The mediators that MCs release are involved in various physiological roles and modulations such as tissue repair, neurogenesis, wound healing, defense against tumors, angiogenesis, emotional behaviors and synaptic plasticity [7, 8, 9, 10]. For their purpose, MCs are armed with a vast repertoire of cell surface receptors enabling them to interact both directly and indirectly with a multitude of activators, pathogens, environmental toxins, allergens, neurotransmitters, neuropeptides and hormones including acetylcholine, calcitonin gene-related peptide (CGRP), corticosteroids, corticotropin-releasing hormone (CRH), substance P (SP) and vasoactive intestinal peptide (VIP). MCs can be activated by agonists that bind receptors or by physical activators or cell-to-cell contact [9, 10]. In particular, MC’s receptors are including FceRI for IgE antibody, FcgR for IgG antibody, Toll-Like Receptors (TLRs 1-7, 9), NOD-like R, pattern recognition receptors (PRRs), c-kit CD117, CD48 and complement receptors [1, 11].
MCs possess the ability to release both mediators performed and neo-synthesized,
alerting the immune response or amplifying an existing response. Two types of
activation are present: 1) direct activation by pathogens via TLRs; 2) indirect
activation by pathogens through the interaction of antigen with its specific IgE
antibody bound to the cell membrane via high-affinity receptor Fc
| Histamine |
| Proteases, such as tryptase, chymase, metalloproteinases |
| Serotonin |
| Heparin |
| Dopamine |
| Adenosine triphosphate |
| Lysosomal enzymes |
| Nitric oxide |
| Lipid mediators, such as prostaglandin, leukotriene and tromboxane |
| Chemokines and Cytokines, such as Interleukin IL-1 |
| Nerve growth factor |
| Vascular endothelial growth factor |
| Platelet derived growth factor |
| Fibroblast growth factor-2 |
| Granulocyte-macrophage colony-stimulating factor |
| Kinins |
| Substance P |
These mediators, individually or in aggregate, can act through different effects
on/and involving immune or structural cells, inducing various responses. Upon
MCs activation, there are two possible primary outcomes: i) release of preformed
molecules stored in granules through the classical anaphylactic degranulation
manner in which the content of each granule is secreted in the acute release.
Histamine, proteases, tumor necrosis factor (TNF)-
MCs interact with other cells of the immune system. Furthermore, MCs establish a wide variety of interactions even with non-immune cells, such as neurons, fibroblasts, smooth muscle cells, bronchial epithelial, etc. This property allows them to be able to influence a multiplicity of functional activities. The immune system and the nervous system are in close communication, and MCs are the connecting link as they can respond and release neurotransmitters and immune mediators. Acetylcholine, norepinephrine, SP and histamine modulate immune activity. On the other hand, neuroendocrine hormones such as CRH and alpha-melanocyte-stimulating hormone regulate cytokine balance. In other words, there is a mutual influence between the immune system and brain activity [13].
Beyond degranulation and de novo synthesis, activation of MC involves further modalities of the release of molecules with structural alterations and cell-cell communication. The extreme communicative heterogeneity of the activated MC is a consequence of the position and the chemical and anatomical stimuli present in the local microenvironment and dependent on the functional state. In particular, the release of extracellular vesicles through exosomes, with which MCs transfer their loads of proteins, DNA and RNA, and through which MCs interact with sensory nerve fibers; the formation of tunneling nanotubes through the extension of structures similar to pseudopods with which MCs interact with neural, vascular, and immune systems, and, finally, extracellular traps through which MCs concur in chronic inflammatory conditions have been described [14, 15].
In the gut, there are multiple interactions with the external environment. The intestinal epithelial barrier must usually tolerate millions of microorganisms that live in the intestine. It must also perform intestinal functions; yet, it must establish tolerance or immunity depending on the antigenic it encounters, and the major antigenic component is the ingested food. The intestinal epithelial barrier must integrate external and internal signals and coordinate an adequate immune response to maintain tissue homeostasis.
In the gut, MCs are in close interaction with the nerves and small vessels, representing the emblem of the neuroimmune network that sees MCs as central players. At the intestinal level, MCs are differentially functional in the various traits. For example, the colon’s MCs have a greater abundance of TLR4 than the MCs of the small intestine since at the level of the colon, the bacterial load is higher [16]. In the gut, MCs may play a role in visceral sensitivity. From a physiological point of view, MCs’ role induces an increase in blood flow and an increase in propulsive motor activity, which constitutes a gastrointestinal defense strategy aimed at washing and eliminating luminal antigens microbes, toxins or harmful substances, as suggested by Wood [17]. So, MCs are decisive in establishing physiological and pathological conditions, ranging from the clearance of pathogens to the maintenance of the intestinal epithelium. MCs can alarm other immune cells, perpetuating inflammatory mediators’ release and affecting the epithelial barrier’s permeability through feedback loops. For example, tryptase can activate the proteinase-activated receptors (PAR-2) on epithelial cells by increasing permeability through tight junctions (TJs). PAR-2 is also expressed on nerve endings, and their activation may result in neurogenic inflammation [18] (see below).
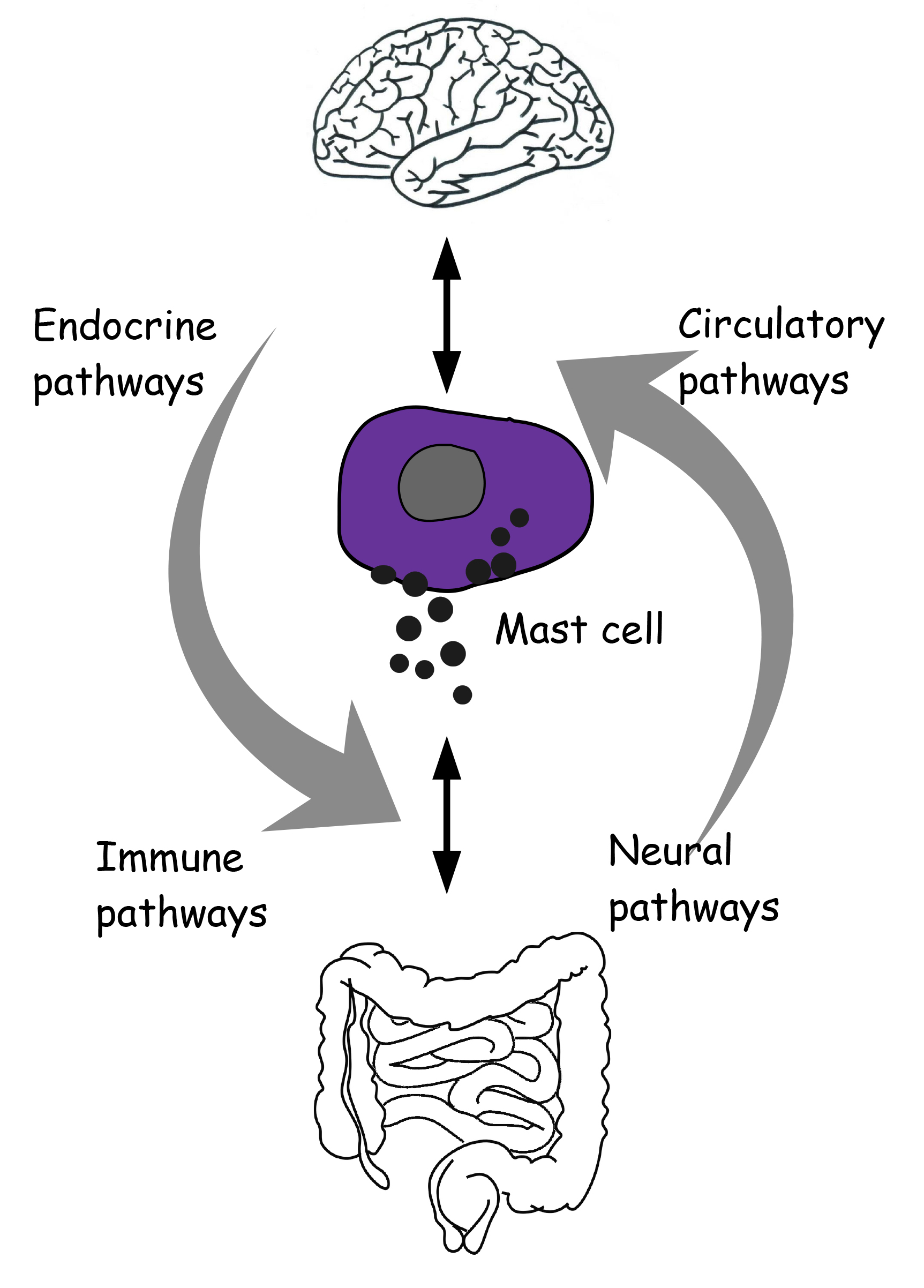
The brain communicates with the gut in a bidirectional manner through neural, endocrine and circulatory messages across an integrative system, the brain-gut axis (Fig. 1).
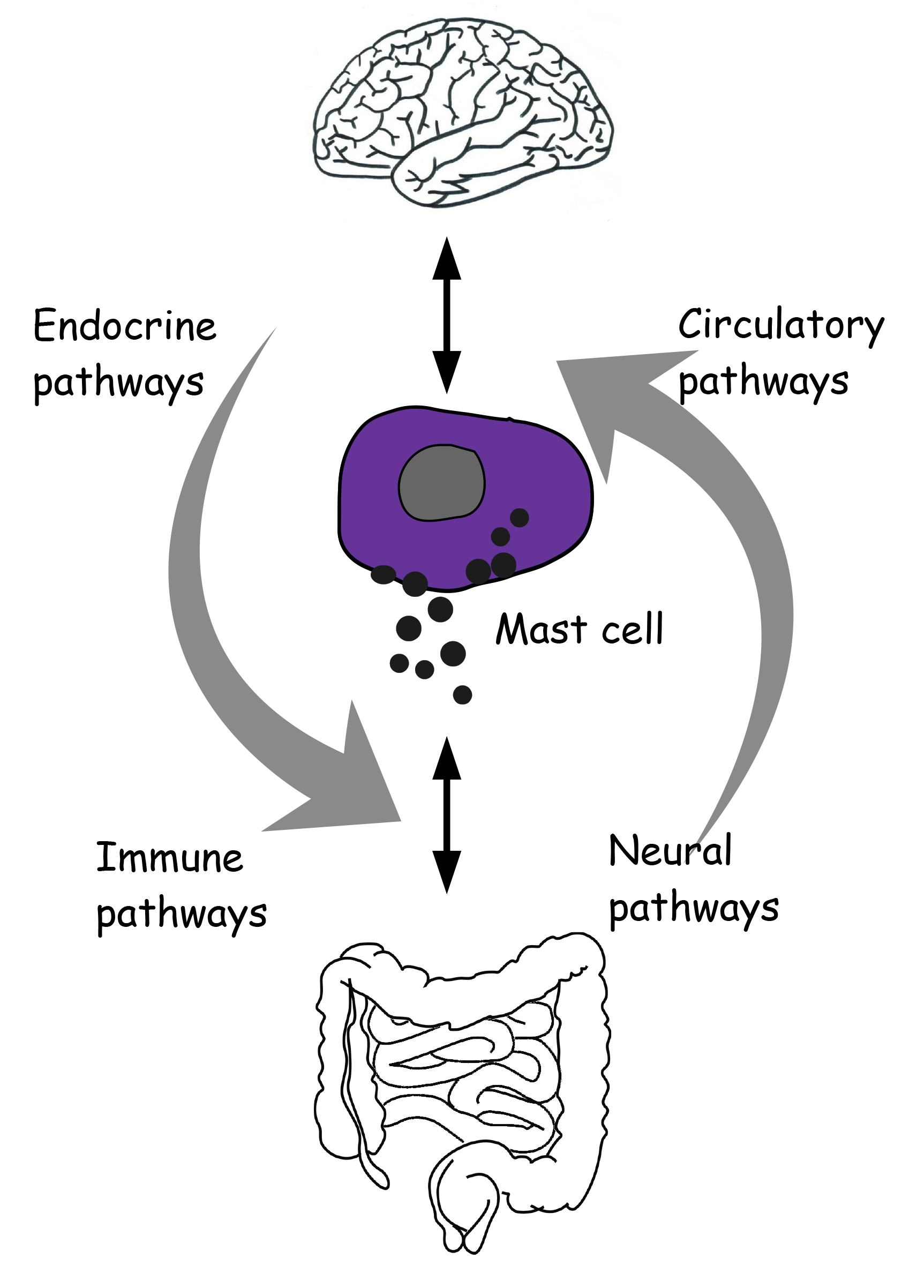 Fig. 1.
Fig. 1.Reciprocal neuro-immune-circulatory communication between brain and gut. Mast cells are proposed as an integral and unifying part of the microbiota-gut-brain axis.
The intestinal microbiota influences behaviors and neural functions [19]. A large body of evidence indicates that the gut microbiota influences diverse physiological and behavioral responses through neuroendocrine pathways’ modulation. The intestinal microbial ecosystem is the first effective barrier for the organism against pathogens. The microorganisms present in the intestine could interact with the host’s neuroendocrine system elements, causing changes in the host’s behavior. Mediators produced in the gut microbiota and the microbiota itself can affect the brain, and, conversely, emotional conditions can affect the microbiota [13]. In the absence of friendly microorganisms, the aggression of pathogenic microorganisms would otherwise be devastating. The microbiota-gut-brain axis plays a crucial role in several nervous conditions, including stress, anxiety, learning and memory, addiction, sexual behavior, social interaction and depression, as well as in neuroinflammation and neurodegeneration [19]. Peripheral inflammation can lead to inflammation of the CNS, causing neurodegeneration. The inflammation resulting from innate immune system activation in the periphery can influence CNS behaviors, as cognitive performance [20, 21, 22]. The dysbiosis condition is an alteration in the intestinal microbiota that can contribute to neurodegeneration. Excellent communication between CNS and the immune system is present. The gut microbiota interacts with CNS, influencing brain activity through neuroimmune cells. There is evidence of bidirectional communication between MCs and neurons in the gastrointestinal tract, and MCs can be considered the classic immune cell activated by neuronal factors and neurotransmitters [13, 23] (Fig. 1).
MCs are a crucial component of interaction between the enteric nervous system (ENS) and central nervous system (CNS) [24, 25]. MC activation has also been implicated in several intestinal stress disorders, such as ulcerative colitis and intestinal inflammation. Although the pathophysiology of inflammatory bowel disease (IBD) is not entirely understood yet, evidence suggests that it can be a consequence of dysregulation of the microbiota-gut-brain-axis, and a correlation with MCs levels has been suggested [26, 27, 28]. An increase in MCs number in terminal ileum and colon of IBD’s patients has been reported. IBD includes Crohn’s disease and ulcerative colitis characterized by abdominal pain, change in gut motility and secretion, change in bowel habit with an increase of mucosal permeability. In the GI tract, MC plays an important role in maintaining homeostasis in the intestine by regulating various functions such as ion transport and secretory activity of the mucosal epithelial cell, vascular permeability and intestine motility. Patients with ulcerative colitis and Crohn’s disease manifest increased histamine metabolite levels in urine compared to normal individuals [29].
In the gut, MCs are the major source for TNF-
The microbiota composition influences the integrity and permeability of the
intestinal mucosa, the maturation of the immune system, and the acquisition of
tolerance. Evidence report that specific probiotic strains induce expression of
transforming growth factor (TGF)-
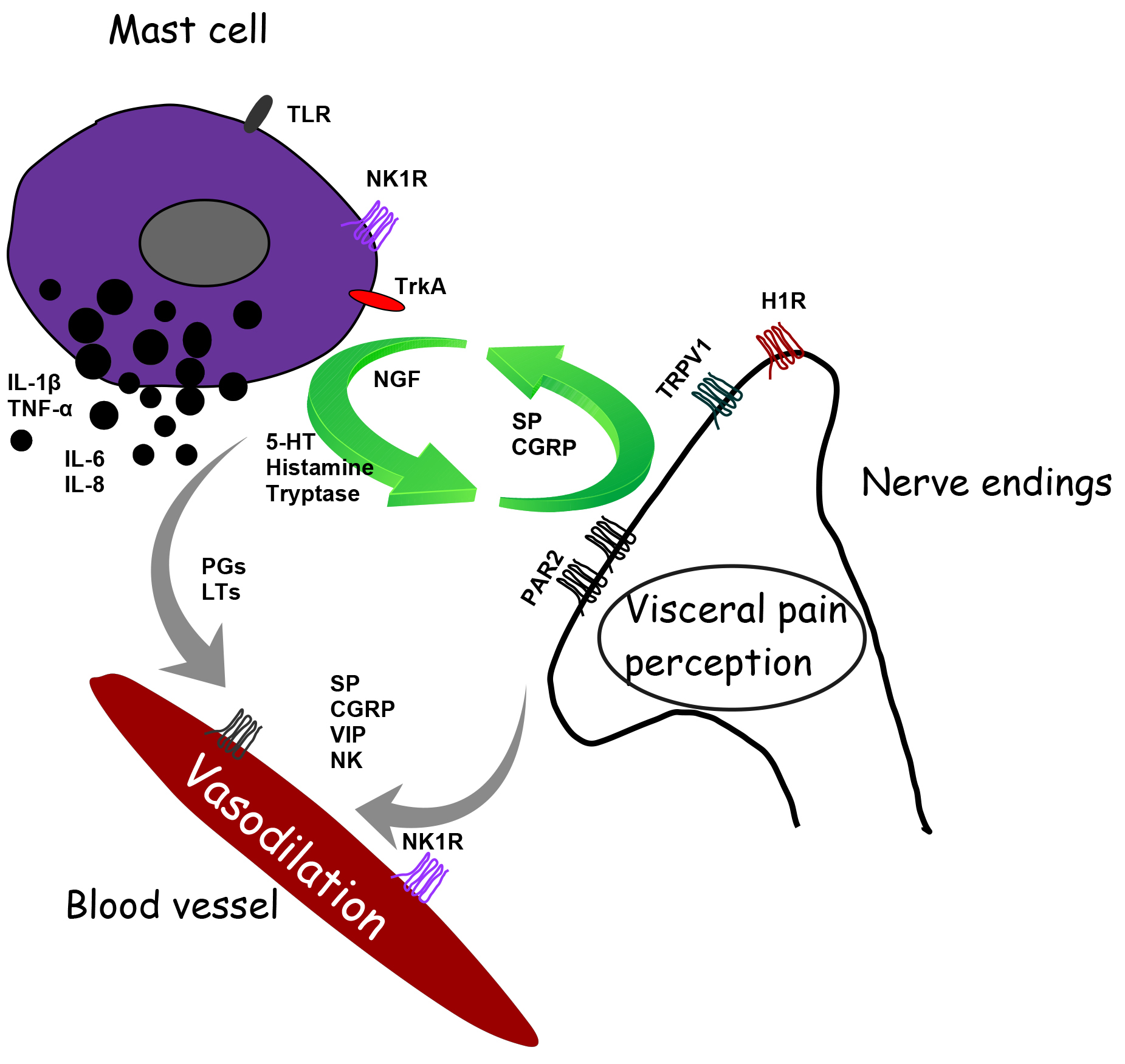
Increasing evidence reports a role of MCs in neurogenic inflammation leading to pain. Inflammatory cytokines and neuropeptides released by MCs at the peripheral level orchestrate neurogenic inflammation giving rise to an inflammatory cascade. In particular, activated MCs give life and fuel a feed-forward cycle that leads to neuropeptides’ release. Tryptase activates PAR2 on peripheral nerve endings. The activation of PAR2, in turn, sensitizes the potential transient receptor potential vanilloid 1 (TRPV1). These polymodal receptors are activated by a wide variety of stimuli that, when coupled with MC activation, have functional relevance for transmission of visceral pain signals in response to insult such as infection and/or stress, stimulating the release of algogenic, vasoactive and inflammatory mediators [18, 52]. Stimulated nociceptors lead to the release of CGRP, ATP and SP, which can exist as co-transmitters from sensory nerve endings. Activation of TRPV1 channels on primary sensory neurons stimulates the release of SP and CGRP in peripheral tissues, contributing to neurogenic inflammation and hyperalgesia, plasma extravasation and granulocyte infiltration [53]. CGRP also interacts with the CGRP type 1 receptor at the level of vasodilating arterioles. Substance P activates plasma extravasation via neurokinin 1 (NK1) receptors. On the other hand, SP also acts on the MCs themselves, giving rise to MC activation’s vicious cycle [13] (Fig. 2).
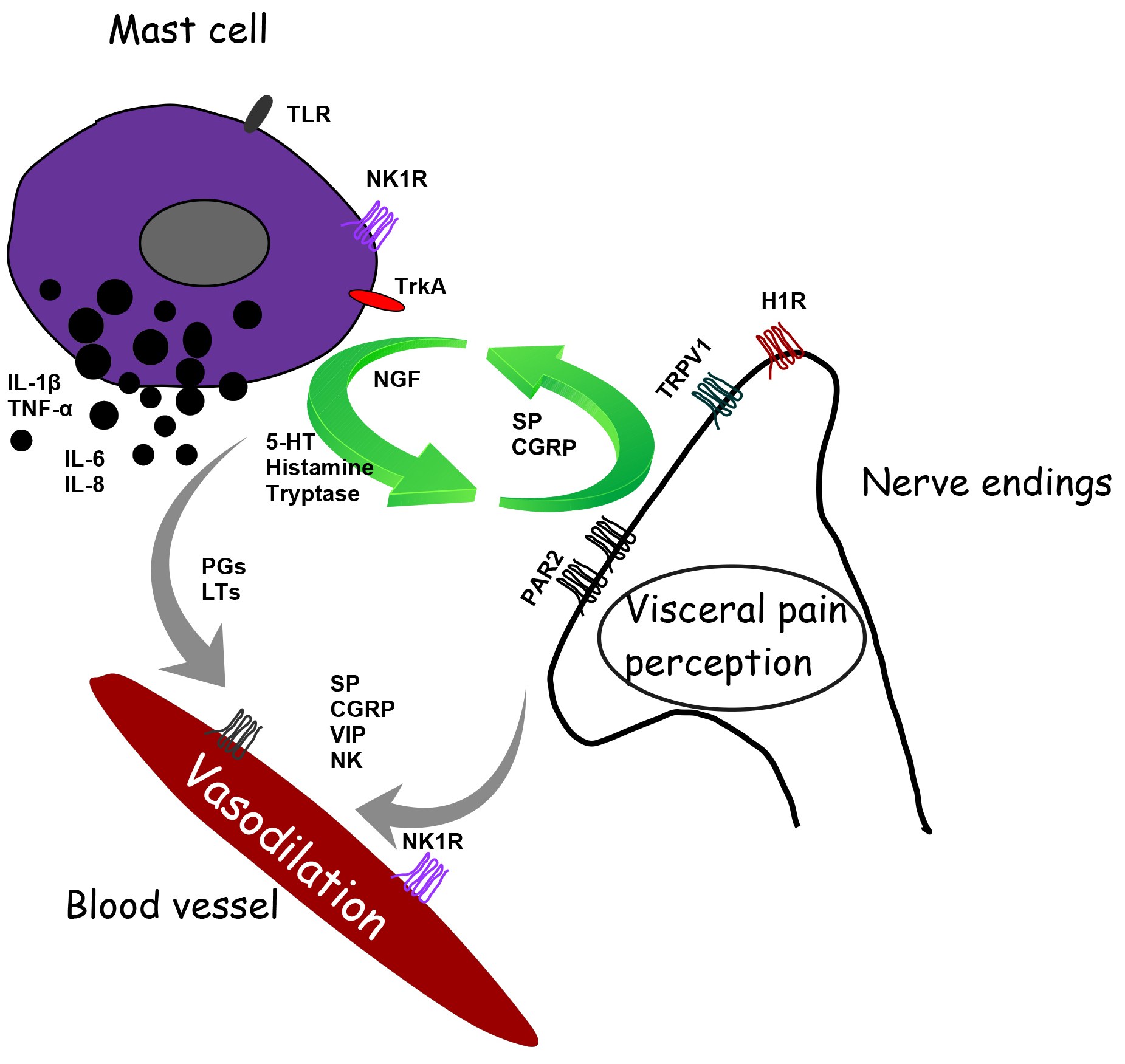 Fig. 2.
Fig. 2.Schematic representation of the interaction between mast cell and ending nerve fibers and blood vessels in the gastrointestinal tract’s mucous tissue to modulate motility and pain signaling. H1R, histamine-1 receptor; TRPV1, transient receptor vanilloid 1; 5-HT, serotonin; 5-HT3, 5-hydroxytryptamine receptor; PAR2, proteinase-activated receptor-2; TrkA, receptor for nerve growth factor; TLR, toll-like receptor; NK1, neurokinin 1 receptor; SP, substance P; CGRP, calcitonin-related gene peptide; NGF, neuronal growth factor; PGs, prostaglandins; LTs, leukotrienes; VIP, vasoactive intestinal polypeptide.
Afferent innervation of enteric MCs can trigger histamine release and proteases, increasing the sensitivity of spinal afferent terminals in a paracrine manner [23]. Moreover, MCs synthesize and store nerve growth factor (NGF) that, in an autocrine manner, stimulates MCs to release pro-nociceptive mediators such as histamine and NGF, establishing a positive feedback loop. In particular, NGF binds to its receptor TrkA evoking pain hypersensitivity through TRPV1 channels [54] (Fig. 2).
MCs are also present in the brain. MC progenitors enter the brain from the leptomeninges during the first stages of development by penetrating blood vessels and residing in CNS [30]. The rats’ neurons secrete stem cell factor, a cytokine necessary for MC survival, proliferation, and differentiation [3]. Peptidergic neurons modulate MC activity by secreting neuromediators; for example, SP is very useful in inducing histamine release from brain MCs [18]. In physiological conditions, the total number of MCs present in the CNS is limited [50].
Nevertheless, they are potent cells, and even a few MCs can release a sufficient quantity of inflammatory mediators that can affect the BBB integrity and activate glia and neurons in the CNS [55]. MCs are present in different brain areas, including area postrema, parenchyma of the thalamus and hypothalamus, leptomeninges, pineal organ, infundibulum, choroid plexus and in dura mater of the spinal cord [50, 56]. Most MCs are positioned on the abluminal side of the blood vessels, where they can interact with neurons, glia and endothelial cells. However, the exact number of MCs in the brain is difficult to calculate, as well as the extent of their activation, because it changes correlated with age, sex, and animal species and also in response to external environmental conditions, such as trauma and stress [55]. In particular, manipulating the rat pup reduced the number of MCs in the brain, possibly with an increase in degranulation [57]. In the rat thalamus, the number of MCs is more significant in females’ brains than in males and greater in the left hemisphere than in the right. These data suggest that MCs may have a specialized function in the thalamus and/or that the local microenvironment is suitable for MC accumulation [58]. On the other hand, newborn male rats have a higher number of activated MCs in the preoptic area, a brain region crucial for male copulatory behavior. Histamine induces the masculinization of microglia and stimulates it to release PGE2, which drives the masculinization of the male’s preoptic area and typical sexual behavior [59].
Once activated, MCs can start, amplify and prolong immune and nerve responses. However, the contents of the granules have also been reported to have anti-inflammatory effects. In particular, the protease chymase can prevent the damaging effects of pro-inflammatory mediators, such as TNF, likely through the degradation of some pro-inflammatory mediators, such as IL-6, IL-13, IL-33, CC-chemokine ligand 2, CCL3, CCL5 as well alarmin molecules [60, 61, 62].
At basal levels, both pro-and anti-inflammatory mediators might be essential in neuroplasticity phenomena [63, 64, 65], whereas, at high levels, they bring about an acute inflammation that, in turn, can induce a chronic inflammation state that can achieve neurodegeneration. Both glia and activated cells (MCs and monocytes/macrophages) produce soluble inflammatory molecules including cytokines, chemokines, reactive oxygen species (ROS) and NO, which are crucial mediators of persistent neuronal damage, oxidative stress and death of neurons, enhancing brain sensitivity to stress [66]. Prolonged neurologic inflammation may have harmful effects involving brain parenchyma changes, BBB alterations, neuronal hyperexcitability, and neuronal death. Adhesion molecules, cytokines, chemokines, and metalloproteases contribute to developing the brain’s inflammatory response by the degradation of extracellular matrix and tissue remodeling. Persistent neuroinflammation can cause CNS injury and cancer [6, 12, 56]. Otherwise, it is possible to assume that cytokines’ net synaptic and neuronal effect results from a delicate balance between pro-and anti-inflammatory molecules.
In the brain, MCs and microglia are innate immune cells, and also astrocytes are
immunocompetent cells since, when stimulated, they can release pro-inflammatory
signaling molecules [67, 68]. The brain is an immune privilege organ because it
has BBB, an active interface between the circulatory system and CNS, which
restricts the free movement of substances between the two compartments. BBB plays
a crucial role in maintaining homeostasis in the CNS. The BBB endothelium
presents TJs, consisting of transmembrane proteins that limit paracellular
transport. It functions to protect the brain from unwanted blood-born materials,
supporting its unique metabolic needs, and it defines a stable environment
crucial for brain homeostasis [67, 69]. Pericytes, endothelial cells, glial
cells, and end-foot of astrocytes form the neurovascular unit where MCs and
neurons are co-localized on the abluminar side of BBB. Infections and
inflammation conditions can induce BBB disruption, resulting in ion unbalance,
entry of immune and plasma molecules and an unstable CNS environment. The passage
of autoreactive T cells into the CNS is under the influence of MCs. MCs exert
such an effect by altering vascular permeability by releasing the histamine and
SP and recruiting inflammatory cells. MCs are residents in the CNS and skillful
to cross BBB into the brain from the peripheral tissue in neuroinflammatory
conditions and physiological conditions [69]. MCs can recruit and activate other
inflammatory cells and glial cells at the inflammation site and induce
vasodilation in neuroinflammation. TNF-
Neurons can activate and modulate MCs. The released mediators from MCs can
modulate the function of microglia, astrocytes, neurons and immune system cells
[67, 68]. In particular, MCs can directly communicate with neurons through CADM1,
N-cadherin, and the transgranulation process [73]. The neurons can
acquire products from MCs through a mechanism of neuron-immune
communication. The outcome is to alter the responsiveness of neurons or
supply material derived from MC that the neuron can then re-release. It has been
reported that MCs trans-granulate heparin, which, in turn, can interfere with
calcium homeostasis, resulting in inhibition of neuronal response [73]. Microglia
are activated by ROS, pro-inflammatory cytokines, chemokines, TNF-a, IL-6, and
IL-12, which are neurotoxics and activate astrocytes. In turn, astrocytes
activate MCs that degranulate and/or transgranular in brain parenchyma with a
consequent abnormal release in inflammatory and neurotrophic mediators according
to a positive feedback loop that may eventually result in neuronal damage [12].
Astrocytes can release IL-33 upon injury. This interleukin is an alarmin cytokine
that orchestrates microglia and MCs, which, in turn, release IL-6, IL-8, IL-13
and chemokines. Both microglia and astrocytes express histamine receptors H1R,
H2R and H3R (microglia also has H4R). Finally, leukocytes are attracted in the
area inducing an adaptive immune response [30, 74]. The MCs multiphasic pattern
by which granular preformed material is released in a few minutes and new
synthesis mediators in the next hours allows them to act as catalysts that
amplify and extend many cellular vasoactive, neuroactive, immunoreactive and
endocrine responses. Also, glia and MCs reactivate each other in the brain
through co-stimulating a wide variety of molecules or inflammatory mediators,
including TNF-
From what has been presented, MCs’ role in neural disorders is central and of great interest and is supported by numerous recent scientific evidence [75]. Acute and chronic stressful conditions can activate MC, and this activation can initiate, fuel and progress neurodegenerative diseases through neuroglia activation and increased permeability to the BBB. Dysregulation of the histaminergic system of the brain leads to neuropsychiatric disorders. The H3R receptor, which is mainly expressed in the CNS, can be a promising pharmacotherapeutic target. For example, histamine dysregulation is likely involved in Tourette’s syndrome and tic disorders; preclinical studies of H3R antagonists in schizophrenia, attention deficit disorder and narcolepsy have shown promising results [76]. MCs are directly involved in various other disorders ranging from autoimmune diseases to fibromyalgia, from AD to stroke and intracerebral hemorrhage [77, 78]. Various evidence suggests the involvement of MC in multiple sclerosis (MS) and rheumatoid arthritis (RA) [79, 80]. In a stroke, MCs are activated after blockage of the cerebral blood vessels, releasing vasoactive and proinflammatory molecules. These result in vasodilation, immune cell recruitment, and BBB damage [81]. Studies reported a significant distribution of MCs in brains with amyloid deposits bringing them to degranulation [82].
Interestingly, it has recently been reported that diseases associated with MCs, including allergy and neuroinflammatory pain disorders, are sexually biased, with women at greater risk [83]. Perinatal gonadal androgens, but not adult androgens, appear to play an important protective role in the severity of MC-mediated anaphylaxis in adults. In particular, these protective effects would manifest themselves through the programming of MC precursors of the bone marrow that would be guided towards a phenotype with reduced release of histamine, 5-HT, protease and, in general, of the granules. This evidence focuses on perinatal life as a critical period for potential interventions to alleviate the risk of MCs’ diseases.
MS is a chronic neurological disease characterized by demyelination and axonal loss. Various studies have reported that cytokines are involved in myelin destruction and remyelination and repair, and there is a close relationship between inflammation and exacerbation of MS [71]. Levels of tryptase and protease are increased in the cerebrospinal fluid of MS patients, and histamine is elevated in the blood. It has been reported that myelin can activate MCs that play a role in the demyelination process [84]. Degranulation of MCs results closely associated with demyelination in MS patients [85]. Stabilization of MCs has provided promising treatment of plaques in MS patients’ brains [86]. Finally, the gut microbiota strongly influences the immune responses and inflammatory profiles of MS and modulating the microbiota could be a therapeutic strategy for MS patients [87]. Butyrate treatment would lead to suppression of demyelination and facilitate remyelination in in vitro models [88].
Autism Spectrum Disorder (ASD) collects a specific combination of behavioral and communication impairments that appear early in life. It is an incredibly multifaceted disorder that affects the gastrointestinal, immune tract and nervous systems. The worldwide incidence is about 1%. Small anatomical and functional differences were observed in post-mortem and neuroimaging studies. Factors that can induce the breakdown of epithelial barriers, the loss of tolerance of the immune system and therefore, immune dysfunction can be involved in the pathogenesis of ASD [89]. Psychosocial interventions can improve certain behaviors by reducing the severity of symptoms. Various subgroups have been outlined within the ASDs, including gastrointestinal problems, mitochondrial dysfunction e allergic symptoms, such as food intolerances and eczema [90]. However, none of these were identified by unique biomarkers. Evidence suggests that brain inflammation is crucial in the pathogenesis of neuropsychiatric disorders, including ASD [91, 92]. Activation of microglia has been reported in the brains of patients with ASD. MCs can activate the microglia. Indeed, the risk of ASD appears to be 10 times higher in children with mastocytosis, a condition characterized by an increase in the number of activated MCs [91].
Finally, an association between ASD and intestinal microbiota composition was highlighted. Subjects with ASD show a reduction in some bacterial Phyla, such as Akkermansia, Bacteroides, Bifidobacterium, E. coli and Enterococcus, and more significant quantities of Faecalibacterium and Lactobacillus, and an increase of Ruminococcus and Clostridium [93]. Studies confirm that an alteration of the intestinal microbiota in children with ASD, including abnormalities in colonization by B. longum and F. prausnitzii, are present [13, 94]. The concomitant development between gut microbiota and brain circuits, including circuits involved in social and emotional cognition, supports a role for gut microbiota and its metabolites in the symptoms and progress of ASD. A product of microbial metabolism such as butyrate has been suggested to have important beneficial effects on the human gut homeostasis, preside over immune functions and play a role in the control of various neurological and neuropsychiatric disorders, including ASD [95, 96, 97].
In RA, MCs can produce pro-arthritogenic molecules and support the aberrant survival of human rheumatoid synovial fibroblasts [98]. Interestingly, MCs are highly tunable cells, capable of influencing immune responses to both pro-inflammatory and anti-inflammatory responses, depending on the type of environment and the triggers to which they are exposed [99]. In the context of arthritis, MCs produce pro-inflammatory molecules [100]. However, in response to IL-33 and immune complexes, MCs can also reduce the activation of monocytes. For example, histamine can also induce immunomodulatory and anti-inflammatory effects. MCs’ immunomodulatory action in RA is supported by evidence that serum levels of tryptase and synovial tryptase mRNA are inversely correlated with inflammatory markers [81]. Most neurological complications in RA are related to joint inflammation and damage due to compression of adjacent structures of the central or peripheral nervous system [101]. It has been observed that the intestinal microbiota is altered before the onset of RA, particularly Prevotella spp in patients with pre-rheumatoid arthritis, supporting the concept that the host genotype is related to the gut microbiota profile [102].
Numerous neurological, cognitive and psychiatric conditions are frequently associated with patients suffering from mastocytosis, including headaches, migraine, sleep disturbances, attention and memory changes, anxiety, depressive-like symptoms [57, 103, 104, 105, 106]. Stress conditions activate MCs, and the MC-derived molecules can increase the BBB’s permeability [92, 107]. The increased IL-6 release from mouse leukocytes is closely related to the likelihood that mice exhibit a chronic stress-sensitive phenotype. Stress increases vascular permeability by stimulation of MC from CRH [92]. This leads to an ionic imbalance, and therefore the entry of immune molecules and an unstable CNS environment [67]. The entry of reactive T lymphocytes is under the influence of MC. Increased CRH results in sensitization of nerve endings [108]. Finally, a lasting increase in glucocorticoid levels damages neurons by altering neurogenesis and is also associated with a reduction in the hippocampus’s volume and impaired memory, perception and attention [109].
Evidence suggests that major depression is mainly prevalent in subjects affected by chronic infections. This observation suggests that a chronic inflammation condition can increase depression incidence [110]. Interferons and interleukins have been causally involved in depression. In particular, proinflammatory cytokines can induce the enzyme indoleamine 2,3-dioxygenase (IDO) responsible for the catabolism of tryptophan to kynurenine, and higher levels of kynurenine are associated with depression [111, 112]. While the role of inflammation in the pathophysiology of depression has been evidenced, MCs’ involvement in this field remains unexplored. However, some studies have reported that the prevalence of depression is evident in patients with mastocytosis. These subjects show low levels of tryptophan and 5-HT and high IDO1 and acid kynurenine activity. MCs can be activated by kynurenine metabolites [103, 113], while molecules released by the MC can influence the IDO pathway leading to an imbalance between kynurenine and 5-HT ratio. Yet, pro-inflammatory cytokines increase monoamine reuptake by further reducing 5-HT levels [50, 67, 97, 112]. Gut microbiota produces neurotransmitters such as GABA, 5-HT, and dopamine. Unlike 5-HT, the tryptophan produced by the intestinal microbiota can permeate the BBB and access the brain and positively affect mood by increasing the levels of 5-HT in the brain [13, 68]. Moreover, the intestinal microbiota can directly use tryptophan and regulate the availability of tryptophan for the metabolism of the kynurenine pathway [114].
Macrophages, microglia, and MCs present in the CNS when activated release pro-inflammatory cytokines that cause increased levels of arachidonic acid product and lead to various neurological manifestations, including migraine [115]. ATP released by astrocytes, neurons, platelets, endothelial cells, and MCs acts as a potent pro-nociceptive and pro-inflammatory agent. Moreover, it is known that MCs communicate with microglia and astrocytes, giving rise to positive feedback and amplification of the phenomenon [68]. Released during a migraine attack, ATP directly excites trigeminal nerve terminals through the degranulation of local MCs and the action on nerve endings [116, 117, 118]. Finally, migraine is closely related to the microbiota-gut-brain axis [119].
However, MCs and neuroinflammation also have important functional significance
in health conditions. Neuroinflammation can influence neurogenesis. During
neurogenesis, new neuronal cells are generated from progenitor cells mainly in
two areas of the hippocampus, the subgranular zone and in the subventricular
zone. MCs are a source of 5-HT in the mouse hippocampus [120]. As a result,
MC-deficient mice exhibit impairment in hippocampal neurogenesis, which was
reversed after chronic treatment with fluoxetine, a selective 5-HT reuptake
inhibitor. MC’s granular contents can also cross a big deal, and their location
in leptomeninges enables the release of MCs mediators into the cerebral spinal
fluid, allowing access to hippocampal parenchyma [104, 121]. Also, they supply
5-HT and possibly other products to hippocampal tissue and affect cell
proliferation and behavior, suggesting that MCs can contribute to the
hippocampus’s behavioral and physiological functions. Mice with MC deficiency
exhibit hippocampal-dependent spatial learning and memory deficits, as found in
Morris water maze and radial arm maze behavioral tests [120]. MCs play a
physiological role in neuroimmune interactions, even in the absence of
inflammatory responses [37, 122]. On the other hand, neuroinflammation modulates
neurogenesis negatively and positively [123]. Histamine can play a neurogenic
role via H2R and differentiation through H3R. Also, serotonin promotes
hippocampal neurogenesis. MC-deficient mice have reduced the volume of
hippocampal granule cells and cell proliferation [120]. Also, TNF-
An alteration of synaptic transmission is a crucial measurement of
neurodegeneration. Long-term potentiation (LTP) is a form of synaptic plasticity,
and it is considered important for learning and memory processing in the
hippocampus [125]. During normal synaptic transmission, glutamate released from
the presynaptic terminal acts on AMPA receptors in dendritic spines, resulting in
an excitatory postsynaptic potential, whereas NMDA receptors are blocked by
magnesium. Tetanic stimulation in the afferent pathway induces LTP by increasing
synaptic transmission. During activity-induced depolarization, magnesium blockage
is removed, and calcium flows into the post-synaptic neuron. LTP can be modulated
by various brain mediators, including cytokines that can exert their action in
both physiological and pathological conditions [126, 127]. A cytokine can induce
differential, even opposite cell responses [128]. When expressed at basal levels
in a healthy brain, cytokines have an essential role in bidirectional
communication and synaptic plasticity modulation. IL-6 and Il-1
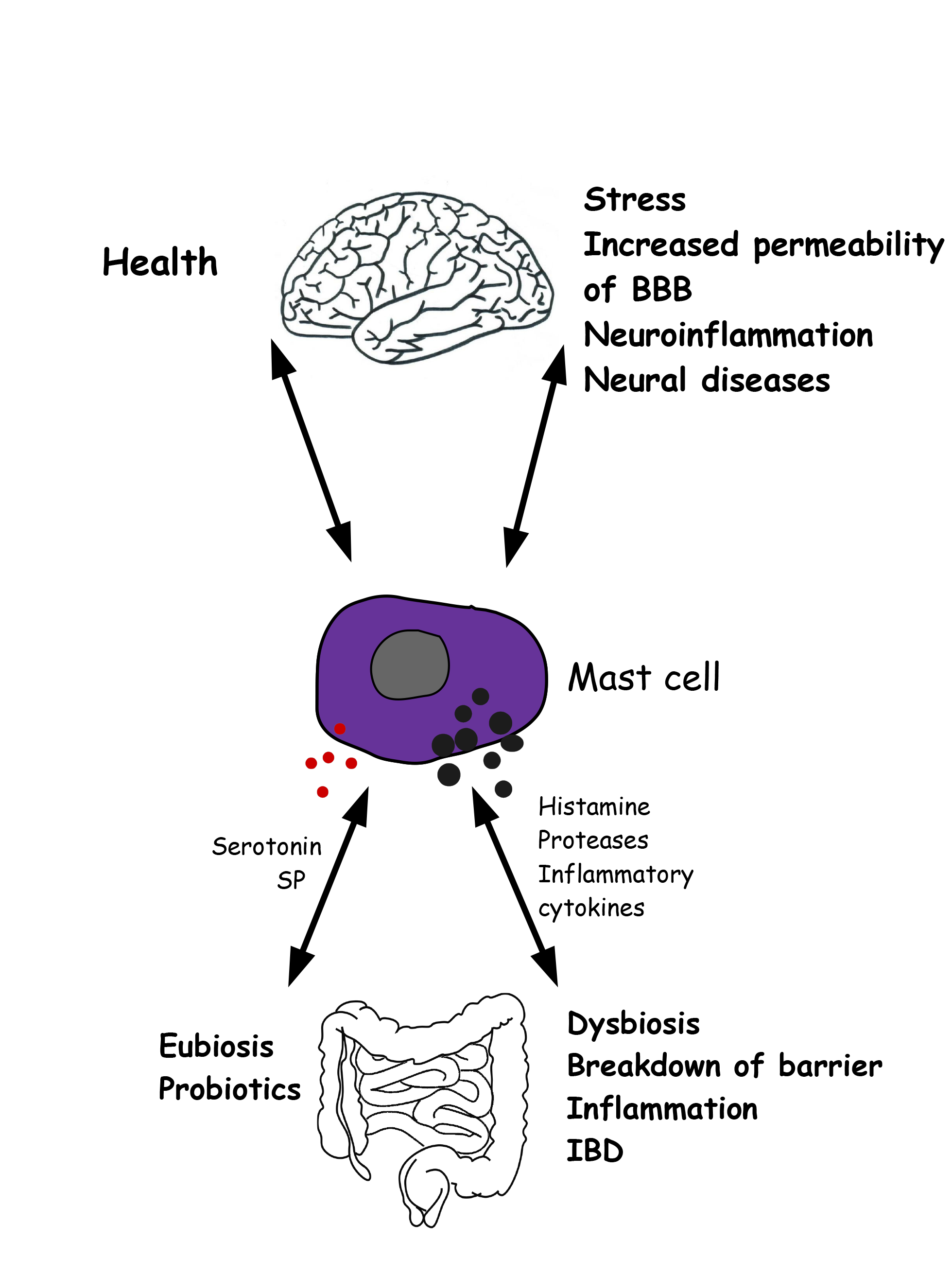
As MCs are an essential source of a wide variety of cytokines and growth factors, it appears, from the above, that they play a significant role in both health and disease. Future studies will allow us to reveal how crucial their contribution is to these phenomena (Fig. 3).
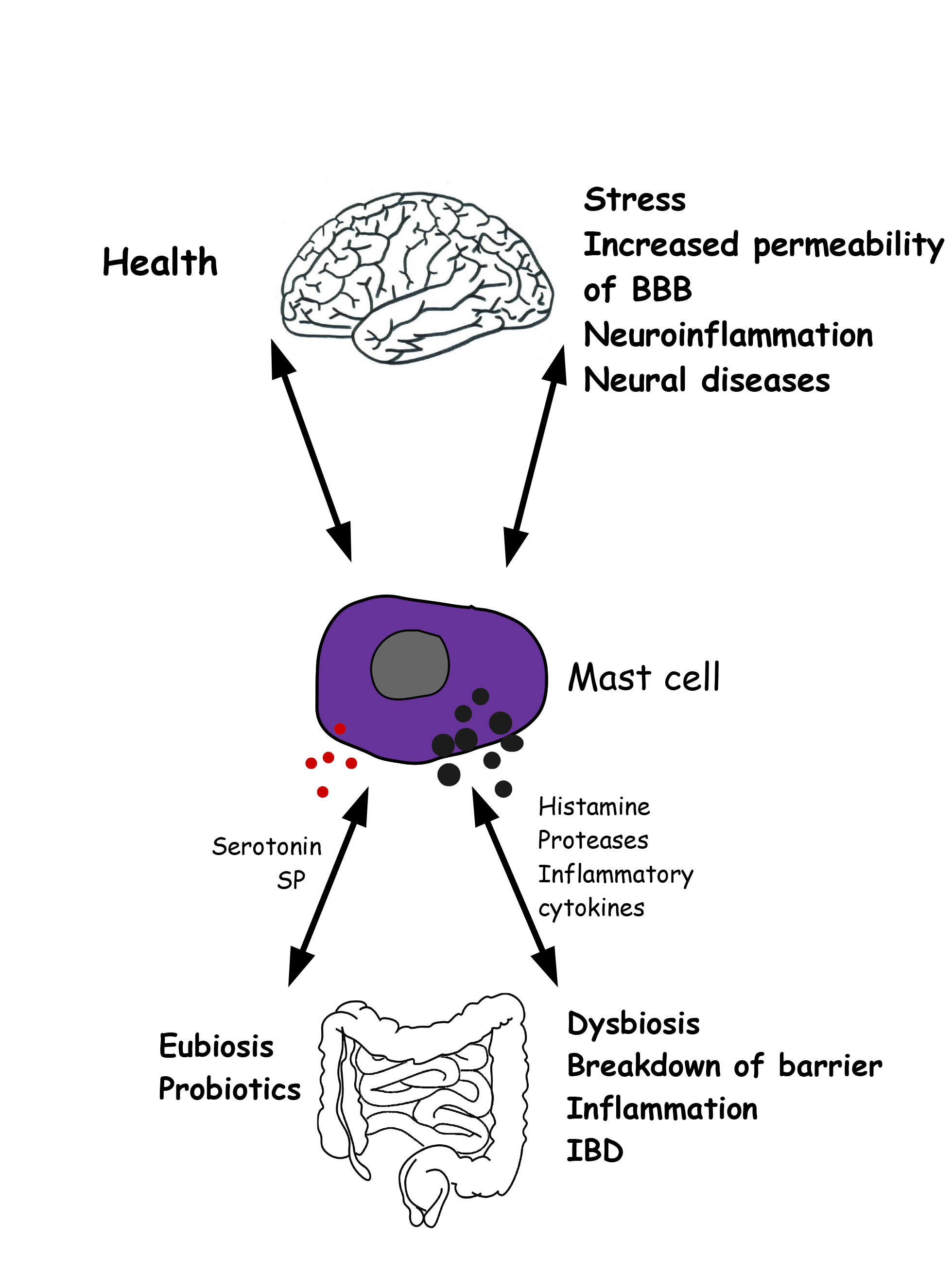 Fig. 3.
Fig. 3.Gut microbiota-mast cell-brain interconnections in health and disease. Left) In healthy condition, reciprocal interations between mast cells, gut microbiota and brain garantee the correct function of gut and brain. Right) Altered conditions involve mast cell activation, release of inflammatory molecules, intestinal dysbiosis, increased permeability of both the blood brain barrier and the intestinal epithelial barrier, neural disorders.
The immune system has become a focal point of novel therapeutic targets for treating neurological disorders, including psychiatric diseases. The dysregulation of the immune system can have a negative impact on the functioning of the brain. Cytokines released in the periphery during an immune response enter the brain and modulate brain systems. Manipulation, sex and stress involve a change in the number of brain MCs, and it is interesting to note that all these manipulations increase the excitation of the CNS.
The microbial composition may play a role in several conditions involving the brain, and the microbiota-gut-brain axis may affect emotions, motivation and other complex cognitive functions. The microbiota can serve its host to neutralize drugs and carcinogens, modulating motility and affecting visceral perception. MCs exacerbate neuroinflammation, functioning as proinflammatory cells. Stabilizing the MCs provides interesting tools to control the permeability of the intestinal epithelial barrier. Therefore, MCs are excellent indicators and health tools and can provide suggestions for therapeutical interventions.
5-HT, 5, hydroxytryptamine, serotonin; AD, Alzheimer’s disease; ASD, autism spectrum disorder; BBB, blood-brain barrier; CGRP, calcitonin gene-related peptide; CNS, central nervous system; CRH, corticotropin-releasing hormone; HPA, hypothalamic-pituitary-adrenal; HR, histamine receptor; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; ICAM-1, intercellular adhesion molecule-1; IDO, indoleamine 2,3-dioxygenase; IL, interleukin; LTP, long term potentiation; MC, mast cell; MS, multiple sclerosis; NGF, nerve growth factor; NK1, neurokinin 1; PAR-2, proteinase-activated receptors; PD, Parkinson’s disease; PGE, prostaglandin; RA, rheumatoid arthritis; SCFA, short-chain fatty acid; SP, substance P; TGF, Transforming growth factor; TJ, tight junction; TLR, Toll-Like Receptor; TNF, tumor necrosis factor; TRPV1, Transient Receptor Potential Vanilloid 1; VIP, vasoactive intestinal peptide.
GT researched and summarized the information and wrote the paper.
Thanks to all the peer reviewers for their opinions and suggestions.
The author declares no conflict of interest.