1 Department of Exercise and Health Promotion, College of Kinesiology and Health, Chinese Culture University, 111, Taipei, Taiwan
2 Department of Plastic Surgery, Taipei Medical University Hospital, Taipei Medical University, 110, Taipei, Taiwan
3 Department of Surgery, School of Medicine, College of Medicine, Taipei Medical University, 110, Taipei, Taiwan
4 Graduate Institute of Biomedical Optomechatronics, College of Biomedical Engineering, Taipei Medical University, 110, Taipei, Taiwan
5 Department of Plastic and Reconstructive Surgery, Chang Gung Memorial Hospital, Chang Gung University, 333, Taipei, Taiwan
6 Division of Neurosurgery, Department of Surgery, Kuang Tien General Hospital, 433, Taichung, Taiwan
7 Department of Biotechnology and Animal Science, College of Bioresources, National Ilan University, 260, Yilan, Taiwan
8 Department of Biotechnology, College of Medical and Health Care, Hung Kuang University, 433, Taichung, Taiwan
9 Department of Health Business Administration, College of Medical and Health Care, Hung Kuang University, 433, Taichung, Taiwan
Abstract
Therapeutic strategies for traumatic spinal cord injury generally involve rectifying concomitant destruction to the spinal cord from inflammation, mitochondrial dysfunction, and eventual neuronal apoptosis. Elevating the expression of spinal cord injury-attenuated CDGSH iron-sulfur domain-2 has been shown to mitigate the pathologies above. In the current work, hypothermia was induced via continuous cryogen spray cooling in a rat spinal cord hemisection model. Spinal cord injury was shown to elevate the mRNA expression of proinflammatory mediators, including NF
Keywords
- Cryogen spray cooling
- hypothermia
- CISD2
- inflammatory response
- astrocyte activation
- apoptosis
- neuronal loss
- spinal cord injury
The incidence of acute spinal cord injury (SCI) in the general population ranges from 15-40 cases per million worldwide. The pathophysiology of acute SCI initially involves significant insult to the spinal cord, resulting in immediate structural damage, including cell membrane rupture, myelin and axon damage, and microvascular destruction. Secondary injuries include profound proinflammatory responses, excitotoxicity, hyperoxidation, mitochondrial dysfunction, and eventual apoptotic cell death (Houle and Tessler, 2003; Bethea and Dietrich, 2002). Several molecular mechanisms have been implicated in SCI’s pathogenesis, including hypoxia, ischemia, lipid peroxidation, free radical production, neutral protease activation, prostaglandin production, and programmed cell death (Bethea and Dietrich, 2002; Hung et al., 2007; Tator and Fehlings, 1991). Therapeutic strategies targeting secondary damage focus on reducing neuronal loss.
Amino acid CDGSH iron sulfur domain 2 (CISD2) plays an important
role in calcium metabolism (Shen et al., 2017) as well as anti-apoptotic
(Chang et al., 2010) and anti-inflammatory activities (Lin, 2020; Kung et al., 2020). CISD2 helps preserve the mitochondrial membrane’s integrity and
thereby prevents mitochondrial malfunction and ultimate cell death. CISD2
depletion has been linked to mitochondrial breakdown and dysfunction accompanied
by autophagic cell death in CISD2 knockout mice (Chen et al., 2009). At the
endoplasmic reticulum, CISD2 has been shown to attenuate calcium-mediated
excitotoxicity through the binding of CISD2 to BCL2 and the inositol 1, 4,
5-triphosphate receptor (Chang et al., 2012). CISD2 has also been shown to
enhance the combination of BCL2 and BECN1, which regulates cellular
autophagy/apoptosis (Kang et al., 2011). CISD2 deficiency has been linked to
an increase in iNOS and a reduction in BCL2 levels in SH-SY5Y cells challenged
with lipopolysaccharide (LPS) (Lin et al., 2017). CISD2 deficiency has also
been linked to augmented nuclear translocation of the NF
Therapeutic hypothermia aimed at halting tumor progression was first reported in 1945 (Kwon et al., 2008). Over the last half-century, therapeutic hypothermia has been evaluated for its neuroprotective effects on the brain and spinal cord in situations such as ischemic or traumatic brain injury (TBI) (Bernard and Buist, 2003; Lo Jr et al., 2009; Hansebout et al., 1984; Yu et al., 2000). Therapeutic hypothermia can be managed systemically or locally. Systemic treatment involves the cooling of external surfaces or internally via the endovascular system. Local hypothermia avoids the side effects of systemic hypothermia while allowing a more pronounced reduction in temperature at the injury site (Jordan and Carhuapoma, 2007). Researchers have demonstrated therapeutic hypothermia’s efficacy in attenuating secondary injury by reducing neuroinflammation and cellular apoptosis in ischemic brain injury (Ohmura et al., 2005). Therapeutic hypothermia has been shown to preserve damaged tissue and promote impaired locomotor function in experiments on SCI rats and SCI patients undergoing systemic hypothermia (Lo Jr et al., 2009; Yu et al., 2000) or local spinal cord cooling (Hansebout et al., 1984).
In 1994, Nelson et al. (1995) introduced cryogen spray cooling (CSC) to induce local hypothermia during the spatially selective photocoagulation of biological tissue. This method involves spraying cryogen on the skin’s surface for a short duration, such that the effects of cooling remain localized in the epidermis and the temperature of deeper blood vessels remains unaffected (Waldorf et al., 1997; Nelson et al., 1996). As mentioned above, neural injuries can lead to CISD2 downregulation, resulting in exaggerated inflammatory reactions. In the current work, we sought to verify the neuroprotective (anti-inflammatory and CISD2-preserving) effects of therapeutic hypothermia induced through the continuous delivery of CSC in a rat spinal cord hemisection model.
Male Sprague-Dawley (SD) rats (Academia Sinica, Taipei, Taiwan) weighing 280-330 g were maintained at a density of two animals per cage for at least 5 days after they arrived at our laboratory. The rats were given ad libitum access to food and water while housed within a room with a 12 : 12 h light-dark cycle. All rats were treated as per the guidelines stipulated by the Experimental Animal Laboratory and approved by the Animal Care and Use Committee of Chang Gung University (IACUC Approval No.: CGU14-052).
The surgical procedure used in the lateral hemisection of the spinal cord in rats can be found in our previous studies (Lin et al., 2011, 2012). Briefly, isoflurane was delivered to the rats as general anesthesia, before the animals were fastened within a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). An adjustable wire knife was used to create a spinal cord lesion on the left side. Control animals were subjected to a sham operation (laminectomy without hemisection). Posterior decompression was achieved via laminectomy at the eleventh thoracic vertebrae using a fine diamond drill. The wire knife guide was positioned vertically adjacent to the lateral surface at the spinal cord’s lower thoracic level. This permitted analysis of the healthy cephalad and caudal segments of the spinal cord. The knife was turned medially and then extended 1.5 mm, at which point the guide was lifted by 4.0 mm to hemi-transect the spinal cord. Iridectomy scissors were then used to cut to ascertain the thoroughness of the hemisection. The wound was closed in layers.
The rats were divided into three groups: (1) Vehicle-treated sham-operated control (sham; laminectomy only) (n = 6); (2) Vehicle-treated SCI group (SCI) (n = 6); (3) SCI rats post-treated with CSC treatment (SCI + CSC) (n = 6).
The cryogen is 1, 1, 1, 2-tetrafluoroethane, an FDA-approved replacement for
Freon, biodegradable, non-toxic, and incombustible. Following spinal cord
hemisection, cryogen spurts were immediately sprayed onto the injured spinal cord
through an electrically powered nozzle from a distance of 3 cm above the dural
surface. Coolant was delivered continuously in spurts of 20 milliseconds at
intervals of 5 spurts per minute for 10 minutes. CSC treatment lowered the
surface temperature in the treatment region to -10
 Fig. 1.
Fig. 1.CSC reduced injury-induced
inflammation following spinal cord hemisection in rats. (A) Schematic
illustration of the CSC technique applied to tissue adjacent to the spinal cord
hemisection under the following conditions: (i) Sham controls; (ii) SCI rats
undergoing SCI; (iii) SCI rats undergoing SCI followed by CSC (SCI + CSC); mRNA
expression levels of (B) NF
At 24 h post-treatment, all of the animals in all groups underwent deep anesthesia via isoflurane. The animals were then sacrificed, and the spinal cord tissue was removed to investigate mRNA expression (n = 3 in each group) and perform immunohistochemical analysis (n = 3 in each group).
Total RNA was prepared through the direct lysis of animal tissue
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Then, reverse transcribing
the mRNA into cDNA using oligo-dT, and SuperScript II reverse transcriptase
(Invitrogen, Carlsbad, CA, USA). Quantitative RT-PCR was performed using the ABI
StepOne sequence detector system (Applied Biosystems, Foster City, CA, USA) in
accordance with the SYBR Green methodology, normalized to the expression of a
housekeeping gene (GAPDH), as previously described (Lee et al., 2015). The
primer sets and product size of each cDNA of interest were as follows: rat CISD2
(Gene ID: 295457, accession number: NM_001191608, 5’- CCCTTCCTTGGTGTACTTGCA -3’
and 5’- TTTCGTTCACCACTTTGGGATT -3’, 129 bp); NF
On the second day after SCI, the animals underwent deep anesthesia
via isoflurane before being sacrificed. They were then thoroughly perfused with
phosphate-buffered saline (PBS), followed by cold 4% paraformaldehyde in 0.15 M
sodium phosphate buffer. The spinal cords were removed immediately, fixed at 4
Independent two-sample t-tests were used to compare data
between the experimental groups. One-way analysis of variance (ANOVA) was used to
determine whether there were significant differences between the means of three
or more independent (unrelated) groups. In cases where the differences were
apparent, multiple comparisons were performed using the Newman-Keuls method. Data
are presented as mean
In the current spinal cord hemisection model, rats were subjected
to CSC treatment involving short spurts (20 milliseconds) at intervals of 5
spurts per min for 10 min, or untreated with CSC (Fig. 1A). qRT-PCR was used to examine the mRNA expression of proinflammatory mediators at the site of SCI.
Compared to the sham control group, animals in the SCI only group presented
higher expression levels of NF
The qRT-PCR analysis revealed that CISD2 expression levels were
significantly lower in the SCI only group than in the sham control group
(P
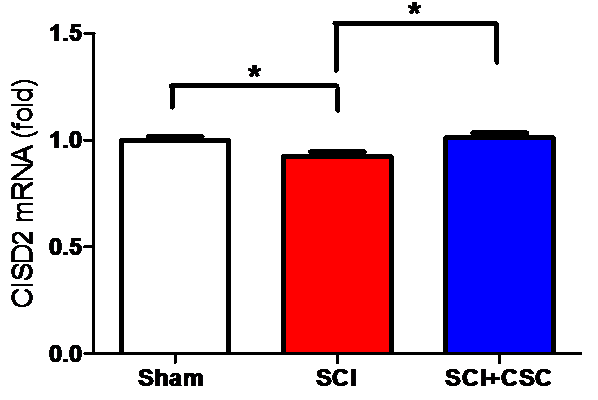 Fig. 2.
Fig. 2.Spinal cord hemisection produced a corresponding decrease in
CISD2 expression. CSC mediated the injury-induced decrease in CISD2 expression.
Vertical bars indicate the mean (
Astrocytes can be stimulated to release various pro-inflammatory
mediators in response to SCI, as indicated by GFAP over-expression.
Immunostaining for GFAP (arrow in Fig. 3C) was more robust in the SCI only
group than in the sham control group (Fig. 3B) (***P
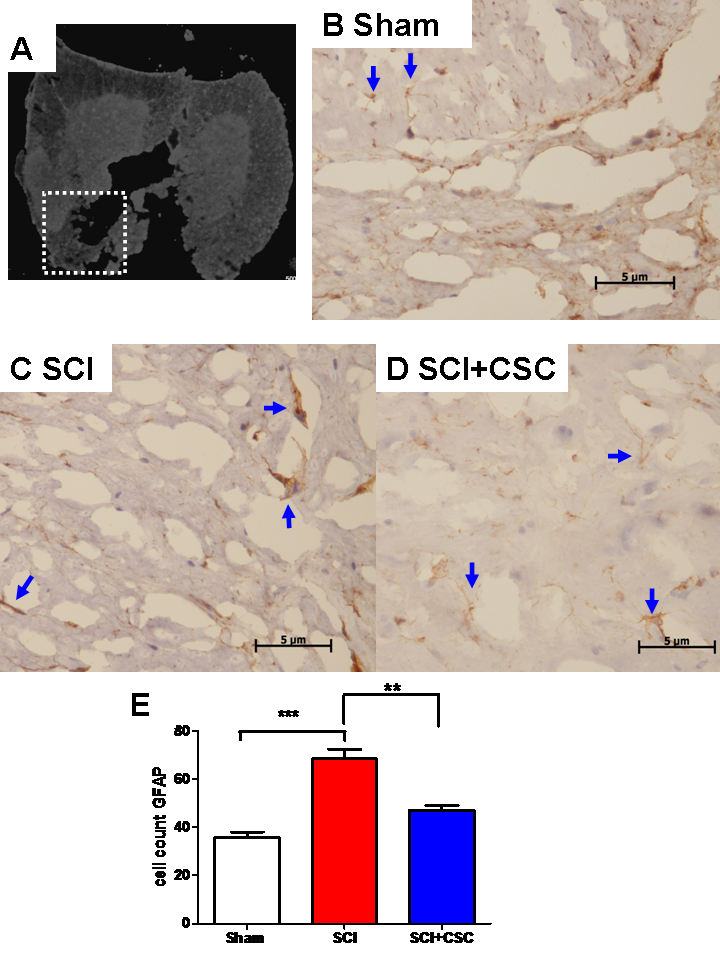 Fig. 3.
Fig. 3.Representative photographs of GFAP-stained sections of tissue
samples were obtained from the spinal cords of rats in the sham control group
(B), SCI (C), and SCI + CSC (D) after SCI. (A) Schematic depiction of ROI
(labeled as the dashed box) in injured spinal cords for all groups. Staining of
GFAP-positive cells was weaker in the sham group (B) than in the SCI group (C).
CSC treatment decreased GFAP immunofluorescence. (A-C: magnification 400
The blue arrows in Fig. 4A-C indicates the immunoreactivity of
caspase-3 as a proxy of cellular apoptosis. Following spinal cord hemisection,
immunostaining for caspase-3 was significantly higher in the SCI only group than
in the sham control group (***P
 Fig. 4.
Fig. 4.Representative photographs of caspase-3-stained
sections of tissue samples obtained from the spinal cords of rats in the (A) sham
control group, (B) SCI only group, and (C) SCI + CSC group (A-C: 400
The blue arrows in Fig. 5A present the immunohistochemistry
indicative of the neuron-specific marker NeuN in the sham group. As indicated by
the blue arrows in Fig. 5B, the morphological integrity was far worse in the
SCI only group than in the sham control group, and neuronal loss was also evident
(***P
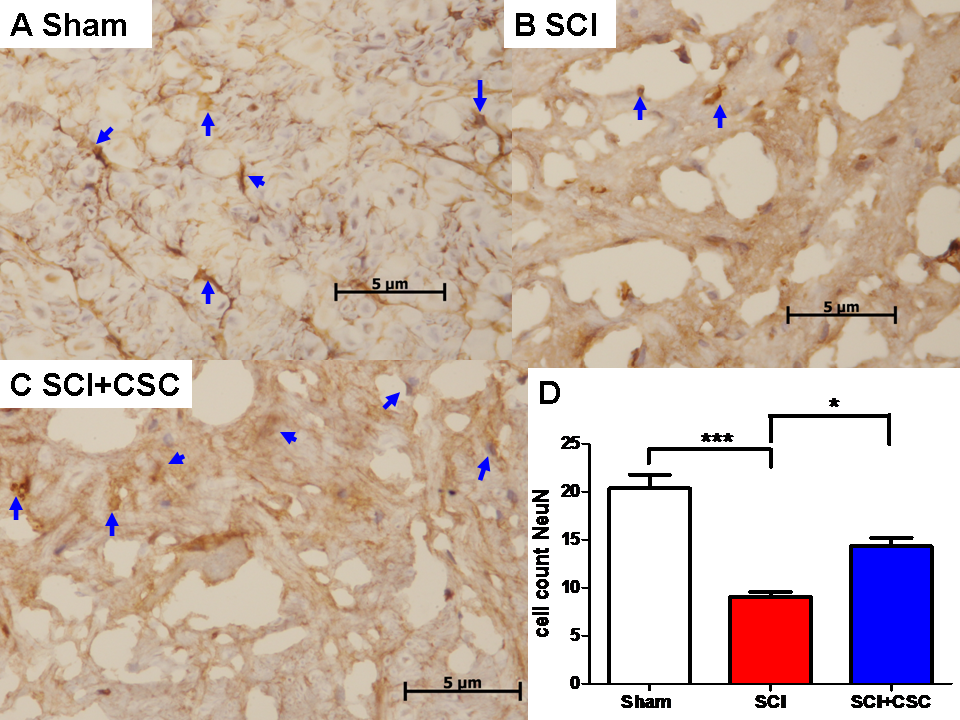 Fig. 5.
Fig. 5.Representative photographs of NeuN-stained sections of tissue
samples obtained from the spinal cords of rats in the (A) sham control group, (B)
SCI only group, and (C) SCI + CSC group (A-C: 400
As in our previous research, we determined in the current study
that spinal injury led to a decline in CISD2 expression, as evidenced by a
reduction in CISD2 immunoreactivity in the SCI only group (***P
 Fig. 6.
Fig. 6.SCI-induced a decrease in CISD2 expression in the injured
spinal cord, the effect of which was attenuated by CSC. Representative
photographs of CISD2-stained sections of tissue samples obtained from the spinal
cords of rats in the (A) sham control group, (B) SCI only group, and (C) SCI +
CSC group (A-C: 400
SCI can induce the activation of immunity-related cells in the spinal cord’s parenchyma and the activation of astrocytes and microglia (Hung et al., 2005; Davies et al., 1996). Reactivated astrocytes produce inflammatory cytokines and chemokines, which trigger microglial activation (favoring the detrimental M1 over the protective M2 phenotype) (Liu et al., 2011; Luo et al., 2002). Injury-activated astrocytes and microglia release considerable nitric oxide and ROS quantities, which amplify the inflammatory cascade (Li et al., 2007). Profound inflammatory responses can eventually lead to mitochondrial dysfunction and the enhanced production of ROS and nitric oxygen species (NOS) (Lucas et al., 2006; van Horssen et al., 2019), which can, in turn, contribute to neuronal apoptosis (Xu et al., 2006) and potential neurological deficits (Hurlbert, 2006). Thus, the therapeutic targeting of acute SCI can help to mitigate secondary insults to the spinal cord.
Therapeutic hypothermia has been shown to exert neuroprotective effects in the central nervous system (CNS) in response to injuries, such as TBI and SCI (Bernard and Buist, 2003). Animal studies have demonstrated that physiological ATP concentrations can be maintained by decreasing cellular consumption or increasing production through glycolysis, thereby preserving the CNS’s neurological function (Arrica and Bissonnette, 2007). In cases of hypoxic-ischemic brain insults, therapeutic hypothermia has been shown to attenuate secondary damage by decreasing proinflammatory responses, such as neutrophil and microglia stimulation. Therapeutic hypothermia has also been shown to decrease the generation of free radicals (which would otherwise lead to oxidative stress) and extracellular glutamate levels (which would otherwise lead to excitotoxicity) (Hachimi-Idrissi et al., 2004). Finally, therapeutic hypothermia has been shown to mediate secondary insults related to SCI, including the release of cytochrome-3 and the activation of caspase-3 and calpain (Ohmura et al., 2005).
One secondary injury related to SCI involves the hypertrophic formation of astrocytes and increased GFAP expression, which can, in turn, lead to glial scarring and a corresponding decrease in axonal and neuronal regeneration (Morino et al., 2003; Stichel and Müller, 1998). Glia-mediated neuroinflammation following acute SCI can lead to mitochondrial dysfunction, cellular apoptosis, and eventual neuron loss (Scholpa and Schnellmann, 2017). Ameliorating injury-induced astrocyte activation has been shown to help prevent pathogenesis in cases of SCI. In the current study, the injury-induced expression of GFAP, caspase-3, and NeuN indicates pathogenesis, including respective astrocytic proliferation, cellular apoptosis, and neuronal damage. We discovered that CSC-induced hypothermia helped attenuate secondary injuries following SCI, including astrocyte-associated inflammatory response, apoptosis, and neuronal deficit.
We found that CSC-induced hypothermia led to the upregulation of
CISD2, even though acute SCI tends to downregulate the expression of CISD2. CSC’s
anti-inflammatory effects can be attributed to the fact that CSC treatment
attenuated the downregulation of CISD2 mRNA and proteins. In previous siRNA
knockdown models aimed at decreasing CISD2 expression levels, we observed
elevated inflammatory responses in SH-SY5Y neuronal-like cells (Lin et al., 2019) and EOC microglia (Lin, 2020). We also confirmed that CISD2 inhibits
inflammation via the NF
The current study has several limitations, which must be considered in the interpretation of our findings. In the current study, we were limited to only three animals in each group. Many inflammation-related inflammatory factors, such as IL-4, were not addressed, and we did not collect samples at other time points. Subsequent in vivo studies will be required to assess CSC treatment’s long-term effects on upstream and downstream inflammatory signaling. Note that we did not perform CISD2 knockdown or knockout; therefore, a causal relationship cannot be determined between CISD2 and injury-induced inflammation following CSC.
Nonetheless, the anti-inflammatory effects of CISD2, including the antagonism of
PPAR-
The results obtained in this study demonstrate that CSC treatment inhibited astrocyte activation, astrocyte-mediated inflammatory responses, cellular apoptosis, and neuronal loss in rats following spinal cord hemisection. These results demonstrate the CISD2-preserving effects of CSC, which could contribute to the prevention of astrocytic activation, astrocyte-mediated neuroinflammation, apoptosis, and neuron loss.
WMK is responsible for the writing of the original draft and formal analysis. CJC was accountable for the resources, supervision, and investigation. TYC conceptualized the study and participated in the experiments and data analysis. MSL acted as the corresponding author and funded, wrote, reviewed, and edited the final draft.
All rats were treated as per the Experimental Animal Laboratory guidelines under the approval of the Animal Care and Use Committee of Chang Gung University (IACUC Approval No.: CGU14-052).
This work was supported by grants from the Health Bureau, Taipei City Government, Taiwan (10401-62-038).
The authors declare no conflict of interest.






