1 School of Kinesiology and Physical Education, Zhengzhou University, Zhengzhou Key Laboratory of Sport Nutrition and Health, Centre for Sport Nutrition and Health, 450001 Zhengzhou, Henan, China
2 School of Life Sciences, Zhengzhou University, 450001 Zhengzhou, Henan, China
3 Henan Sports Science and Technology Centre, 450012 Zhengzhou, Henan, China
†These authors contributed equally.
Abstract
Icariin (ICA) is a flavonoid, that has been shown to exert antioxidant and anti-inflammatory effects. We aimed to explore the effects of acute exhaustive exercise on skeletal muscle injury and inflammatory factor levels, and investigate the anti-injury and anti-inflammatory effects of ICA through gut microbiota modulation.
Thirty C57BL/6J mice were administered ICA by gavage for 8 consecutive weeks, which were randomly divided into 3 groups as follows: solvent gavage control group (CON), 25 mg/kg ICA gavage group (ICA-L), and 50 mg/kg ICA gavage group (ICA-H). Serum biochemical and skeletal muscle antioxidant indicators were measured. Antioxidant enzyme activities and anti-inflammatory factor levels were determined. Additionally, gut microbiota were sequenced and analyzed by 16S rDNA and the correlations between metabolic indices and microbial species were assessed using Spearman correlation analysis.
ICA alleviated oxidative stress in skeletal muscle by reducing malondialdehyde (MDA) levels and upregulating the activities and mRNA expression of antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-PX)). In addition, ICA suppressed inflammation through downregulation of tumor necrosis factor (TNF-α), nuclear factor-κB (NF-κB), and inflammatory cytokines (interleukin-6 (IL-6) and interleukin-1β (IL-1β)). Gut microbiota analysis revealed ICA enriched short-chain fatty acid (SCFA)-producing bacteria while inhibiting pathogens, with microbial shifts significantly correlated with muscle injury and antioxidant parameters, including Lachnospiraceae_NK4A136_group, Mucispirillum, and Harryflintia.
Our study demonstrated that ICA ameliorated exercise-induced acute muscle injury and inflammation in mice by modulating gut microbiota composition and regulating NF-κB signaling pathway along with related antioxidant enzyme gene expression.
Keywords
- icariin
- acute muscle injury
- inflammation
- gut microbiota
- antioxidant enzyme
Exercise and physical activity have been shown to enhance overall health and decrease the risk of obesity, cardiovascular disease, and even cancer [1]. However, it is important to strike a balance between levels of exercise that confer health benefits and levels that lead to muscle and tissue damage. When the body is subjected to exhaustive exercise, it becomes overstimulated [2], triggering a stress response that can cause tissue damage. Specifically, exhaustive exercise puts a significant strain on skeletal muscle, triggering overproduction of reactive oxygen species (ROS) in the cells, which can damage the muscle tissue [3]. Over time, the accumulation of oxidative stress and inflammation can result in damage to cell membrane integrity, protein oxidation, and even changes in cell function [4]. Although the body’s natural capacity to produce antioxidants can partially offset oxidative damage due to free radicals, it may not be sufficient; consequently, excessive exercise can impair skeletal muscle function and lower exercise performance. It is therefore important to find ways to supplement the body with external sources of antioxidants to enhance its ability to combat oxidative stress, protect tissues from damage, and ultimately improve exercise performance [5].
Accumulating evidence demonstrates that flavonoid supplementation upregulates endogenous antioxidant defense systems, particularly through activation of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX) enzymatic pathways [6]. This supplementation can improve exercise performance and protect against exercise-induced oxidative stress, inflammation, and tissue damage [7, 8]. A perennial herb known as Epimedium koreanum Nakai, belonging to the Berberidaceae family, has been identified as a potent source of flavonoid glycosides. In particular, icariin (ICA), a flavonol glycoside, is the primary bioactive compound in Epimedium koreanum Nakai and exerts protective effects against acute exhaustive exercise [9, 10].
ICA has been demonstrated to possess a robust capacity for scavenging free
radicals. It has been shown to inhibit lipid peroxidation and to stabilize the
structure and function of cell membranes, increasing antioxidant enzyme activity
and reducing oxidative damage. Recent research has further shown that ICA has the
ability to reduce the level of superoxide anion in human mesangial cells and
enhance the expression and activity of antioxidant enzymes [11]. The
supplementation of ICA increases SOD, CAT, and GSH enzyme activity, consequently
reducing ROS and malondialdehyde (MDA) levels in the paw. ICA has also been shown
to inhibit cigarette smoke-induced oxidative stress in human lung epithelial
cells, thereby controlling the development of airway diseases [12]. Additionally,
the anti-inflammatory effects of ICA are its most well-known feature.
Experimental evidence from animal studies demonstrates icariin’s
anti-inflammatory efficacy. ICA (12.5 and 25 mg/kg/day, gavage) ameliorated
experimental autoimmune encephalomyelitis in a mouse relapse-remission model by
suppressing the inflammation-related signal pathways [13]. A study using
streptozocin-induced diabetic nephropathy rats, showed that ICA (150 mg/kg/day,
gavage) attenuated renal inflammation via TLR4/NF-
The advancement of microbiome research has provided increasing evidence linking
disturbances in intestinal flora to systemic oxidative stress and inflammation.
Indeed, the gut microbiota contributes significantly to regulating oxidative
stress, inflammation, metabolism and energy expenditure during strenuous
exercise. Strenuous exercise may also result in intestinal ischemia, compromised
intestinal barrier function, and heightened oxidative stress. It has been
suggested that elevated concentrations of beneficial bacteria in microorganisms
can counter the oxidative stress resulting from exercise while effectively
regulating immune dysfunction and inflammatory responses that may occur
post-exercise [20]. The ingestion of probiotics and protein has been demonstrated
to reduce indices of muscle damage, accelerate recovery processes, and sustain
exercise performance after muscle injury [21]. A preliminary study on fifteen
healthy, resistance-trained males discovered that supplementation with
Streptococcus thermophilus FP4 and Bifidobacterium
breveBR03 can diminish the loss of exercise capacity and muscle function, as
well as promote muscle recovery following injury. The collective evidence
indicates that the judicious regulation of gut microbiota, coupled with the
augmentation of beneficial bacteria, has the potential to mitigate skeletal
muscle injury [22]. ICA, a compound found in plants, can improve the health of
the brain, liver, kidney, and intestines in aged mice by altering the microbiota
composition to resemble that of younger mice [23]. Specifically, this
intervention boosted beneficial bacteria like Akkermansia while reducing
harmful bacteria such as Mucispirillum. This modulation of the
microbiota resulted in the regulation of aging-related signaling molecules, such
as NF-
Previous research has demonstrated that ICA exhibits antioxidant and anti-inflammatory properties, but its impact on exercise-induced oxidative stress remains not fully elucidated. Hence, this study explored how ICA affects oxidative stress and inflammatory damage in mouse skeletal muscle induced following acute exercise. Additionally, we analyzed correlations between microbial composition changes and oxidative stress and inflammatory factor levels to explore potential associations.
Thirty male C57BL/6J mice, aged 8 weeks, were purchased from Beijing HFK
Laboratory Animal Company (Beijing, China). The mice were housed individually in
ventilated cages at the Centre for Sport Nutrition and Health, Zhengzhou
University, Zhengzhou Key Laboratory of Sport Nutrition and Health. Throughout
the experiment, a conventional 12 h light/12 h dark cycle was maintained to
sustain natural circadian rhythms. Ambient temperature was controlled at 22
After ICA intervention for eight weeks, all mice underwent a three-day treadmill (XR-PT-10B, Shanghai, China) adaptation training at a speed of 10 m/min for 10 minutes prior to the exhaustive exercise test. On the test day, one hour following the administration of the last gavage, exhaustive exercise was induced using a progressive running protocol. The initial speed was at a speed of 10 meters per minute for 8 minutes, followed by 15 meters per minute for another 10 minutes. Thereafter, running speed was increased by 1.5 m/min every three minutes until reaching a speed of 22 m/min. Mice then ran for 10 min at 22 m/min, then the speed was increased by 1 m/min every 1.5 min until exhaustion [27, 28]. The exhaustion standard was that the overall state of the mice at the end of the exercise was weak and limp, and they could not maintain the original running speed, even when repeatedly tapped on their backs [29, 30]. The exhaustion time was recorded. At the end of the experiment, 18-week-old mice were humanely euthanised through pentobarbital sodium solution (200 mg/kg, intraperitoneal) overdose. Blood was extracted from the retro-orbital sinus, allowed to clot at room temperature for one hour, and subsequently centrifuged. The upper serum layer was rapidly transferred and stored at –80 °C. The muscle tissue samples were snap-frozen using liquid nitrogen, and subsequently maintained in a –80 °C refrigerator.
The serum concentrations of lactate dehydrogenase (LDH), blood urea nitrogen (BUN), and creatine kinase (CK) were determined using reagent kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Oxidative stress markers in skeletal muscle, such as MDA, SOD, CAT, and GSH-PX,
were measured using commercial assay kits from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). Levels of IL-1
Gastrocnemius muscle samples were rinsed with cold saline, blotted dry with filter paper, partly fixed in 4% paraformaldehyde, dehydrated through a graded ethanol series, and subsequently embedded in paraffin. Sections were prepared from paraffin-embedded blocks and stained with hematoxylin and eosin (H&E). Finally observed it using light microscopy (Nikon Corp., Nishioi, Shinagawa-ku, Tokyo, Japan).
Total RNA isolated from the muscle was obtained using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and subsequently reverse transcribed following the protocol of a commercial kit manufacturer (Takara, Shiga, Japan) after the genomic DNA was eliminated. The PCR amplification was conducted on a quantitative real-time PCR platform (CFX96 Deep Well Dx) utilizing the UltraSYBR Mixture (Taizhou, Jiangsu, China). The 18S ribosomal RNA served as the reference gene, and the expression level of the target gene was assessed using the 2-ΔΔct method. Primer pairs were designed with NCBI’s Primer-BLAST and confirmed for specificity via a BLAST search. Primers were synthesized by Beijing Liuhe BGI Technology Co., Ltd. (Beijing, China). Forward and reverse primers corresponding to the target genes are listed in the Table 1.
| Target gene | Forward | Reverse |
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGTAGCG |
| Sod1 | CCACCATGTTTCTTAGAGTGAGG | ACCAGTTGTGTTGTCAGGAC |
| Cat | AGCHACCAGATGAAGCAGTG | TCCGCTCTCTGTCAAAGTGTG |
| Gsh-px1 | TTCCCGTGCAATCAGTTCGGACA | AGCCTTCTCACCATTCACTTCG |
| TNF- |
TGACAAGCCTGTAGCCCACG | TTGTCTTTGAGATCCATGCCG |
| NF-κB | ATGGCAGACGATGATCCCTAC | TGTTGACAGTGGTATTTCTGGTG |
Abbreviations: 18S, 18S ribosomal RNA; Sod1, superoxide
dismutase 1; Cat, catalase; Gsh-px1, glutathione peroxidase 1;
TNF-
A fecal DNA extraction kit (Model D3141, Guangzhou Meiji Biotechnology Co., China) was used to extract the DNA of cecum contents. The V3-V4 region of rDNA was amplified with primers 341F CCTACGGGNGGCWGCAG and 806R GGACTACHVGGGTATCTAAT. After amplification, PCR products were purified and quantified before being sequenced on an Illumina PE250 [31]. Chimeric sequences were filtered out using UCHIME [32], and the remaining sequences were grouped into operational taxonomic units (OTUs) based on 97% similarity through the UPARSE pipeline (v.9.2.64) [33]. Taxonomic classification was performed using a Bayesian classifier with the RDP (v.2.2) [34] and aligned with the SILVA database (v.132) [35]. Krona (v.2.6) [36] was used for abundance visualization.
The stacked bar plot depicting the community composition was generated using the ggplot2 package from the R project (v.2.2.1) [37]. For comparing species among groups, the Tukey’s HSD and Kruskal-Wallis H tests were conducted using the Vegan package in R (v.2.5.3, Auckland, Auckland Region, New Zealand) [38]. The calculation of the Alpha diversity index was executed via QIIME (v.1.9.1) [39]. Principal coordinate analysis (PCoA) was conducted and visualized by both the Vegan and ggplot2 packages in R [37]. Redundancy analysis (RDA), performed with the Vegan package (v.2.5.3) [38], was used to evaluate the impact of environmental factors on community composition. To examine the relationships between environmental factors and species, Pearson correlation coefficients were computed using the psych package from R (v.1.8.4) [40]. Additionally, heatmaps were generated with Omicsmart (http://www.omicsmart.com).
All results are presented as the mean
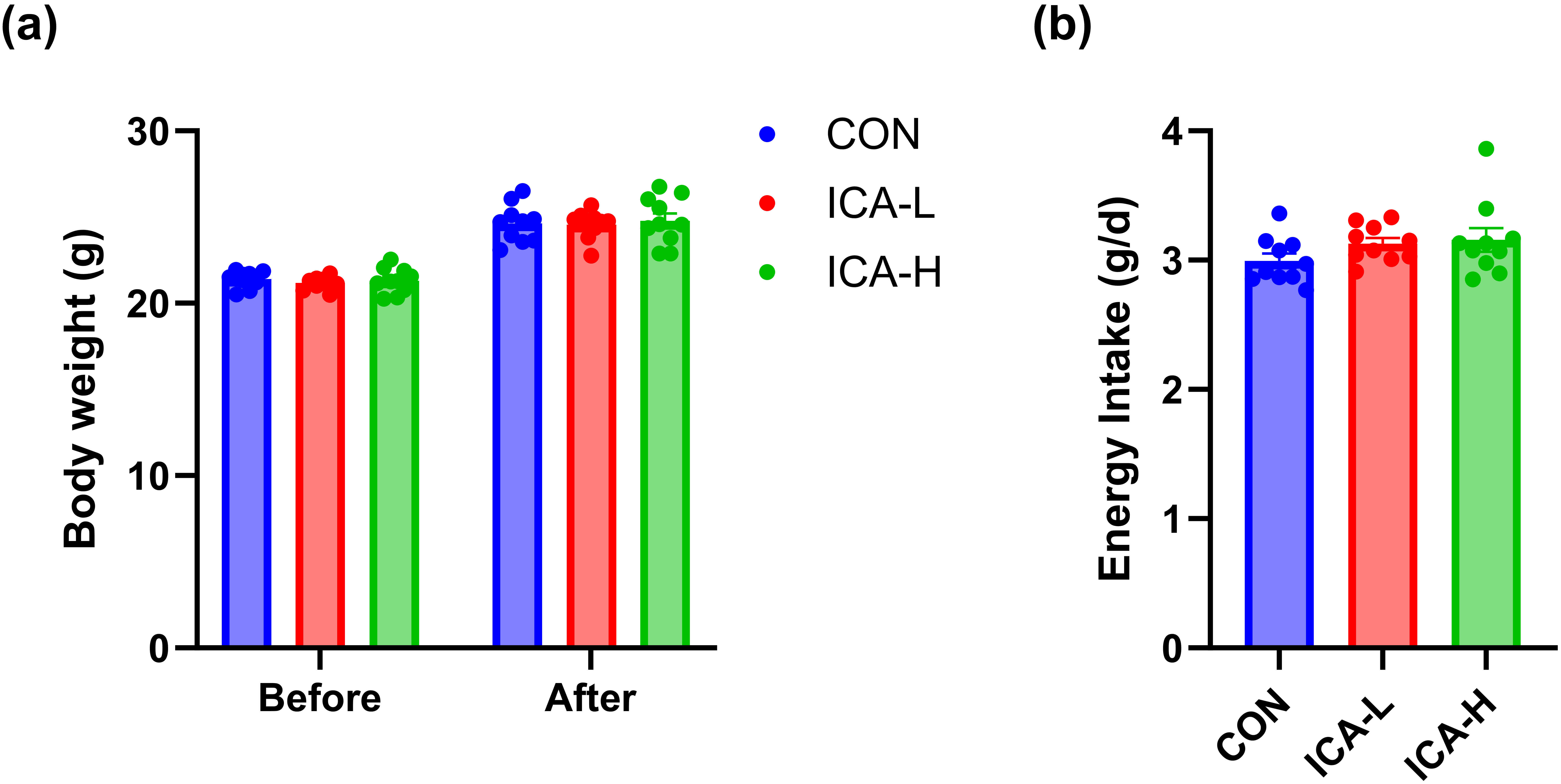
At the conclusion of ICA treatment, we assessed the changes in body weight and energy intake of the mice. No significant differences were observed in the body weight of the mice across the three groups, as shown in Fig. 1a. Furthermore, energy consumption did not differ significantly among the groups (Fig. 1b). These results indicate that dietary intake and growth of the mice were not affected by ICA intervention.
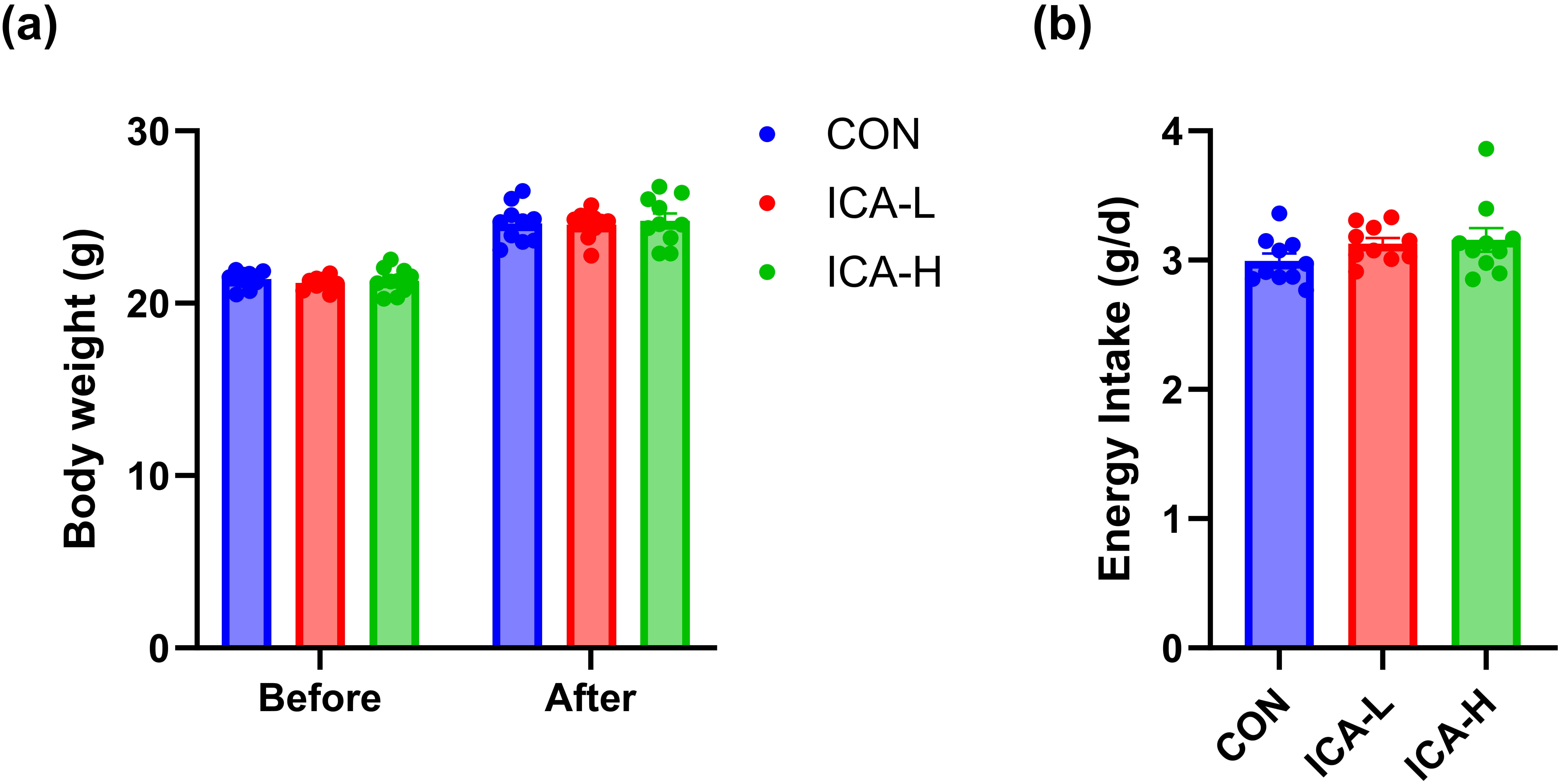 Fig. 1.
Fig. 1.
Effects of ICA treatment on body weight and energy intake in different groups of mice. (a) Body weight. (b) Energy intake. n = 10. Abbreviations: the CON group, solvent gavage control group; the ICA-L group, 25 mg/kg ICA gavage group; the ICA-H group, 50 mg/kg ICA gavage group.
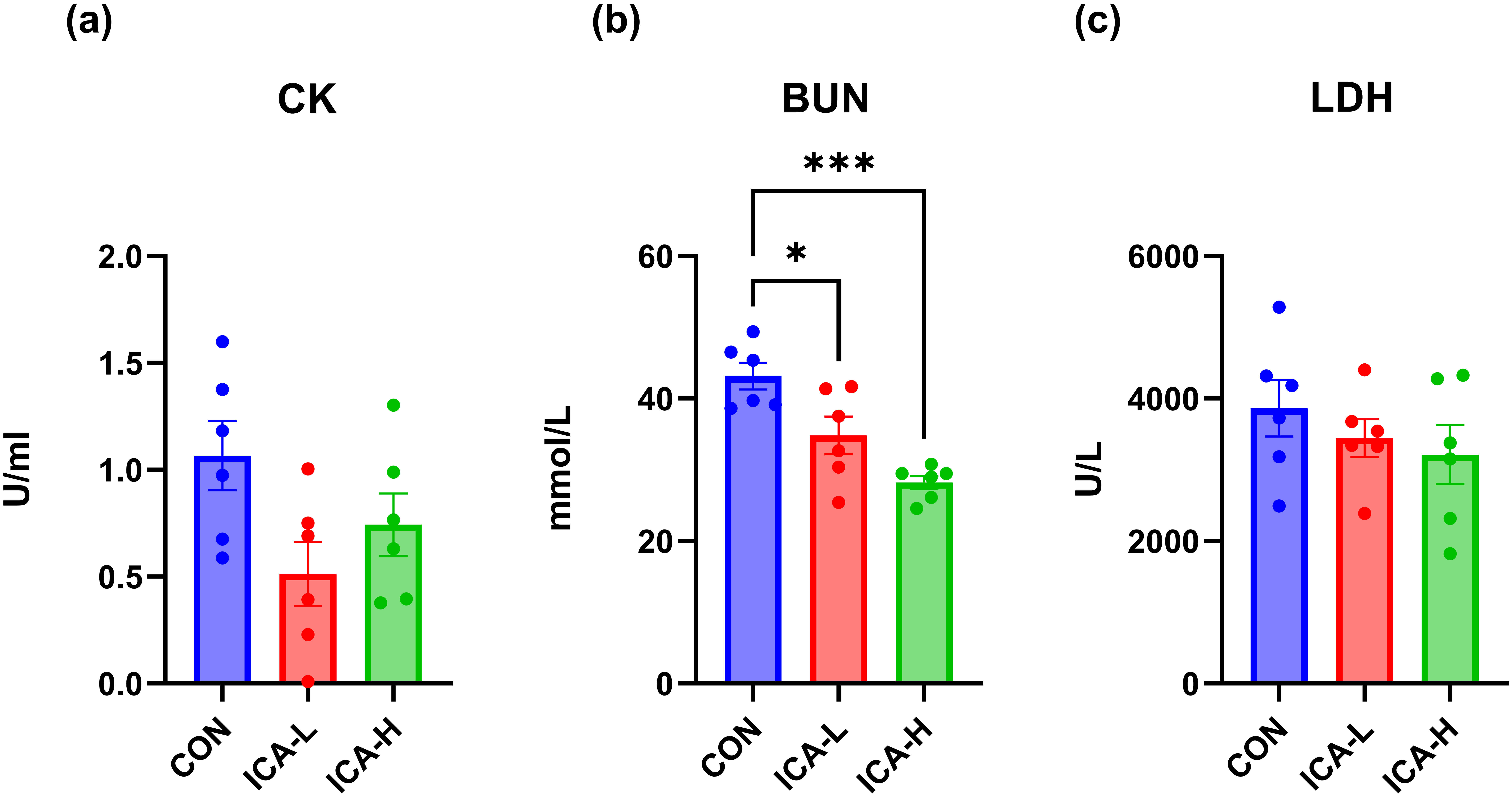
The exhaustive exercise led to elevated in serum levels of CK and BUN, however,
treatment with ICA significantly attenuated these increases when compared to the
CON group. Specifically, the CK level in serum was reduced in the ICA-L group
(p = 0.05), and BUN levels in both ICA intervention groups were
significantly lower than those in the control group (p
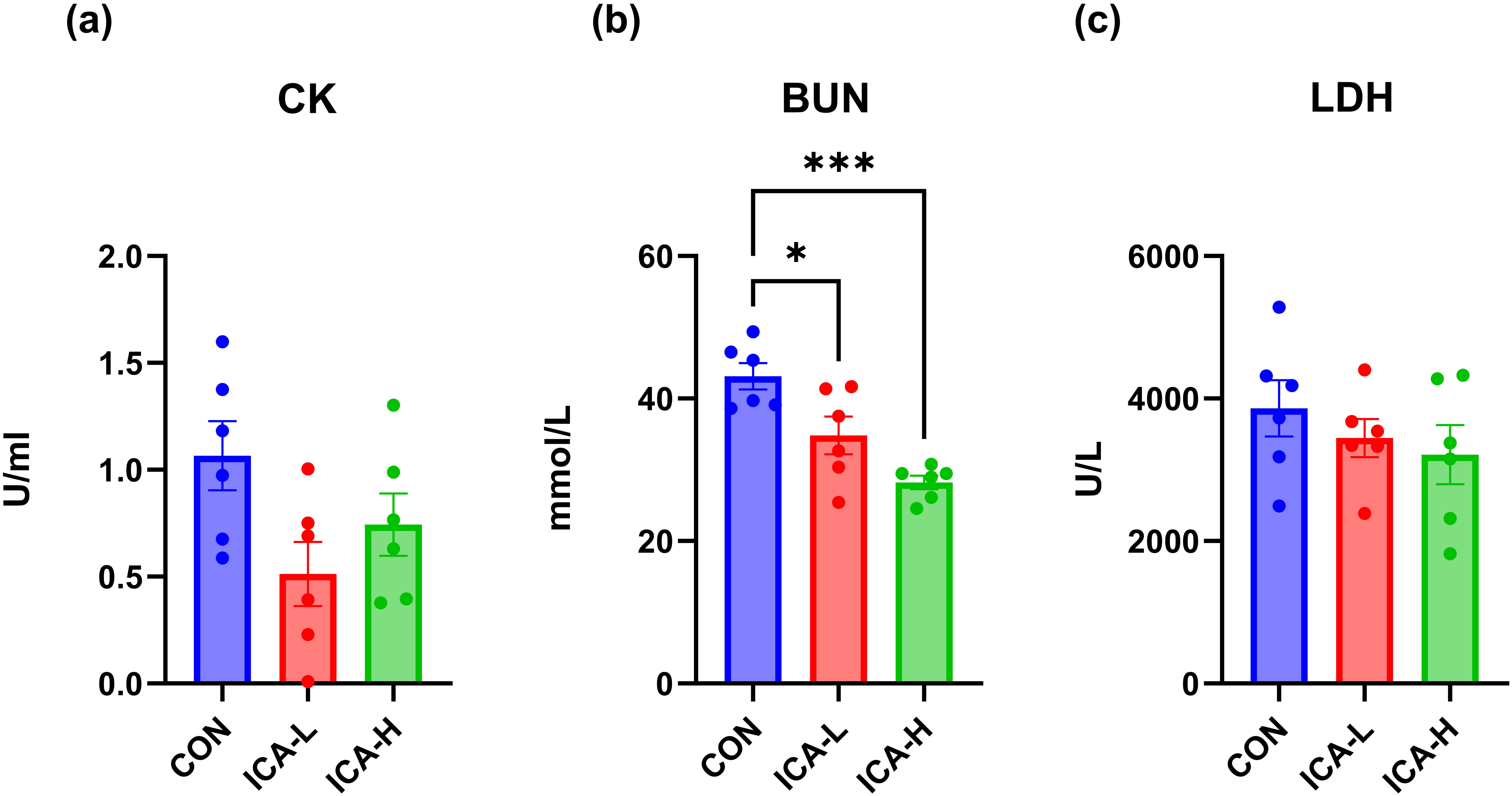 Fig. 2.
Fig. 2.
Effects of ICA treatment on blood biomarkers levels in exhausted
mice. (a) CK. (b) BUN. (c) LDH. n = 6, * p
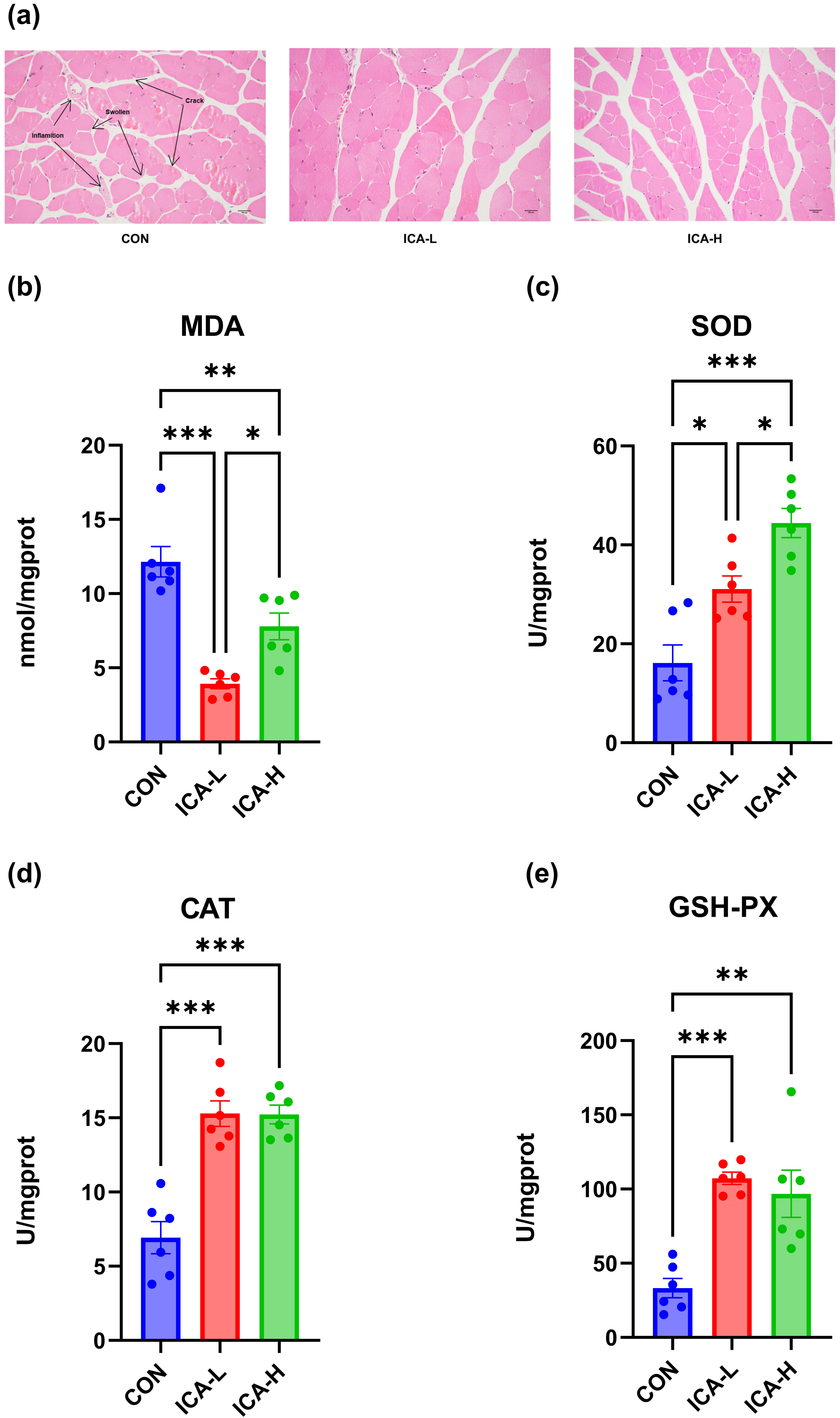
The H&E staining results of muscle sections revealed that exhaustive exercise induced structural damage in the muscle tissue of control mice. This damage included large areas of cracks, inflammatory cell infiltration, swelling, and even necrosis of muscle cells (Fig. 3a). However, these pathological symptoms were ameliorated to varying degrees in the ICA treatment groups. In the ICA-L group, local muscle fibers were slightly swollen and slightly cracked (Fig. 3a), whereas in the ICA-H group skeletal muscle cells were closely packed and had tiny cracks (Fig. 3a), suggesting that ICA intervention could significantly relieve the oxidative muscle damage induced by exhaustive exercise.
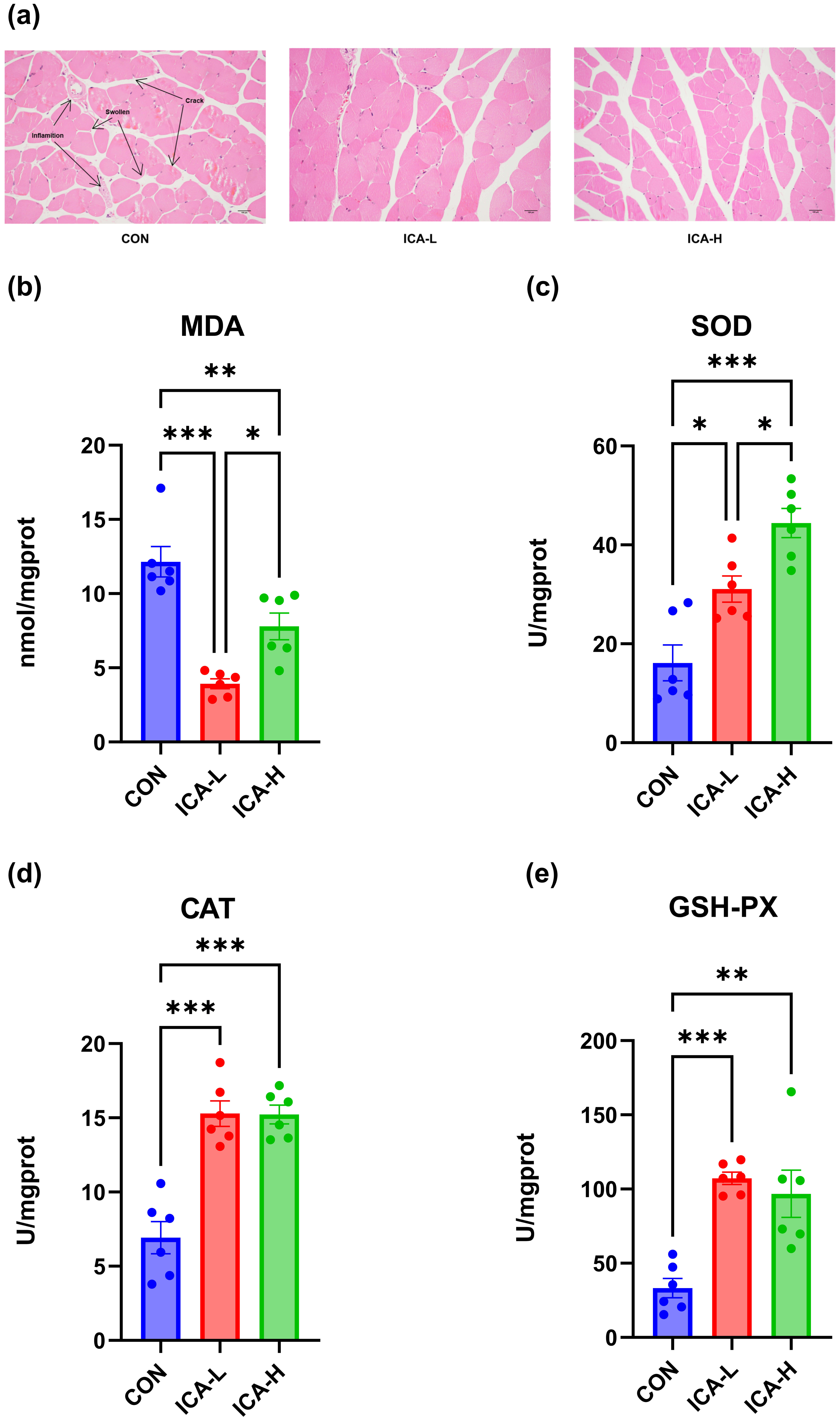 Fig. 3.
Fig. 3.
Effects of ICA on morphology and antioxidant capacity of mouse
myocytes. (a) Histological sections of skeletal muscle stained with H&E (scale:
100 µm). (b–e) Effects of ICA treatment on MDA, SOD, CAT, and GSH-PX
levels in skeletal muscle of exhausted mice. n = 6, * p
After exhaustive exercise, MDA levels in the muscle tissue of all three groups
were elevated, but to a lower extent in the ICA-L (p
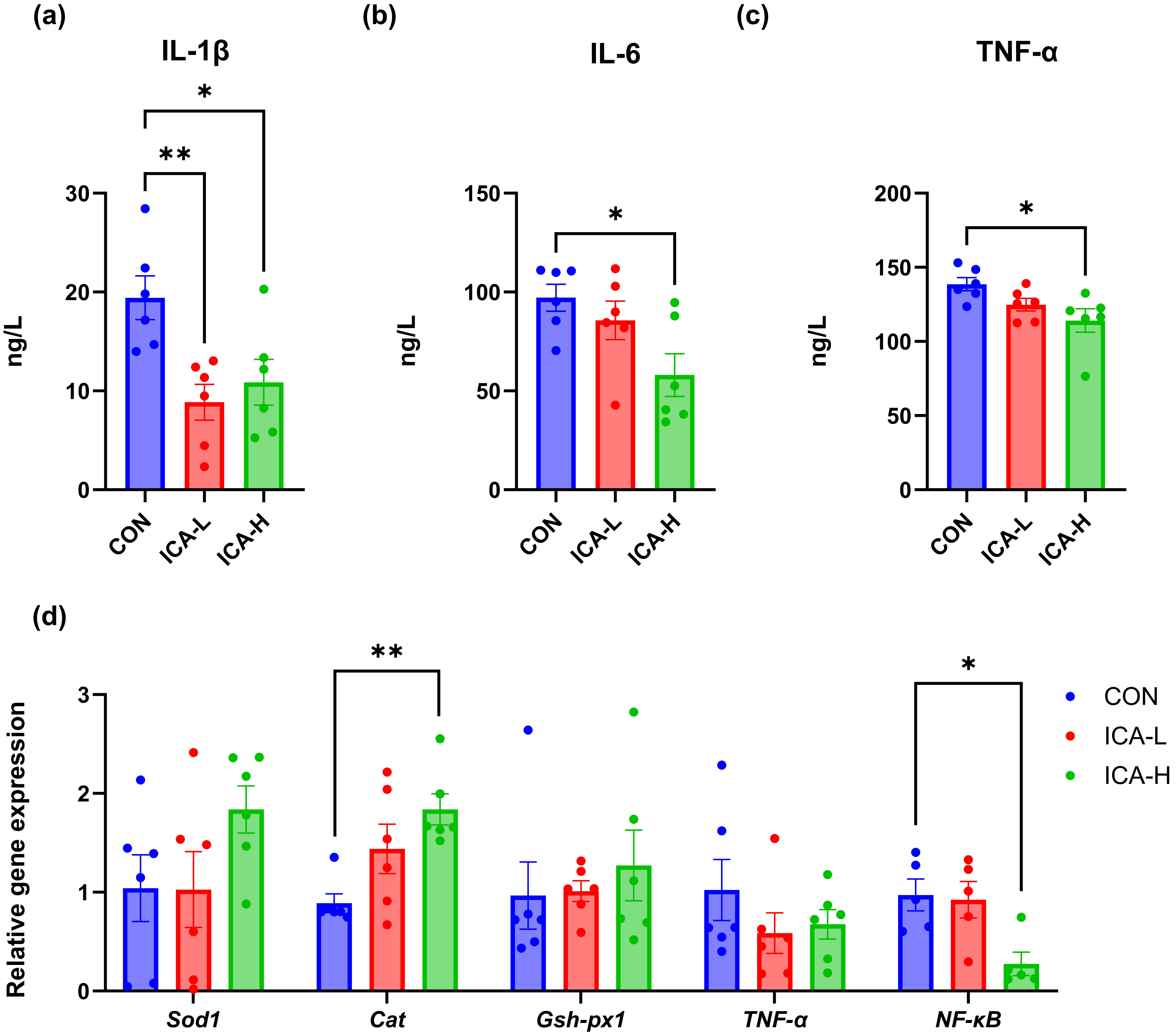
The impact of ICA treatment on skeletal muscle was assessed by evaluating the
levels of inflammation-related cytokines in muscle. The results showed that one
instance of exhaustive exercise stimulated significantly higher IL-1
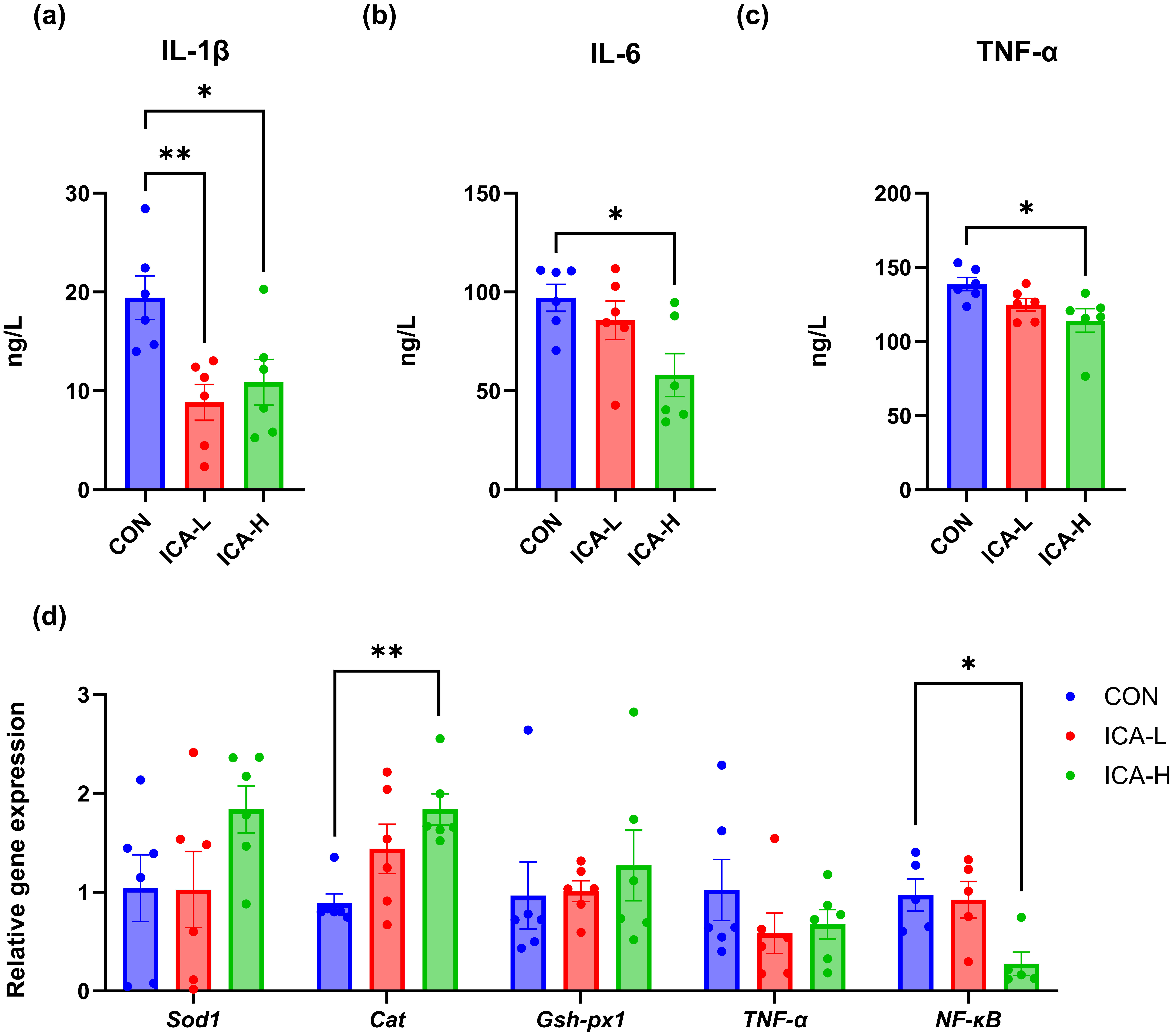 Fig. 4.
Fig. 4.
Effects of ICA treatment on inflammatory cytokine levels of
IL-6, IL-1
The ICA intervention reduced the pro-inflammatory cytokines levels and increased
intramuscular antioxidant enzyme activity. In order to evaluate even further the
effects of ICA on mice exercised to exhaustion, we examined the mRNA expression
related to muscle inflammation and damage. Compared to the control group, mice
receiving ICA intervention showed a significant upregulation of Cat mRNA
expression levels in the ICA-H group (p
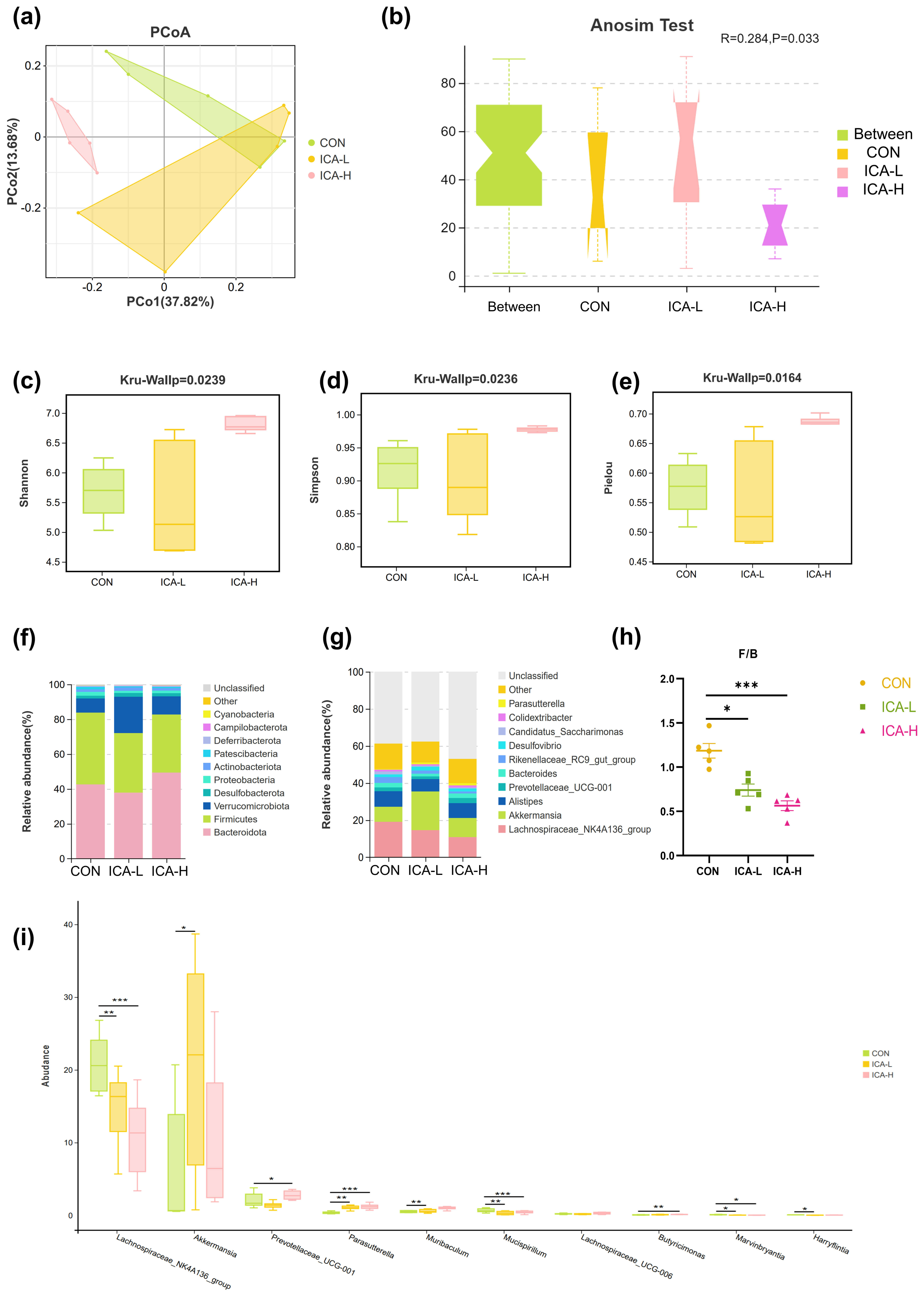
The dilution curves for all samples showed that the discovery rate of new OTUs levelled off as the sequencing volume increased, and the goods_coverage indices were all above 0.99 (indicating that at least 99% of the species had been detected), all of them reflecting that the amount of sequencing data was sufficient to cover the vast majority of microorganisms. Sob dilution curves, and list of goods_coverage data are in Supplementary Fig. 1 and Supplementary Table 1.
Next-generation sequencing of the 16S rDNA gene was performed to assess the
effect of ICA treatment on the gut microbiota structure. Principal coordinates
analysis (PCoA) based on Bray-Curtis distance exhibited the discrepancy in the
structure of the microbiota between the control and ICA intervention groups. The
ANOSIM Test results implied that ICA treatment significantly altered the gut
microbiota structure of mice (Fig. 5a,b). The
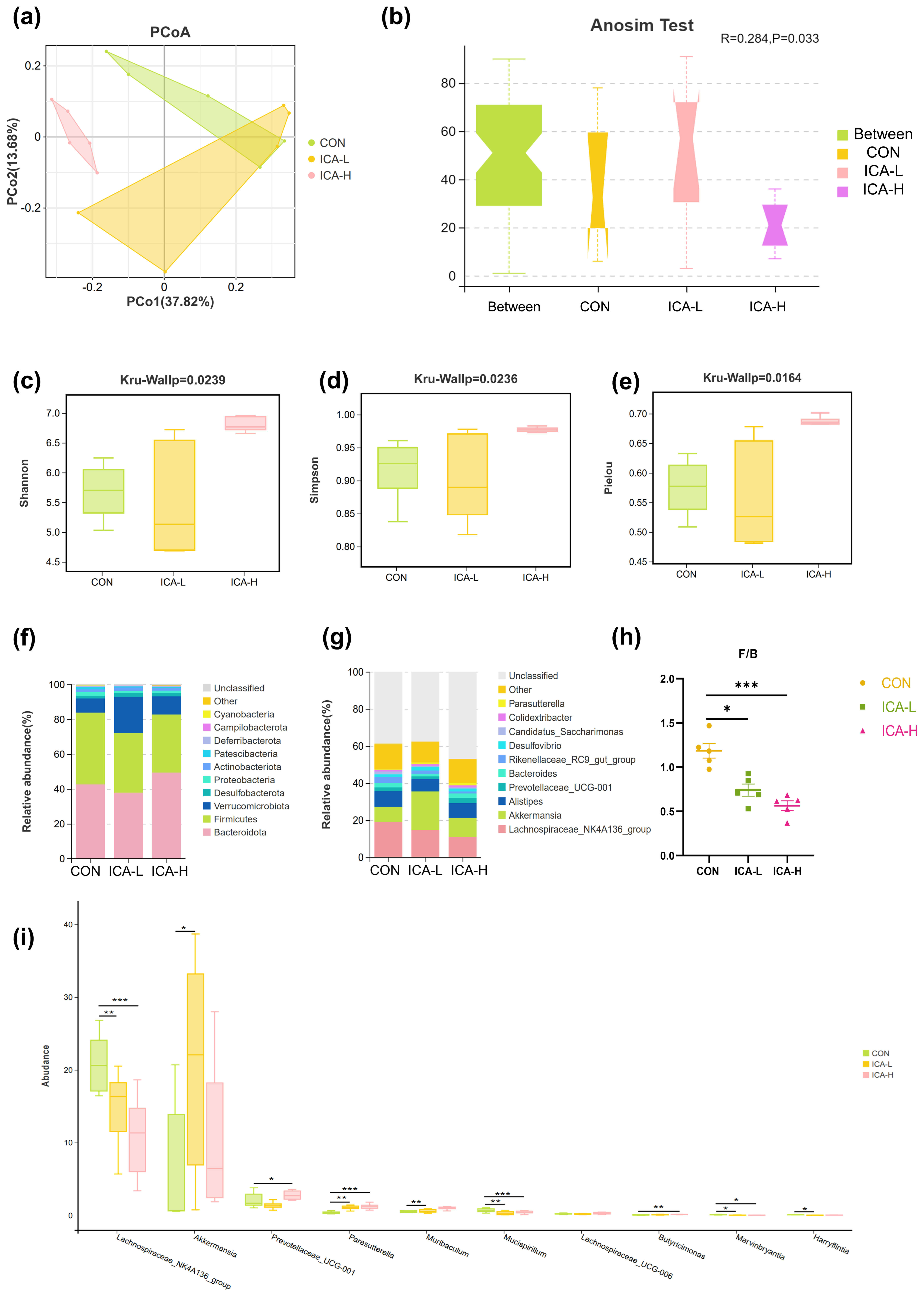 Fig. 5.
Fig. 5.
Influence of ICA treatment on the composition of intestinal
microbiota in mice of different groups. (a) Principal coordinates analysis
(PCoA) based on Bray. (b) Anosim (Analysis of similarities). (c) Shannon index.
(d) Simpson index. (e) Pielou index. (f) Species distribution stacking map
(phylum). (g) Species distribution stacking map (genus). (h) The ratio of
Firmicutes to Bacteroidetes. (i) Indicator species analysis. n = 5, * p
Fig. 5f,g display the differences in taxonomic composition, revealing
significant changes between the top ten microorganisms in abundance at the phylum
and genus levels. Bacteroidetes, Firmicutes, and Verrucomicrobiota emerged as the
dominant phyla of the microbiota (Fig. 5f). The ICA intervention group exhibited
a reduction in the abundance of Firmicutes compared to the control group, while
Verrucomicrobiota abundance increased in both the ICA-L and ICA-H groups compared
to the control group. Furthermore, notable variations were observed in the ratio
of Firmicutes to Bacteroidetes among the three groups. Both doses of ICA
intervention decreased the F/B ratio (p
To determine the differences in intestinal flora composition caused by ICA, we
performed an Indicator Species Analysis and applied the Tukey HSD rank sum test.
Significant differences were found in 10 microbial species at the genus level
among the three groups. Specifically, the abundance of
Lachnospiraceae_NK4A136_group and Mucispirillum was higher in
the CON group compared to the ICA groups (p
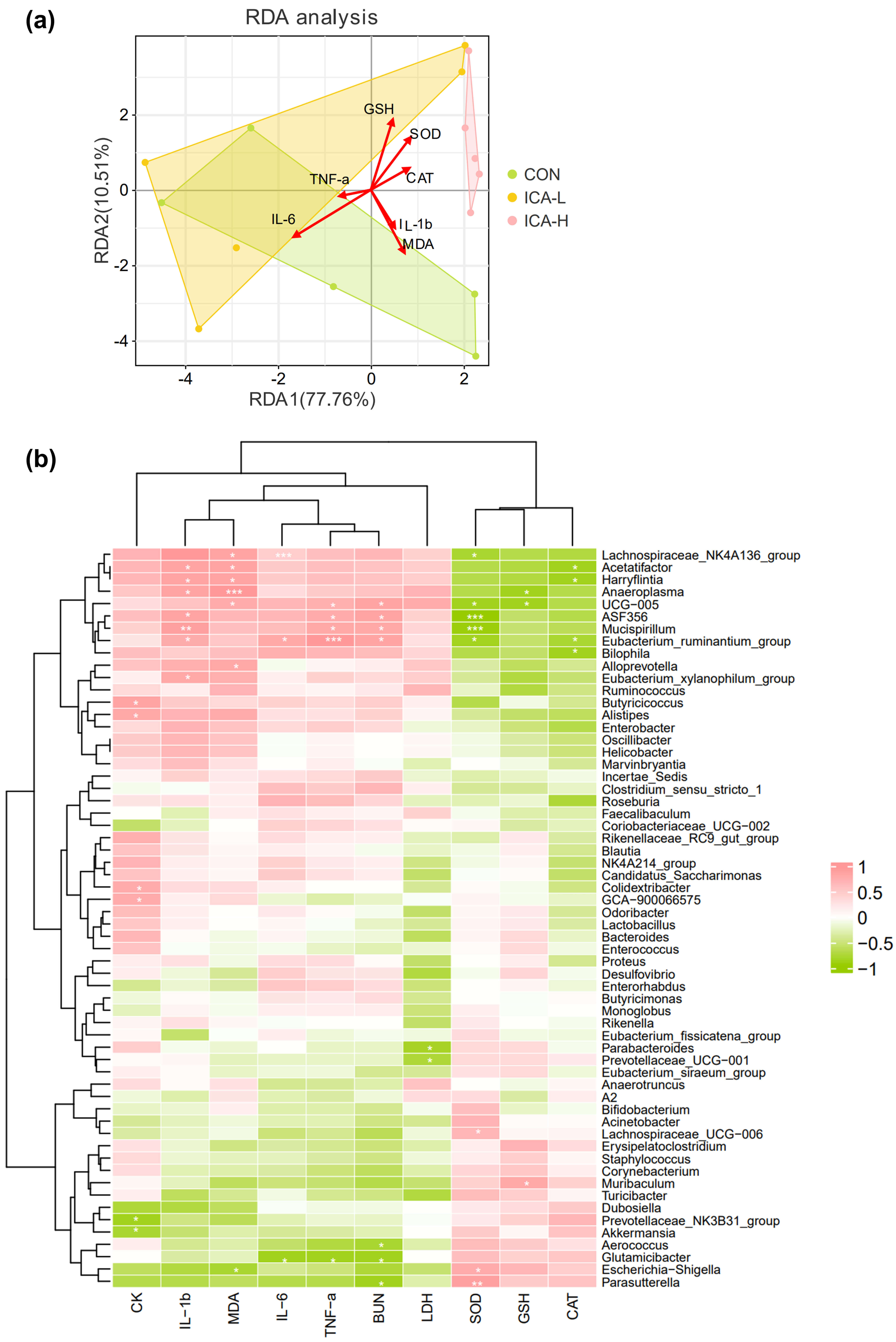
To delve deeper into the relationship between key gut microbial taxa and parameters related to skeletal muscle injury and inflammation, redundancy analysis was first conducted to delineate the relationship among microbiota, samples, and environmental factors. Our results indicated that markers of inflammation and muscle damage were more closely related to microbial composition in the CON group, especially MDA. However, the activity of antioxidant enzymes had a great influence on the structure of microorganisms in the ICA-H group, especially the activity of SOD (Fig. 6a). Then, we performed Spearman correlation analyses. The analysis showed significant correlations between muscle damage indicators and the abundance of the top 60 gut microbial genera.
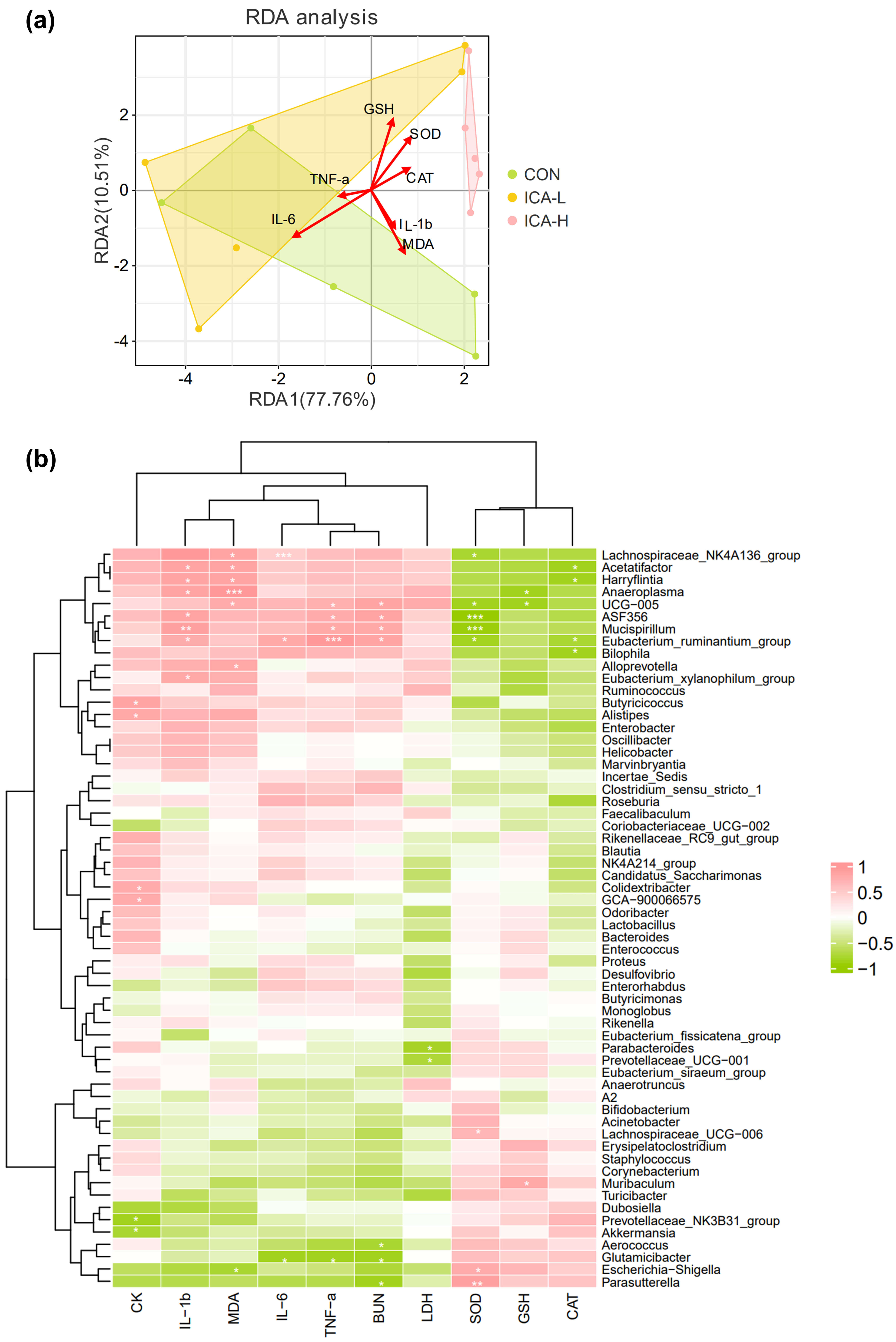 Fig. 6.
Fig. 6.
Relationship between intestinal microflora and proinflammatory
cytokine levels. (a) Redundancy analysis. (b) Heatmap of Spearman’s correlation.
The intensity of the color represents the degree of association. n = 5, *
p
Lachnospiraceae_NK4A136_group was negatively correlated with SOD
(p
This study assessed the impacts of two levels of ICA treatment on oxidative stress and inflammation triggered by exercise in skeletal muscle of mice. Exhausted mice were used as models to investigate the potential mechanism underlying any protective effects of ICA on exercise-induced muscle injury. To assess these effects, we measured serum injury markers, muscle antioxidant capacity, levels of inflammatory cytokines, expression of relevant mRNA, and intestinal microbial composition.
In contrast to moderate physical exercise, intense and prolonged contractions of skeletal muscles during exhaustive exercise induce fatigue, inflammation, and oxidative stress in various tissues within the body [41]. Skeletal muscle injury is a prevalent occurrence within the domain of sports medicine. Vigorous exercise triggers a surge in free radical production. Oxygen free radicals bind with unsaturated fatty acids on the cell membrane of skeletal muscles, prompting lipid peroxidation. MDA, a free-radical byproduct, is often used as a biomarker for tissue damage. Acute exhaustive exercise is well-regarded for causing oxidative stress damage in skeletal muscle, leading to elevated levels of MDA [42] and associated structural harm to muscle cells. Our study aligns with these findings, as one instance of exhaustive exercise resulted in heightened levels of MDA within gastrocnemius muscle of the control group following a single exhaustive exercise session.
Furthermore, numerous studies have confirmed that strenuous exercise increases oxidative damage markers in skeletal muscle and blood [43, 44], indicating the presence of oxidative stress. This type of exercise results in damage to the sarcoplasmic membrane and Z-discs of the skeletal muscle, increasing myocyte permeability [45]. Consequently, CK and LDH from the muscle enter the tissue fluid and pass through the lymphatic system into the bloodstream. Increased CK and LDH levels in both serum and muscle tissue are recognized indicators of muscle damage [44]. We observed that LDH and CK levels in the serum in the CON group were higher after a one-time exhaustive exercise. However, both doses of ICA intervention alleviated this effect, demonstrating the protective ability of ICA intervention against muscle injury induced by acute exhaustive exercise. Additionally, forceful exercise induces fatigue in the body, leading to the accumulation of metabolites [46]. BUN is the final byproduct of protein metabolism during high-intensity exercise when the energy demand exceeds the availability of carbohydrates and fats [47]. BUN serves as a sensitive indicator of body fatigue. In our study, ICA intervention significantly reduced the accumulation of BUN relative to the CON group. This suggests that ICA may exert a protective effect on the decline in muscle performance induced by the accumulation of metabolites.
Maintaining redox homeostasis is crucial for proper functioning. To prevent oxidative damage resulting from an increase in ROS, cells, including muscle cells, possess endogenous antioxidant defense mechanisms [48]. Intracellular antioxidants, both enzymatic and non-enzymatic, work together to regulate ROS levels in the body [49]. One important antioxidant enzyme is SOD, which is the first line of defense against free radical damage [50]. SOD helps in dissociating oxygen-free radicals. Additionally, CAT and GSH-PX also play a vital role in protecting cells from oxidative stress. They assist in the decomposition of hydrogen peroxide [51, 52]. Our study investigated the impact of ICA treatment on the activities of antioxidant enzymes in skeletal muscle. Our findings showed that ICA treatment significantly increased the activities of SOD, CAT, and GSH-PX enzymes. Furthermore, we observed an enhancement in the expression of Sod1, Cat and Gsh-px1 mRNA, especially in the ICA-H group. Consistent with previous studies, ICA administration effectively enhanced antioxidant enzyme activity and mitigated hypoxia-induced apoptosis in osteoblasts in a dose-dependent manner [53].
Another distinct feature of the skeletal muscle injury and associated
inflammatory response caused by strenuous exercise is the elevation of
pro-inflammatory cytokines, specifically IL-6, IL-1
Gut microbiota can mitigate the impact of exercise-induced oxidative stress and inflammatory responses. It also makes a vital contribution to increasing energy expenditure during intense physical activity [60]. To assess intestinal dysbiosis, the high F/B ratio is commonly utilized as a substitute [61]. In our study, after exhaustive exercise, the F/B value of the control group was significantly higher compared to the group that received intervention with ICA. This indicates that ICA intervention plays a vital role in maintaining intestinal homeostasis. Our findings reveal that treatment with ICA had a marked effect on gut microbiota composition. It notably reduced the abundance of Lachnospiraceae_NK4A136_group, Mucispirillum, Marvinbryantia, and Harryflintia, which had been increased due to exhaustive exercise. Importantly, a positive correlation was found between the abundance of these genera and the levels of inflammatory cytokines in muscle as well as injury-related markers in serum. The presence of Lachnospiraceae_NK4A136_group has been linked to brain inflammation in Alzheimer’s disease. However, it has also been found to be useful as it enhances butyrate production, and alleviates liver lipid accumulation and oxidative stress [62]. Furthermore, the presence or absence of non-specific disease-related genera seems to be a more reliable indicator of the transfer of disease-associated microbes compared to changes in their relative abundance [63]. Mucispirillum is widely distributed in the digestive tract and has the ability to cross the mucosal barrier by flagellar movement, increasing the risk of host inflammation [64]. This agrees with earlier research, which has established a high association of its prevalence within the gut microbiota and inflammatory bowel disease as well as inflammation-driven obesity [65]. In our study, exhaustive exercise led to an enrichment of Mucispirillum, elevating its risk of crossing the intestinal barrier into the circulation and causing muscle inflammation.
In addition, ICA intervention increased the proportion of beneficial bacteria,
including Akkermansia, Parasutterella, Muribaculum, and Butyricimonas. Previous
studies have revealed that astragalus, a type of flavonoid, can significantly
mitigate the decline in Akkermansia levels in mice with colitis induced by
dextran sulphate sodium [66]. This suggests that astragalus has an important role
in reducing intestinal inflammation. Parasutterella is an integral component of
gut microbiota of both humans and mice. It actively participates in bile acid
metabolism and produces succinic acid to maintain the symbiotic relationship
among intestinal microbiota [67]. Furthermore, the abundance of Parasutterella is
inversely correlated with metabolic conditions induced by a high-fat diet, such
as obesity, diabetes, and fatty liver, which includes inflammation [68].
Muribaculum is a commensal bacterium that has been previously shown to hinder
pathogen colonization and preserve gut homeostasis. It is the predominant flora
in healthy mice [67, 69] and exhibits a negative correlation with
aging and oxidative stress processes [70]. It is widely known that an increase in
short-chain fatty acids (SCFAs), particularly butyrate and propionate, inhibits
the activation of NF-
Our research aimed to investigate the effects of ICA on oxidative stress and inflammatory injury in mouse skeletal muscle caused by acute exercise, as well as to determine whether changes in microbial composition are associated with oxidative stress and inflammatory factor levels. Although the present study revealed associations between the gut microbiota and oxidative stress and inflammatory factors, the causal relationship needs to be further verified by mechanistic studies. We focused on measuring the gene expression of specific cytokines and enzymes. However, it is important that further studies investigate the signaling mechanisms by which ICA supplementation alleviates exercise-induced muscle damage. While there is existing research on the use of ICA to mitigate tissue oxidative damage, there is limited understanding about its effects on exercise-induced oxidative damage. Given the potential for gender differences, it is also crucial to explore whether similar outcomes would arise in female animals. It is recommended that future studies concentrate on investigating the signaling mechanisms that underpin the effects of ICA on the gut microbiota and the specific associations between gut microbes and muscle health. This will allow for further refinement and optimization of the use of ICA in acute muscle injury.
Overall, ICA intervention significantly modulated the gut microbiota, enhancing those associated with SCFAs production, while reducing pathogenic bacteria. Post-exercise, this shift was strongly linked with an enhancement of muscle antioxidant capacity and a decrease in expression of inflammatory cytokine. Consequently, our hypothesis is that ICA treatment has the potential to alter the gut microbiota composition and lower the risk of both acute muscle injury and inflammation.
This study has limitations. First, employing single-bout exhaustive exercise may not fully reflect varied exercise-induced injury patterns influenced by intensity, duration, or modality. Future work should explore resistance or high-intensity interval training to clarify exercise-specific effects. Second, while focused on gene expression of select cytokines/enzymes, ICA’s signaling mechanisms against muscle injury remain unclear. Moreover, potential sex-related differences were not addressed in this experimental design. It is essential to examine whether female animal models exhibit comparable outcomes to ensure broader applicability of the findings.
In the present study, ICA treatment was demonstrated to ameliorate skeletal muscle inflammation and gut microbial dysbiosis in mice subjected to acute exercise-induced injury. Specifically, ICA was able to alleviate muscle damage caused by acute exhaustive exercise. This was mediated through the upregulation of antioxidant gene expression and enzyme activity while downregulating inflammatory factor expression. Furthermore, ICA was shown to significantly shifted the gut microbiota composition, enriching beneficial bacteria and reducing harmful bacteria. This study provides evidence suggesting that ICA is an effective antioxidant for protecting skeletal muscle during exercise, and warrants future investigation in human studies.
Data will be made available on request.
SW, RCR and CZW designed the research; RCR, DYL, TF, WY, YJB and ZY performed the experiment; SW, RCR, DYL, THY and LDL collected and assayed the data, with LDL specifically responsible for mathematical and statistical analysis. All authors contributed to editorial changes in the manuscript. All authors have read and approved the final submitted version. They have all made substantial contributions to the work and have agreed to be responsible for all aspects of the research.
The study was carried out by the guidelines of the NIH for care and use of lab animals. Additionally, the animal experimental procedures were approved by the Life Sciences Institutional Review Board of Zhengzhou University (Approval number: ZZUIRB 2023-203).
We thanks to Prof. Dr. David Raubenheimer for his guidance and revision of the writing, thanks to Donglin Liu’s research focuses on the intersection of nutrition and economics, particularly on how purchasing power and nutritional choices impact health outcomes. Thanks to all those who participated in and contributed to this study.
This work was supported by Top-notch Talents of Zhengzhou University (32211502, 32212341, 32340114, 32340438, 35220132), Nutrition and Food Development Funding from Henan De Rui Si Health Technology Co., Ltd. (24120031), the Outstanding Young Science and Technology Talent Project of Zhengzhou City, the Key Research and Promotion Special Project (Science and Technology Innovation) of Henan Province (252102310006) and Startup Funding for scientific research of Zhengzhou University (32212962).
The authors declare no conflict of interest. And Henan De Rui Si Health Technology Co., Ltd. declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/IJVNR39821.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.